Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5420
Peer-review started: September 19, 2021
First decision: December 10, 2021
Revised: December 19, 2021
Accepted: April 22, 2022
Article in press: April 22, 2022
Published online: June 6, 2022
Processing time: 255 Days and 21.5 Hours
Gastric hepatoid adenocarcinoma (GHA) is a rare and aggressive cancer that is characterized by foci with features of both hepatocellular differentiation and adenomatous differentiation. However, there is currently no standard treatment for this disease, which has a poor prognosis.
A 72-year-old male with a body mass index of 20.9 was diagnosed with GHA with perigastric lymph node and liver metastasis. He underwent first-line chemotherapy but that failed. Pembrolizumab and bevacizumab with chemotherapy were used in the second-line treatment. The progression-free survival and overall survival were 14 mo and 16 mo, respectively, after treatment. In addition, the main adverse reaction was tolerable. The patient did not die of tumor progression.
The combination of pembrolizumab and bevacizumab with chemotherapy is an effective and safe regimen for GHA and may be recommended as a new choice for GHA treatment. Further studies should evaluate this treatment in a larger cohort or a randomized controlled trial.
Core Tip: We report a rare case of gastric hepatoid adenocarcinoma in a patient who underwent general chemotherapy that failed. However, pembrolizumab and bevacizumab combination chemotherapy was successful. The overall survival was 16 mo, and the main adverse reaction was tolerable. The patient died of intestinal infection rather than tumor progression.
- Citation: Liu M, Luo C, Xie ZZ, Li X. Treatment of gastric hepatoid adenocarcinoma with pembrolizumab and bevacizumab combination chemotherapy: A case report. World J Clin Cases 2022; 10(16): 5420-5427
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5420.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5420
Gastric hepatoid adenocarcinoma (GHA) is a primary gastric cancer with the characteristics of adenocarcinoma and hepatocellular carcinoma-like differentiation. GHA is a rare and special subtype of gastric cancer with a clinical incidence rate that is less than or equal to 1%[1-3]. GHA is more common in men. Most patients have high serum AFP levels and are prone to lymph node and liver metastasis. The median overall survival time of GHA is 6-17 mo[4-6]. Some studies have confirmed that the prognosis of patients with GHA is worse than that of patients with common gastric cancer. However, there is currently no standard treatment for this disease.
We report a case of GHA who experienced general chemotherapy failure. Pembrolizumab and bevacizumab combination chemotherapy was successful. The overall survival (OS) was 16 mo, and the main adverse reaction was tolerable.
A 72-year-old male was admitted to our hospital in December 2019 with a 3-mo history of epigastric pain.
Three months before admission, the patient began to experience epigastric pain. He had no abdominal distention, diarrhea, nausea, vomiting, etc. Because the symptoms persisted and tended to worsen, the patient visited our hospital for further evaluation.
He had no history of other diseases.
No family history to note.
Upon physical examination, the abdomen was flat and soft. There was tenderness in the upper abdomen without rebound pain or muscle tension. There was no mass in the abdomen, and there was no swelling of the liver or spleen.
Routine blood examination, blood coagulation function, urinalysis, stool analysis, liver chemistry tests, urea, creatinine, uric acid and electrocardiogram results were all within normal limits. His serum AFP was 339.6 μg/L on December 5, 2019.
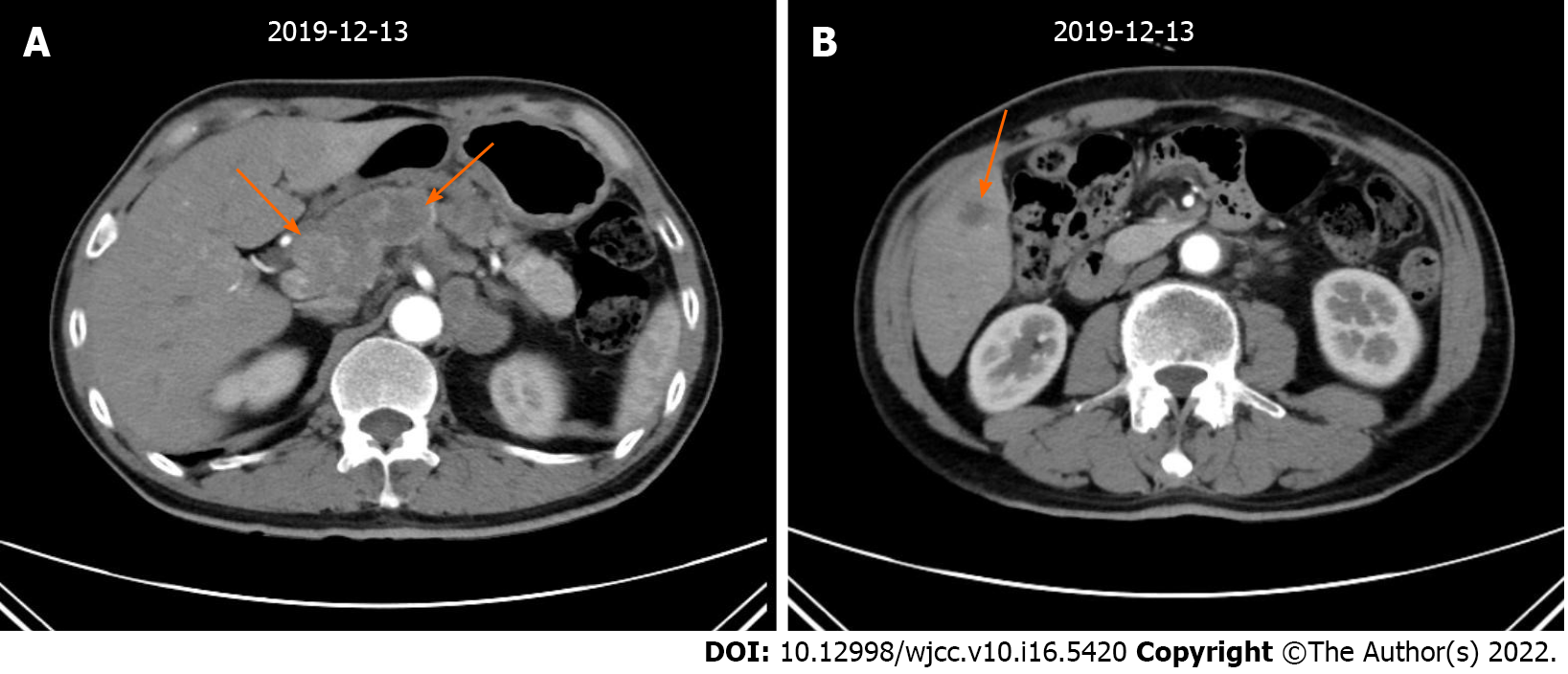
An abdominal enhanced computed tomography (CT) scan revealed the following: (1) The gastric fundus and cardia were occupied malignant tumors and multiple lymph node metastases were found around the stomach; and (2) The right anterior lobe of the liver had a low density and was considered metastatic (Figure 1). PET/CT examination showed gastric cancer with perigastric lymph node and liver metastasis. No obvious abnormality was found in the rest of the abdomen.
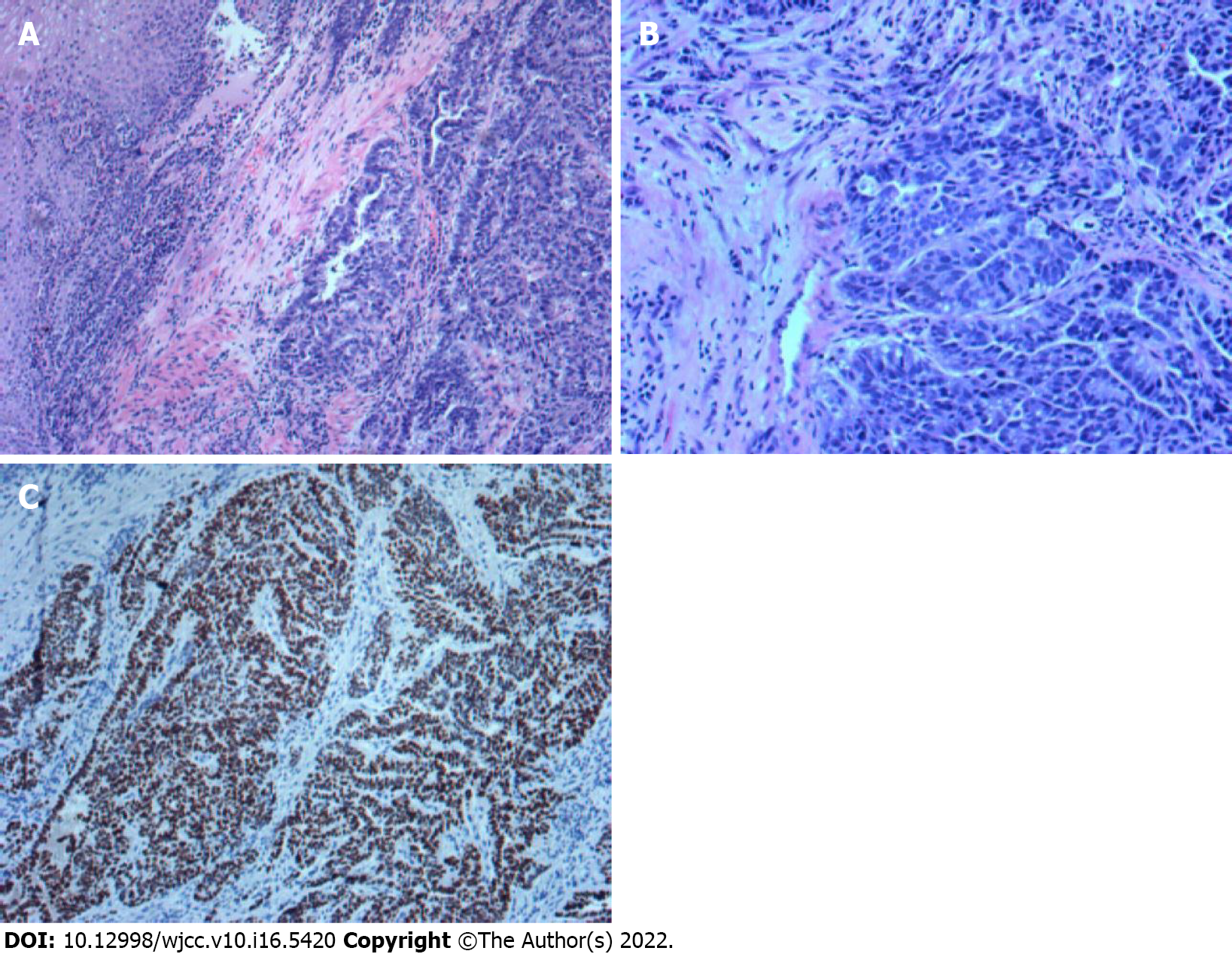
A gastroscopy biopsy confirmed a histopathological diagnosis of gastric hepatoid adenocarcinoma. Immunohistochemistry showed HER-2 (-), Ki-67 (+ > 75%), MSH6 (+ > 75%), MSH2 (+ > 75%), PMS2 (+ > 75%), MLH1 (+ > 75%), AFP (+), hepatocytes (+), GPC-3 (+), and SALL4 (+) (Figure 2). Gene detection was performed (2019-12-27, Baishibo, sample type: plasma and paraffin section, SP142), and programmed cell death-ligand 1 (PD-L1) protein expression in paraffin sections was positive. The percentage of positive tumor cells was 0%. The comprehensive positive score was + (5%). In a paraffin section, microsatellites were stable. The TMB values were 8.94 mutations/MB (high) in plasma and 3.81 mutations/MB (moderate) in paraffin sections, with quantiles of 82.36% and 41.9%, respectively. The TP53 Levels were 57.79% in paraffin sections and 11.98% in plasma.
The patient was diagnosed with GHA, with a classification of clinical stage IV.
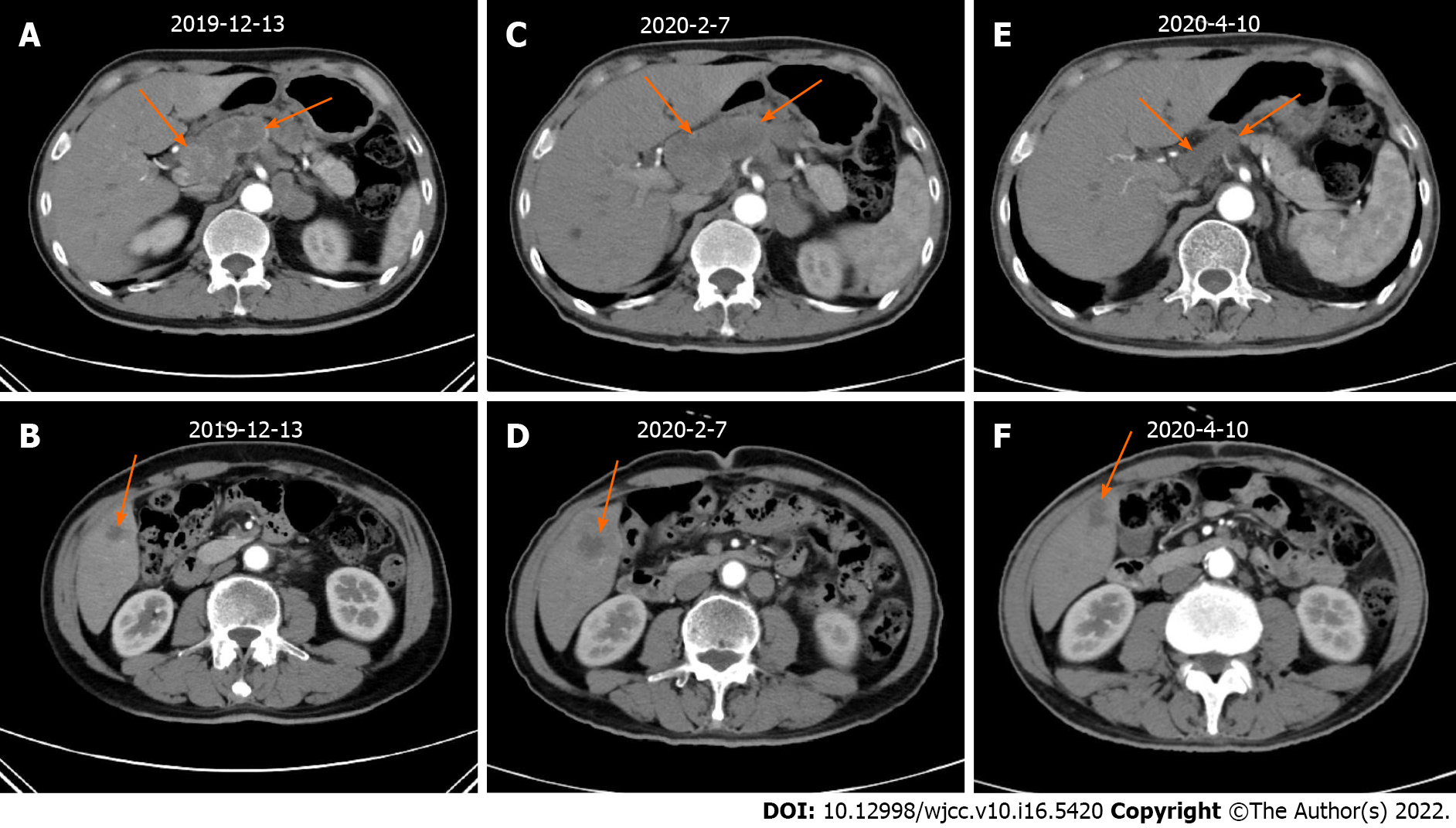
According to the National Comprehensive Cancer Network guidelines, the patient received first-line treatment of 2 cycles of chemotherapy with oxaliplatin + teggio on December 17, 2019. The doses of oxaliplatin and teggio were 130 mg/m2 (Day 1) and 60 mg bid (Days 1-14), respectively. Then, reexamination of abdominal enhanced CT showed the following: (1) the gastric fundus and cardia were occupied, the abdominal cavity and retroperitoneal lymph node showed signs of metastasis, and the previously identified lesions were larger than before; and (2) the range of lesions in the right lobe of the liver was larger than before (Figure 3).
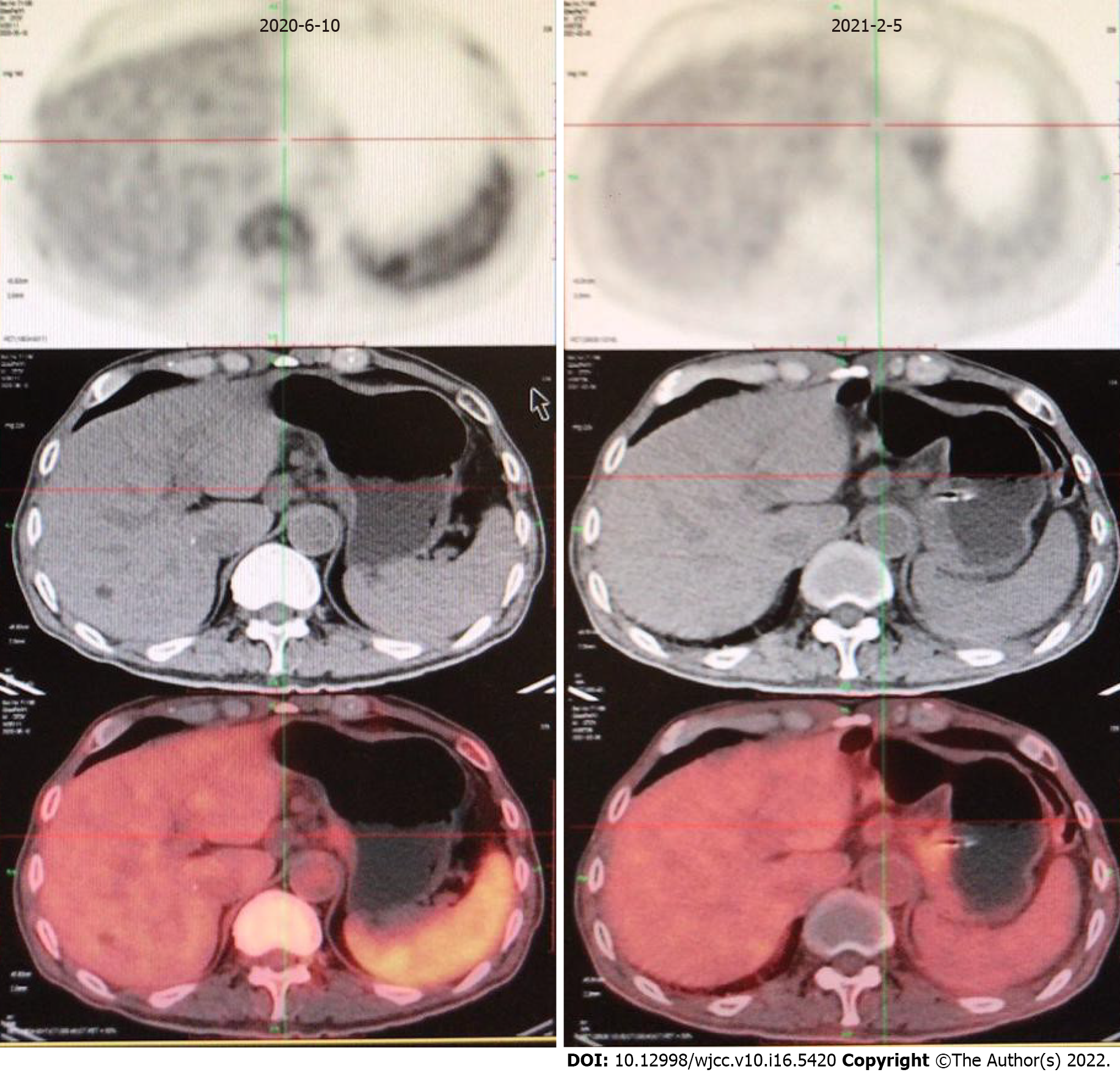
The following evaluation of curative effect is based on the Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.1 standard. We selected liver metastases and the two perigastric malignant lymph nodes (indicated by the arrow in Figure 3) as target lesions, in which the maximum diameter of liver metastases and the minimum diameter of perigastric malignant lymph nodes were measured. The sum of the three was the measurable lesion length. See Table 1 for detailed data. Disease progression (PD) was evaluated. Due to failure of first-line chemotherapy, the effective probability of second-line chemotherapy by itself was not high. GHA has similar components to hepatocellular carcinoma. In recent years, drug treatments for liver cancer have been tried as treatments for GHA. After communicating with the patient and his family and obtaining their consent, we decided to try second-line chemotherapy with pembrolizumab and bevacizumab in February 2020. The doses of epirubicin, albumin binding paclitaxel, pembrolizumab and bevacizumab were 90 mg/m2 (Day 1), 260 mg/m2 (Day 1), 2 mg/kg (Day 2) and 7.5 mg/kg (Day 0). This was repeated every 3 wk. However, the patient developed 4 degrees of myelosuppression and agranulocytosis with fever and 1 degree of gastrointestinal reaction after the first cycle of the above treatment. He returned to normal soon after symptomatic treatment. Therefore, epirubicin was reduced to 80 mg/m2, while other drug doses remained unchanged in cycles 2-4. The above side effects did not reoccur. The patient achieved remission after second-line treatment (Figure 3Evs3C; Figure 3Fvs3D). After general surgery consultation, surgery was recommended. Therefore, we halted bevacizumab treatment in the 5th cycle (June 2020) and recommended that the patient undergo general surgery for surgical treatment after 3 wk. However, due to his advanced age, he did not follow the doctor's advice undergo surgery. In August 2020, he received pembrolizumab by itself for the last time. However, he received no further treatment and recuperated at home for personal reasons. However, in January 2021, he developed diarrhea with fever and could not eat normally. His body mass index dropped to 18. Thus, he returned to the hospital. PET/CT examination showed that the tumor was still stable (Figure 4).
| Date | The minimum diameter of perigastric malignant lymph nodes 1 (cm) | The minimum diameter of perigastric malignant lymph nodes 2 (cm) | The maximum diameter of liver metastases (cm) | The sum (cm) |
| December 13, 2019 | 1.49 | 2.15 | 1.82 | 5.46 |
| February 7, 2020 | 2.25 | 2.82 | 3.19 | 8.26 |
| April 10, 2020 | 1.54 | 1.02 | 1.71 | 4.27 |
Laboratory examinations showed that his inflammatory indices were high, leukocytes and neutrophils were slightly low, and other measures, such as thyroid function and the presence hepatitis B virus, were normal. However, the patient refused to allow examination of the stool routine, culture, etc., thus preventing the collection of etiological evidence. The possibility of intestinal infection was considered in the diagnosis. The patient was given parenteral nutrition, anti-infection therapy, an indwelling gastric tube for enteral nutrition and other supportive treatment. After the symptoms improved, the patient was discharged and returned to his hometown.
The patient's family reported that the patient had diarrhea with fever again in March 2021. He was treated in a local hospital but died in April 2021. The cause of death was intestinal infection. The last telephone follow-up was June 18, 2021. The patient, whose progression-free survival (PFS) and OS were 14 mo and 16 mo, achieved remission after second-line treatment. The main adverse reaction of the treatment was tolerable. He died of intestinal infection rather than tumor progression.
The incidence rate of GHA is low. In addition, there is no standard treatment. Most of the treatment methods follow the principles of general gastric cancer. Surgery and chemotherapy are the main treatments. Molecular targeting therapy and immunotherapy are also being explored. D2 radical resection is the first choice for patients with early-stage GHA. For patients with isolated liver metastasis, palliative gastrectomy plus simultaneous resection of liver metastasis can be considered. Palliative gastrectomy plus local treatment of liver metastasis, such as hepatic artery chemoembolization or radiofrequency ablation, can also be considered. Chemotherapy is the main treatment for patients with advanced GHA that cannot be removed by surgery. Related studies have indicated that the first-line standard chemotherapy plan for GHA includes 5-FU and platinum-based chemotherapy, combined with simultaneous Taxol, irinotecan, methotrexate, mitomycin-C and other chemotherapy[3,7-8]. With the development of molecular detection technology, molecular targeted therapy has also been a topic of major interest in recent years. Trastuzumab combined with chemotherapy in the first-line treatment of HER2-positive common gastric cancer achieved positive results in a phase 3 Large-scale clinical study (ToGA study). Ranuciumab, which is a VEGFR2 antibody, has been approved as the first antiangiogenic drug for the treatment of advanced common gastric cancer[8]. In this case, the patient’s HER-2 status was negative; thus, his cancer was not suitable for anti-Her-2 treatment. In addition, because of drug accessibility, ramucirumab-targeted therapy was not carried out. SOX chemotherapy was carried out as the first-line treatment, but it failed. The selection of the second-line treatment was based on the following four considerations: (1) The patient was diagnosed with GHA, had eating difficulties (medication was inconvenient) and had similar components of hepatocellular carcinoma. However, chemotherapy alone was ineffective. According to the literature, multiple targeted antiangiogenic drugs, such as apatinib and sorafenib[9-10], can be used to treat clinical hepatoid adenocarcinoma; (2) IMbrave150, a phase 3 clinical trial of liver cancer[11], showed that the OS and PFS of atezolizumab + bevacizumab in unresectable HCC patients without previous systemic therapy were statistically and clinically significant and improved compared with those of sorafenib, with controllable safety; (3) PD-L1 protein expression was positive in this patient, with a TMB of 8.94 mutations/MB (high) in plasma and 3.81 mutations/MB in paraffin sections; and (4) Because of the accessibility of atezolizumab and the patient’s financial means, we tried a new kind of "T + A"-like combined chemotherapy: pembrolizumab + bevacizumab combined chemotherapy. Ultimately, the patient achieved an extended PFS. The main adverse reaction was hematological toxicity, which was tolerated. Unfortunately, the patient died of complications: intestinal infection, not tumor progression. His OS might have been longer.
Immunotherapy treatment of GHA is relatively understudied. However, a recent phase III randomized clinical trial, Keynote-062, showed that pembrolizumab was not inferior to chemotherapy for untreated advanced gastric/gastroesophageal junction cancer. In addition, fewer adverse events were observed. pembrolizumab or pembrolizumab plus chemotherapy was not superior to chemotherapy for OS and PFS endpoints[12]. However, the results of the Checkmate 649 study showed that for patients with advanced gastric cancer/gastroesophageal junction cancer/esophageal adenocarcinoma who had not been treated in the past, nivolumab was the first PD-1 inhibitor that was clinically shown to be superior to chemotherapy alone in terms of OS and PFS, with controllable safety[13]. The first-line chemotherapy treatment failed in this patient. However, the second-line attempt of pembrolizumab + bevacizumab combined chemotherapy led to tumor remission, and the side effects were tolerable. Consistent with the results of the Checkmate 649 study, pembrolizumab + bevacizumab combined chemotherapy may be effective. However, in the Keynote-062 clinical trial, pembrolizumab + chemotherapy was not superior to chemotherapy alone for OS and PFS. Possible explanations are as follows: (1) The lack of a synergistic effect of anti-angiogenesis targeted drugs such as bevacizumab; and (2) first-line chemotherapy may increase antigen exposure, and as a result, the benefits of second-line immunotherapy. However, these possible explanations are only speculation; there is no current evidence.
Previous studies have shown that surgery, chemotherapy and targeted therapy can be used in patients with GHA. However, the application of immunotherapy in such patients has not been reported in the literature. In this case of GHA, we tried a new regimen of pembrolizumab and bevacizumab with chemotherapy, and the patient benefited. However, the sample size of this was very small. Further studies should evaluate this treatment in a larger cohort or a randomized controlled trial.
The combination of pembrolizumab and bevacizumab with chemotherapy is an effective and safe regimen for treating this GHA patient. However, the sample size of this study was very small. Further evaluation of this treatment in a larger cohort or a randomized controlled trial is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Abdelbasset WK, Saudi Arabia; Khaled I, Egypt; Solanki SL; India S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Zhao M, Sun L, Lai JZ, Shi H, Mei K, He X, Jin X, Lai J, Cao D. Expression of RNA-binding protein LIN28 in classic gastric hepatoid carcinomas, gastric fetal type gastrointestinal adenocarcinomas, and hepatocellular carcinomas: An immunohistochemical study with comparison to SALL4, alpha-fetoprotein, glypican-3, and Hep Par1. Pathol Res Pract. 2018;214:1707-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Lin CY, Yeh HC, Hsu CM, Lin WR, Chiu CT. Clinicopathologial features of gastric hepatoid adenocarcinoma. Biomed J. 2015;38:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Zhang ZR, Wu J, Li HW, Wang T. Hepatoid adenocarcinoma of the stomach: Thirteen case reports and review of literature. World J Clin Cases. 2020;8:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Wang Y, Sun L, Li Z, Gao J, Ge S, Zhang C, Yuan J, Wang X, Li J, Lu Z, Gong J, Lu M, Zhou J, Peng Z, Shen L, Zhang X. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer. 2019;22:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 5. | Chen EB, Wei YC, Liu HN, Tang C, Liu ML, Peng K, Liu T. Hepatoid Adenocarcinoma of Stomach: Emphasis on the Clinical Relationship with Alpha-Fetoprotein-Positive Gastric Cancer. Biomed Res Int. 2019;2019:6710428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Yang J, Wang R, Zhang W, Zhuang W, Wang M, Tang C. Clinicopathological and prognostic characteristics of hepatoid adenocarcinoma of the stomach. Gastroenterol Res Pract. 2014;2014:140587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Zou M, Li Y, Dai Y, Sun L, Huang T, Yuan X, Qiu H. AFP-producing hepatoid adenocarcinoma (HAC) of peritoneum and omentum: a case report and literature review. Onco Targets Ther. 2019;12:7649-7654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Søreide JA. Therapeutic Approaches to Gastric Hepatoid Adenocarcinoma: Current Perspectives. Ther Clin Risk Manag. 2019;15:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Gavrancic T, Park YH. A novel approach using sorafenib in alpha fetoprotein-producing hepatoid adenocarcinoma of the lung. J Natl Compr Canc Netw. 2015;13:387-91; quiz 391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Petrelli F, Ghilardi M, Colombo S, Stringhi E, Barbara C, Cabiddu M, Elia S, Corti D, Barni S. A rare case of metastatic pancreatic hepatoid carcinoma treated with sorafenib. J Gastrointest Cancer. 2012;43:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Ducreux MP, Cheng AL, Qin S, Zhu AX, Ikeda M, Kim TY, Xu DZ, Verret W, Liu J, Finn RS, Galle PR. Atezolizumab + bevacizumab vs sorafenib in locally advanced or metastatic hepatocellular carcinoma: The randomised phase III study IMbrave150. Annals of Oncology. 2018;29 (suppl 8). [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Shitara K, Van Cutsem E, Bang YJ, Fuchs C, Wyrwicz L, Lee KW, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Mansoor W, Braghiroli MI, Karaseva N, Caglevic C, Villanueva L, Goekkurt E, Satake H, Enzinger P, Alsina M, Benson A, Chao J, Ko AH, Wainberg ZA, Kher U, Shah S, Kang SP, Tabernero J. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer: The KEYNOTE-062 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1571-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 867] [Article Influence: 173.4] [Reference Citation Analysis (1)] |
| 13. | Moehler M, Shitara K, Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T, Bragagnoli AC, Liu T, Schenker M, Yanez P, Tehfe M, Poulart V, Cullen D, Lei M, Kondo K, Li M, Ajani JA, Janjigian YY. Nivolumab (nivo) plus chemotherapy (chemo) vs chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): first results of the CheckMate 649 study. Annals Oncol. 2020;31 (suppl 4):S1142-S1215. [RCA] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |