Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5387
Peer-review started: September 10, 2021
First decision: November 22, 2021
Revised: December 5, 2021
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: June 6, 2022
Processing time: 264 Days and 19 Hours
Minimal change disease is a common cause of nephrotic syndrome (NS) in children and has a good prognosis. Idiopathic membranous nephropathy (IMN), a rare cause of NS in children, may progress to chronic kidney disease. However, there is little data on how to evaluate and treat IMN in children.
In this article, we report the case of a 7-year-old boy with steroid-resistant NS. After cyclophosphamide pulse therapy combined with oral prednisone, the urinary protein results remained positive. Renal biopsy confirmed the patho
IMN is rare in children. The main clinical manifestation is NS. The diagnosis depends on renal biopsy. There is little evidence-based data on the treatment of IMN in children. Therefore, large-sample randomized controlled trials need to be performed. Individualized treatment should be used to improve the prognosis of the disease.
Core Tip: Here, we report the case of a school-age boy with idiopathic membranous nephropathy (IMN). IMN was diagnosed after treatment with steroids and immunosuppressants for several weeks by renal biopsy. This case highlights that individualized treatment can be used to improve the prognosis of the disease. Phospholipase A2 receptor antibody levels may be valuable for early diagnosis, treatment monitoring, and follow-up.
- Citation: Cui KH, Zhang H, Tao YH. Idiopathic membranous nephropathy in children: A case report. World J Clin Cases 2022; 10(16): 5387-5393
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5387.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5387
Idiopathic membranous nephropathy (IMN) is an uncommon cause of nephrotic syndrome (NS) in children, with an incidence of less than 5%[1]. The diagnosis of IMN depends on renal biopsy. IMN is an organ-specific (kidney) autoimmune disease, and its pathogenesis involves circulating antibodies binding to the intrinsic antigen on podocytes, which leads to subepithelial deposits of immune complexes[2]. To date, phospholipase A2 receptors (PLA2Rs) on podocytes have been considered to be the primary antigen, and the rate of PLA2R positivity in the renal tissue of children with IMN is nearly 32%[3]. Circulating autoantibodies to PLA2R have been confirmed in approximately 80% of adults with IMN and are seldom detected in secondary MN[4]. Some studies have indicated that the titer of anti-PLA2R antibodies reflects disease activity and urinary protein levels. A high titer of antibodies is correlated with a great risk of deterioration in renal function and minimal chance of remission[5]. Although the possibility of spontaneous remission in children with IMN is greater than that in adults, personalized therapy must be adopted because there is no evidence-based standard treatment for IMN in children. Steroids and immunosuppressant drugs are popular treatment options for this disease; however, which immunosuppressant drug should be used and for how long still need to be determined[6].
There were no reports of tacrolimus in the treatment of idiopathic nephropathy in children. Here, we describe a child with IMN who presented with NS and was followed for a long period of time. Initially, high-dose methylprednisolone pulse therapy followed by prednisone therapy failed to eliminate the presence of urinary proteins. Renal biopsy and other specific tests were performed to confirm the diagnosis of IMN. Consequently, tacrolimus was added, and the therapeutic effect was satisfactory. By reviewing the literature on the therapeutic strategies for and prognosis of IMN in children, we aimed to better manage this disease.
A 7-year-old boy was admitted to our hospital (West China Second University Hospital of Sichuan University, Chengdu, Sichuan, China) in August 2020 with “repeated edema over the past 6 mo”.
Six months prior to admission, the patient developed facial edema and was diagnosed with idiopathic NS due to nephrotic proteinuria (24-h urinary protein 2.51 g), hypoproteinemia (albumin 20 g/L), and hyperlipidemia (serum cholesterol 6.29 mmol/L). At the same time, hematuria and leukocyturia were observed, without hypertension or abnormal renal function. Initially, the patient was treated with oral prednisone (2 mg/kg∙d) and captopril (0.5-1.0 mg/kg∙d) for 4 wk in another hospital (The First People’s Hospital of Sichuan Liangshan Yi Autonomous Prefecture, Liangshan Yi Autonomous Prefecture, Sichuan, China). However, the presence of urinary proteins persisted. Subsequently, the urinary protein fluctuated between 1+ and 3+ after methylprednisolone pulse therapy (20 mg/kg∙d) for 3 d followed by oral prednisone (2 mg/kg∙d) for 2 wk. Captopril (1 mg/kg∙d) was prescribed as maintenance therapy for the patient. Early-onset steroid resistance was considered; consequently, immunosuppressant drugs were added. The doctor administered cyclophosphamide (CTX) pulse therapy (8 mg/kg, used for two consecutive days, every 2 wk), and the prednisone was gradually tapered by 5-10 mg every 4 wk. After six CTX treatments, the urinary protein results remained positive. Because of the poor therapeutic effects, the patient was transferred to our hospital and was admitted to the Nephrology Department on October 28, 2020.
The patient had no history of other illnesses and no known allergies.
The patient did not have any relevant family history.
The patient’s temperature, arterial pressure, weight, height, respiration rate, and heart rate were 36.5 °C, 110/65 mmHg, 26 kg, 115.9 cm, 22 times per minute, and 95 times per minute, respectively. A moon face and eyelid edema were observed on October 28, 2020.
The laboratory test results revealed heavy proteinuria, hypoproteinemia, hyperlipidemia, leukocyturia, hematuria, and pathological casts (Table 1). The blood count, complement protein levels, and renal function were normal on October 28, 2020.
| Index | Result | Reference range | |
| Blood count | White blood cell count (109/L) | 7.23 | 3.6-9.7 |
| Hemoglobin (g/L) | 120 | 110-146 | |
| Platelet count (109/L) | 379 | 100-450 | |
| Urinary chemistry | Urinary protein (g/24 h) | 2.51 | 0-0.15 |
| Urinary protein | 4+ | Negative | |
| Urinary red blood cell count (cells/HP) | 4+ | 0-3 | |
| Urinary leukocyte count (cells/HP) | 4+ | 0-5 | |
| Pathological casts | Positive | Negative | |
| Serum chemistry | Albumin (g/L) | 20 | 35-50 |
| Total cholesterol (mmol/L) | 6.29 | < 5.18 | |
| Triglyceride (mmol/L) | 1.38 | < 1.7 | |
| Blood urea nitrogen (mmol/L) | 4.76 | 3.2-7.1 | |
| Serum creatinine (μmol/L) | 35 | 17.3-54.6 | |
| Immunology | Immunoglobulin G (g/L) | 2.21 | 5.29-21.9 |
| Immunoglobulin A (g/L) | 0.39 | 0.41-3.95 | |
| Immunoglobulin M (g/L) | 0.96 | 0.48-2.26 | |
| Immunoglobulin E (g/L) | 17.2 | < 90 | |
| Complement C3 (g/L) | 0.98 | 0.7-2.06 | |
| Complement C4 (g/L) | 0.18 | 0.11-0.61 | |
| Complement C1q (mg/dL) | 23.9 | 15.7-23.7 |
The computerized tomography (CT) scan was normal. Urinary ultrasound showed a slightly enhanced echo in the bilateral renal parenchyma on October 28, 2020.
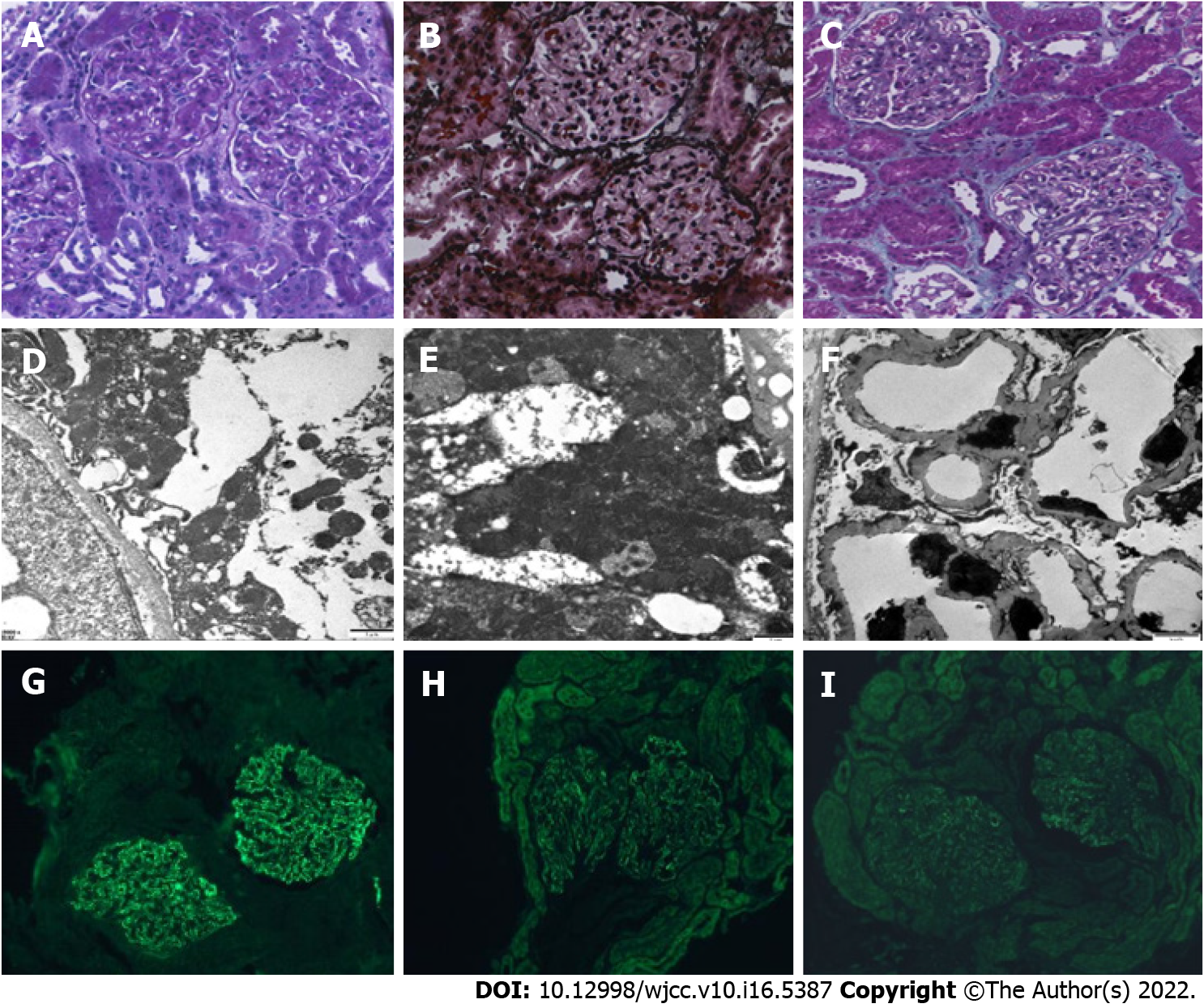
To further evaluate if the patient was steroid-resistant, renal biopsy was performed on October 30, 2020. No glomerulosclerosis, segmental sclerosis, or crescents was found. Obvious diffuse thickening of the basement membrane with several spiky formations, subepithelial deposition of fuchsinophilic protein, and vacuolar and granular degeneration in the renal tubular epithelial cells, without wire-loop lesions, were revealed by light microscopy. Irregular thickening of the basement membrane, along with electron-dense deposits in the subepithelial and intrabasal areas, and diffuse fusion of the foot processes of epithelial cells were suggested based on electron microscopy. The immunofluorescence results were as follows: Immunoglobulin A (IgA) -, IgG +++, IgM +, C3 +, complement C1q -, and fibrinogen -. Immunohistochemical staining for IgG subtypes indicated deposition of IgG1 and IgG4 along the glomerular capillary wall, with PLA2R + and thrombospondin type-1 domain-containing 7A -, as shown in Figure 1.
To exclude secondary MN, we performed analyses of anti-streptolysin O, autoantibodies, anti-neutrophil cytoplasmic antibodies, and complement proteins, tested for the hepatitis virus, syphilis, acquired immunodeficiency syndrome, and tuberculosis, and performed a urine culture, sputum culture, and plain chest CT scan, which were all negative.
Renal biopsy confirmed the pathological diagnosis of stage II IMN. The pathological changes of IMN include thickened glomerular basement membranes under a light microscope, with immunofluorescence examination showing granular staining for IgG, IgM, C3 and PLA2R along the periphery of glomerular capillary loops, and electron-dense subepithelial deposits under an electron microscope. After ruling out other immunological and infectious diseases relevant to secondary MN with other laboratory tests, IMN was identified.
In view of the patient’s poor response to steroids and CTX, we changed the therapeutic strategy to include tacrolimus (0.07 mg/kg∙d), and at the same time, prednisone was gradually decreased referencing 2019 Kidney Disease Improving Global Outcomes.
On January 4, 2021, after 49 d of treatment using tacrolimus and prednisone, the urine protein results and urinary pathologic casts were negative. The red blood cell count in the urine decreased to 59 cells/μL, and the white blood cell count in the urine decreased to 10 cells/μL. Throughout a 5-mo follow-up period, the patient showed an uneventful clinical course, and the steroids were progressively decreased. Kidney function remained normal in the patient during follow-up. The urinalysis results during the follow-up are shown in Table 2.
| Time of follow-up (d) | Urinary proteinuria | Urinary RBC count (cells/μL) | Urinary WBC count (cells/μL) | Urinary pathologic casts (cells/μL) |
| 0 | 4+ | 1066 | 98 | 14 |
| 16 | 2+ | 280 | 9 | 0 |
| 60 | 3+ | 325 | 34 | 0 |
| 77 | 3+ | 1689 | 91.9 | 4.81 |
| 118 | 3+ | 1286 | 14 | 0 |
| 130 | Negative | 53 | 3 | 0 |
| 144 | Negative | 59 | 10 | 0 |
IMN is a rare cause of NS in children. Here, we report the case of a school-age boy with steroid-resistant NS. IMN was diagnosed by renal biopsy, with PLA2R +. The therapeutic effect of tacrolimus plus prednisone was satisfactory. In this article, we described a 7-year-old boy with stage II IMN confirmed by renal biopsy and other laboratory tests. Initially, prednisone and CTX were given for several months, but remission was not achieved. After tacrolimus was substituted for CTX, the proteinuria gradually became negative. IMN patients who present with NS need to receive steroid therapy for several weeks initially. If the therapeutic effect is not satisfactory, immunosuppressant drugs should be added to the treatment regimen for these patients. However, which immunosuppressant drug should be used and for how long are still unclear. Alkylating agents remain the only agents proven to be effective in preventing end-stage renal disease or death[6]. Rituximab may be considered for children who do not respond to hormonal or immunosuppressant therapy[6]. Other studies have reported that tacrolimus and CTX are superior to other immunosuppressant drugs in terms of complete remission rate, and tacrolimus monotherapy is also the safest and most effective choice for IMN with stable renal function[7]. A regimen of prednisone plus tacrolimus could undoubtedly yield a higher remission rate and long-term safety. More than 60% of patients can reach complete remission or partial remission in 24 mo, and relapse is rare[8]. In terms of other immunosuppressant drugs, the remission rate associated with the use of glucocorticoids and CTX in IMN is only 58.6%[9].
Minimal change disease (MCD) is a common cause of NS in children and has a good prognosis. However, IMN is rare and has a poor prognosis in children. Especially in children with IMN who present with NS, it is necessary to carefully evaluate and select a reasonable therapeutic strategy as early as possible to improve the prognosis. Due to the lower morbidity of IMN in children, there are few relevant data on therapeutic strategies for and evaluation of children with IMN, and there is a notable lack of large-sample randomized controlled trials (RCTs). Most of the current studies were retrospective studies or case reports. IMN in children is more common in men and in adolescents. Studies indicated that the most prominent clinical manifestation was NS, which was accompanied by various degrees of edema, hyperlipidemia, hypoalbuminemia, and nephrotic proteinuria[7]. Hypertension, hematuria, and renal injury were also observed[9]. All reported cases were resistant to hormones[10,11]. In children with MN, PLA2R-associated MN appears to be common. PLA2R antibody levels are closely associated with disease activity, whereas PLA2R antibody-negative patients often have a good prognosis[4]. MCD in children is common in male preschool children. NS is the main clinical manifestation, and it can be mitigated by hormone therapy in 90%-95% of cases[12]. We summarize the differences between IMN and MCD to better identify and address IMN at the early stage, as shown in Table 3.
| IMN | MCD | ||
| General features | Sex | Male | Male |
| Age | Adolescence | Preschool age | |
| Clinical manifestations | Nephrotic syndrome | Common | Common |
| Leukocyturia | Some | Rare | |
| Hematuria | Some | Rare | |
| Hypertension | Some | Rare | |
| Renal failure | Some | Rare | |
| Laboratory tests | Anti-PLA2R, antibody | Elevated | Normal |
| Anti-THSD7A, antibody | Elevated | Normal | |
| Treatment strategy | Prednisone | Mostly resistance | Mostly sensitive |
| Immunosuppressant | Tacrolimus, cyclophosphamide, mycophenolate mofetil | Rare | |
| Biological agent | Rituximab | Rare |
IMN is rare in children. The main clinical manifestations are edema and NS. The diagnosis depends on renal biopsy. PLA2R antibody should be used to monitor treatment and follow-up. There is little evidence-based data on the treatment of IMN in children. Therefore, large-sample RCTs need to be performed. Individualized treatment should be used to improve the prognosis of the disease. In the future, for children who are not simple NS, we hope to detect serum PLA2R antibody to early predict MN and its prognosis.
We sincerely thank the Department of Nephrology and Renal Pathology.
Provenance and peer review: Unsolicited article; Externally peer reviewed
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Islam SMRU, Bangladesh; Kilis-Pstrusinska K, Poland; Meneghesso D, Italy; Tarris G, France S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, Balkrishnan R, Dietrich X, Eckard A, Eggers PW, Gaipov A, Gillen D, Gipson D, Hailpern SM, Hall YN, Han Y, He K, Herman W, Heung M, Hirth RA, Hutton D, Jacobsen SJ, Jin Y, Kalantar-Zadeh K, Kapke A, Kovesdy CP, Lavallee D, Leslie J, McCullough K, Modi Z, Molnar MZ, Montez-Rath M, Moradi H, Morgenstern H, Mukhopadhyay P, Nallamothu B, Nguyen DV, Norris KC, O'Hare AM, Obi Y, Park C, Pearson J, Pisoni R, Potukuchi PK, Rao P, Repeck K, Rhee CM, Schrager J, Schaubel DE, Selewski DT, Shaw SF, Shi JM, Shieu M, Sim JJ, Soohoo M, Steffick D, Streja E, Sumida K, Tamura MK, Tilea A, Tong L, Wang D, Wang M, Woodside KJ, Xin X, Yin M, You AS, Zhou H, Shahinian V. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2018;71:A7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 531] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 2. | Nie S, He W, Huang T, Liu D, Wang G, Geng J, Chen N, Xu G, Zhang P, Luo Y, Nie J, Xu X, Hou FF. The Spectrum of Biopsy-Proven Glomerular Diseases among Children in China: A National, Cross-Sectional Survey. Clin J Am Soc Nephrol. 2018;13:1047-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 3. | Zhang D, Wu Y, Zhang C, Zhang W, Zou J, Jiang G. Compared staining of the phospholipase A2 receptor in the glomeruli of Chinese adults and children with idiopathic membranous nephropathy. Pathol Res Pract. 2019;215:952-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Dhaouadi T, Abdellatif J, Trabelsi R, Gaied H, Chamkhi S, Sfar I, Goucha R, Ben Hamida F, Ben Abdallah T, Gorgi Y. PLA2R antibody, PLA2R rs4664308 polymorphism and PLA2R mRNA levels in Tunisian patients with primary membranous nephritis. PLoS One. 2020;15:e0240025. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Dettmar AK, Wiech T, Kemper MJ, Soave A, Rink M, Oh J, Stahl RAK, Hoxha E; Pediatric MN Study Group. Immunohistochemical and serological characterization of membranous nephropathy in children and adolescents. Pediatr Nephrol. 2018;33:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Safar-Boueri L, Piya A, Beck LH Jr, Ayalon R. Membranous nephropathy: diagnosis, treatment, and monitoring in the post-PLA2R era. Pediatr Nephrol. 2021;36:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 7. | Malatesta-Muncher R, Eldin KW, Beck LH Jr, Michael M. Idiopathic membranous nephropathy in children treated with rituximab: report of two cases. Pediatr Nephrol. 2018;33:1089-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Wang R, Wang M, Xia Z, Gao C, Shi Z, Fang X, Wu H, Peng Y. Long-term renal survival and related risk factors for primary membranous nephropathy in Chinese children: a retrospective analysis of 217 cases. J Nephrol. 2021;34:589-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Gong L, Xu M, Xu W, Tang W, Lu J, Jiang W, Xie F, Ding L, Qian X. Efficacy and safety of tacrolimus monotherapy vs cyclophosphamide-corticosteroid combination therapy for idiopathic membranous nephropathy: A meta-analysis. Medicine (Baltimore). 2021;100:e26628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Ram R, Guditi S, Kaligotla Venkata D. A 10-year follow-up of idiopathic membranous nephropathy patients on steroids and cyclophosphamide: a case series. Ren Fail. 2015;37:452-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Bhimma R, Naicker E, Ramdial PK. Mycophenolate mofetil therapy in children with idiopathic membranous nephropathy. Clin Nephrol. 2013;80:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Hansrivijit P, Puthenpura MM, Ghahramani N. Efficacy of abatacept treatment for focal segmental glomerulosclerosis and minimal change disease: A systematic review of case reports, case series, and observational studies. Clin Nephrol. 2020;94:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |