Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5352
Peer-review started: July 24, 2021
First decision: December 17, 2021
Revised: December 21, 2021
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: June 6, 2022
Processing time: 313 Days and 2 Hours
Renal involvement in lymphoma is commonly associated with widespread nodal or extranodal lymphoma. Primary renal diffuse large B-cell lymphoma is an extremely rare extranodal lymphoma, accounting for fewer than 1% of all renal masses. Interestingly, the patient in this study had a renal vein tumor thrombus that was observed after laparoscopic radical nephrectomy.
We report the case of a 56-year-old female patient with primary renal lymphoma and a renal vein tumor thrombus whose first symptom was right pain in the back and gross hematuria. Histopathology revealed primary renal diffuse large B-cell lymphoma. The patient received 8 standard cycles of rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy after surgery, and no obvious signs of recurrence were observed during the one-year follow-up.
We evaluated comprehensive treatment of primary renal diffuse large B-cell lymphoma and multidisciplinary management of this malignancy.
Core Tip: Primary renal diffuse large B-cell lymphoma is an extremely rare extranodal lymphoma. This case also had a simultaneous right renal vein tumor thrombus whose first symptom was right waist pain and gross hematuria. Clinical features and treatment options may contribute to good prognoses. When surgery is combined with chemotherapy, the overall survival is relatively better.
- Citation: He J, Mu Y, Che BW, Liu M, Zhang WJ, Xu SH, Tang KF. Comprehensive treatment for primary right renal diffuse large B-cell lymphoma with a renal vein tumor thrombus: A case report. World J Clin Cases 2022; 10(16): 5352-5358
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5352.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5352
Renal involvement in lymphoma is generally associated with widespread nodal or extranodal lymphoma, which is classified as secondary renal lymphoma. Primary renal lymphoma (PRL) is rare and may involve the kidneys alone without evidence of disease elsewhere[1]. Very few patients have been diagnosed with PRL, and the present case is particularly rare due to simultaneous right renal vein tumor thrombus. PRL and other renal cancers share a number of key features; thus, making a diagnosis is difficult. The standard management of PRL is radical or partial nephrectomy. Although aggressive, PRLs respond well to rituximab with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Currently, surgery or systemic chemotherapy are widely used for the treatment of PRL. The overall survival rate and long-term disease-free interval of PRL patients are relatively better when surgery and chemotherapy are combined.
Here, we report the case of a 56-year-old female with PRL and a renal vein tumor thrombus. After laparoscopic radical nephrectomy, R-CHOP chemotherapy was given, and satisfactory results were achieved.
A 56-year-old female presented to the hospital on October 3, 2019 due to right waist pain and gross hematuria that had persisted for 15 d.
The patient presented with urgency and dysuria accompanied by nausea and vomiting. The patient’s vomiting comprised stomach contents, and her pain was slightly relieved after vomiting.
The patient’s past medical history was insignificant.
The patient admitted a previous history of hypertension and denied any medical history of coronary heart disease, diabetes or tuberculosis. The patient had no relevant family history.
The physical examination revealed only tenderness in the right renal area.
The laboratory examination showed normal coagulation function, kidney function and electrolyte levels with only slightly higher serum creatinine and uric acid levels. Hepatitis B serology revealed a previously unknown hepatitis B infection.
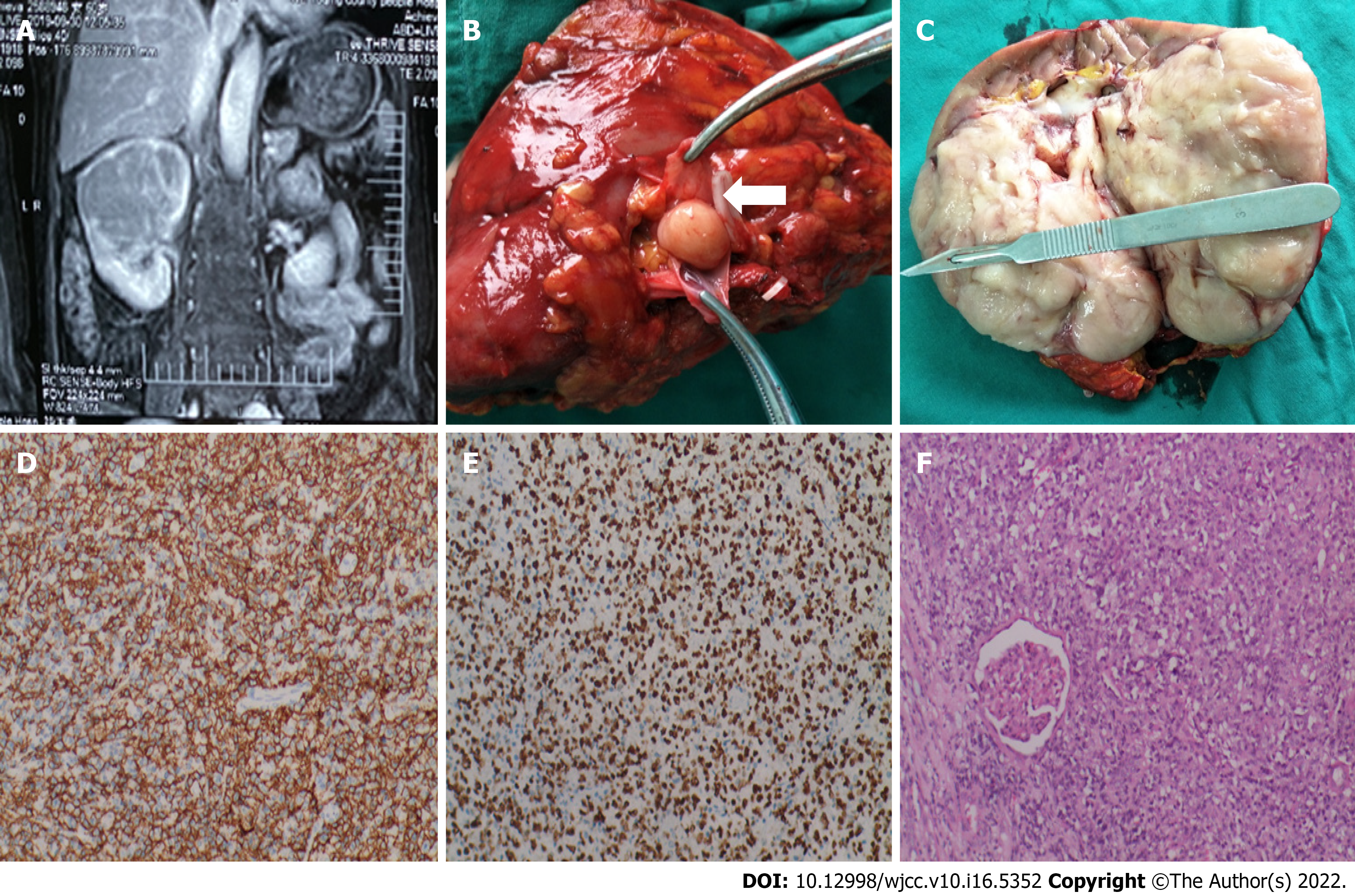
The ultrasound showed a large hypoechoic mass in the upper pole of the right kidney, 9.3 cm × 7.4 cm × 8.0 cm in size, with a regular shape, clear boundary, and uneven internal echo, which had a high-speed and high-block blood flow spectrum, indicating multiple perforating blood flow signals. Computed tomography (CT) and magnetic resonance imaging (MRI) revealed a space-occupying lesion in the right kidney that did not invade the surrounding tissues (Figure 1A).
According to the pathological examination, the gross specimen was a total kidney with partial ureteral resection, and its volume was 14.0 cm × 9.0 cm × 8.2 cm (Figure 1C). The contiguous ureteral length was 5.5 cm, and the diameter was 0.4 cm-0.5 cm. The boundary between the renal tissue and the medulla was unclear, and the renal pelvis and calyces were extruded and deformed. A gray–white mass was observed on the cut surface (Figure 1C). In addition, the ureter and blood vessels were cut without tumor involvement, and metastatic lymph nodes were not found in the gray parenchyma of the cut surface. Immunohistochemical profiling revealed diffuse and intense expression of CD20 and CD79, along with expression of Bcl-6, Vim, INI-1, ALK1, Ki-67 and PAX-8. The tumor was negative for CD30, CD56, NSE, S100, desmin and WT expression (Figure 1D-1F). The pathological diagnosis was non-Hodgkin's lymphoma of the right kidney, which was consistent with a diffuse large B lymphocyte tumor and tended to be the source of germinal center cells.
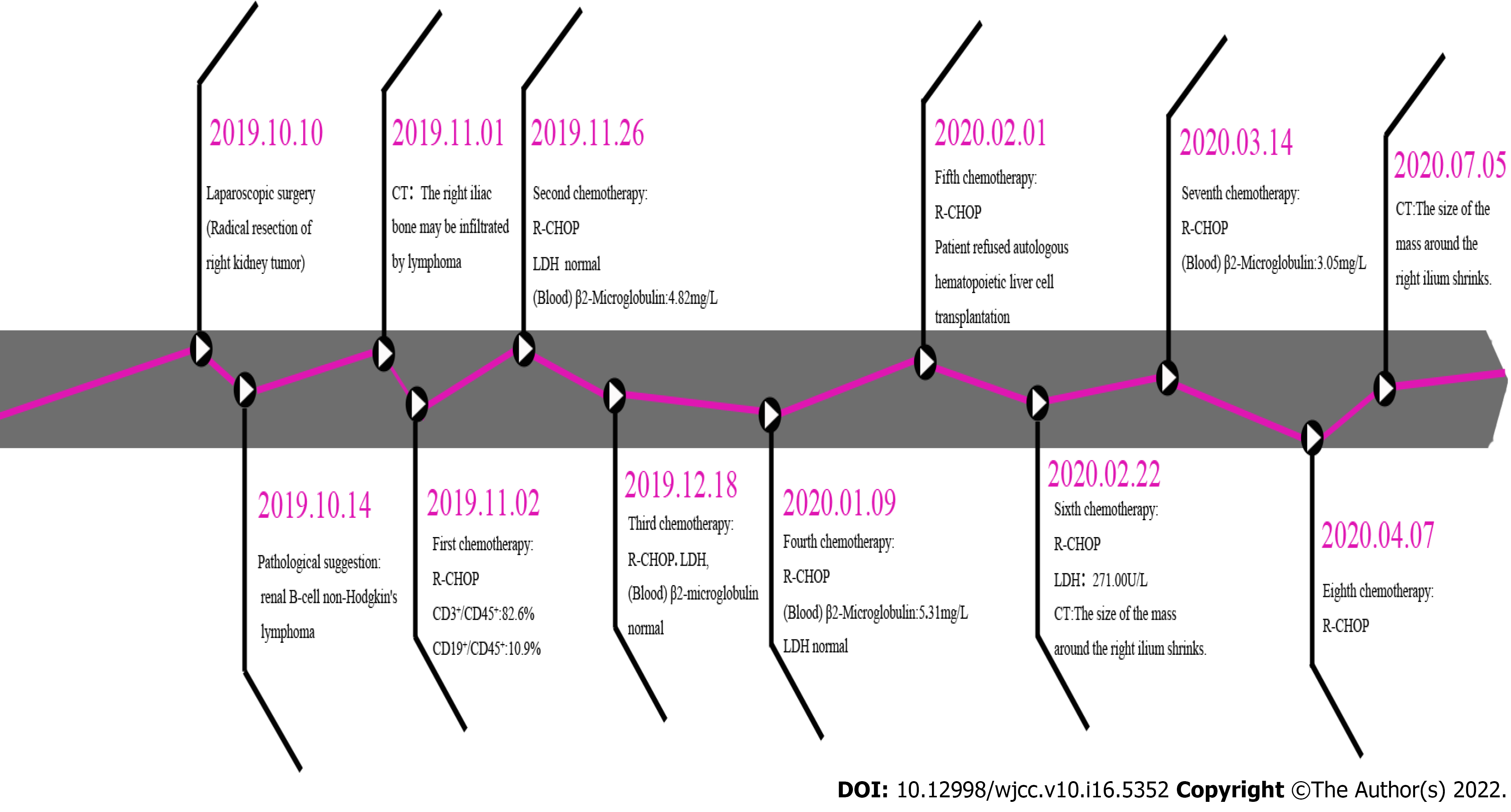
Laparoscopic radical resection of the right renal tumor was performed on October 10, 2019. Under laparoscopy, we observed that a large mass had compressed the inferior vena cava and part of the liver. During surgical exploration, it was found that the tumor thrombus continued to the right, not involving the inferior vena cava. The thrombus was graded 0 using the Mayo system (Figure 1B).
This patient was stable after surgery. She presented at the Department of Hematology on October 30, 2019, and was diagnosed with diffuse large B-cell lymphoma of the right kidney (stage IV Group A). Her IPI score was low-medium-risk; thus, the patient received R-CHOP treatment. The initial regimen was rituximab 100 mg d1, cyclophosphamide 1260 mg d1, epirubicin 100 mg d1, vindesine 4 mg d1, and dexamethasone 15 mg d1-d5. Subsequently, the dose of rituximab was adjusted based on the patient's condition. The patient was followed up until the completion of 8 cycles of R-CHOP chemotherapy. In the fourth month after surgery, a CT scan indicated that the right renal lymphoma resection was successful and without obvious signs of recurrence (Figure 2A-2C). The patient attended a follow-up appointment on July 24, 2020, and presented with no complications; the images did not show obvious abnormalities (Figure 2D-2F). Satisfactory results were maintained over the past year.
Primary renal diffuse large B-cell lymphoma is an extremely rare extranodal lymphoma, accounting for fewer than 1% of all renal masses. It is a unique clinical and pathological entity that is highly aggressive. These tumors present as solitary nodules (10%-20%), multiple nodules (60%), adjacent retroperitoneal lesions with renal involvement (25-30%), diffuse infiltration (20%) or perirenal involvement (10%). The origin of primary renal lymphoma remains unclear because a normal kidney is an extranodal organ without lymphatic vessels. The studies that have been conducted to date indicate that the use of immunosuppressive drugs for transplant patients is a risk factor for the development of B-cell lymphoma. Chemical agents such as dyes and pesticides may also increase the risk[1].
Proliferating B cells in or around germinal centers is a principal determining factor of lymphoma, and systemic symptoms become more obvious due to cytokine production. Lymphoma enlarges and compresses the surrounding renal parenchyma, which forms a solitary nodule or multiple nodules. In addition, heterogeneous infiltrative growth may cause extracapsular dilation into the perirenal space. Primary renal diffuse large B-cell lymphoma patients who present with typical symptoms of renal cell carcinoma, such as gross hematuria, lower back pain, abdominal masses or acute or chronic renal failure, are often misdiagnosed with the more common renal cell carcinoma or nephroblastoma. In previous studies, lymphoma parenchymal infiltration and hypercalcemia caused by excessive production of vitamin D in the ureter have been found to be related to renal failure[2].
Central to the diagnosis of lymphoma is the concept of detailed physical examination and laboratory tests. Whole-body lymph node examination should be performed, including the head, neck, supraclavicular, axillary, femoral and inguinal lymph nodes. Abdominal examination should also be performed to assess liver and spleen enlargement. Simultaneously, there is a major need to address the abnormal problems caused by complete blood count with differential, lactate dehydrogenase (LDH), HIV and hepatitis B and C serologies. In this patient, hepatitis B antigen levels were elevated without her knowledge, and the patient was not treated in time, decreasing the ability of her immune system to regulate malignant cells. The current study highlights the importance of blood urea nitrogen and creatinine levels. The results of one study indicated that swollen retroperitoneal lymph node enlargement causes ureteral obstruction or adverse reactions to nephrotoxic chemotherapy drugs as their level increases[3]. An intermediate relationship between LDH and lymphoma has been reported in the literature, and high LDH indicates a high turnover rate of lymphoma cells and the release of degradation products into the blood (Figure 3). Therefore, LDH may be a predictor of lymphoma patient survival[4].
In clinical practice, doctors should emphasize the importance of imaging in renal lymphoma diagnoses, as imaging allows for the most complete diagnosis, including the tumor grade and stage. On CT scans, primary renal diffuse large B-cell lymphoma is a homogeneous or slightly low-density mass with unclear borders. Enhanced CT shows mild enhancement, and the enhancement degree is lower than that of normal renal parenchyma. Primary renal diffuse large B-cell lymphoma lacks a blood supply and rarely invades the inferior vena cava, as was also observed in the present case. If both kidneys are extensively infiltrated, CT enhancement will show uneven kidney enhancement, normal cortical and medulla differentiation enhancement, and enveloping and deforming glomerular systems. It has been suggested that primary renal diffuse large B-cell lymphoma shows low to moderate signal intensity on T1- and T2-weighted MRI sequences[5]. New findings with positron emission tomography provide further evidence that primary renal diffuse large B-cell lymphoma with a high standard intake value may indicate aggressive disease. Using this approach, imaging experts have been able to show abnormal uptake in the mass for staging and perform follow-up analyses after treatment using fluorodeoxyglucose-positron emission tomography[6]. Morphology, immunophenotyping, and staining for B-cell markers are required to obtain the diagnosis, as the imaging findings were nonspecific. The current staging system classification is based on the Ann Arbor classification[7]. In this patient, PRL had spread into one lymphatic organ, and as the postoperative images suggested, the tumor was stage IV (Figure 3).
The standard treatment for primary diffuse renal large B-cell lymphoma is radical nephrectomy and postoperative systemic chemotherapy. Data from several studies suggest that multidrug chemotherapy is often necessary for high-grade lymphoma before surgery. The R-CHOP regimen (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) has been studied using several animal models. Many patients receive 8 cycles of standard treatment, and a greater degree of relief is achieved for most patients. For our patient, vincristine was replaced by vindesine because vincristine was unavailable. In general, the prognosis of patients with primary renal lymphoma is improved after rituximab is added to the CHOP regimen[8]. Emerging immunotherapy options and targeted therapies may provide chemotherapy-free first-line approaches for DLBCL[9]. However, recent evidence suggests that COO, concurrent rearrangements of MYC/BCL2/BCL6, the characterization of DH/TH HGBL, and the overexpression of MYC/BCL2 result in highly aggressive clinical behavior, resistance to standard chemotherapy and extremely poor outcomes[10,11]. Side effect management and challenges to effective therapy, including unfavorable biological features, individual vulnerabilities and toxicities of cytotoxic chemotherapy, are the main concerns.
The prognosis of renal diffuse large B-cell lymphoma patients depends on the stage of the disease, histopathology, extranodal involvement, and the patient’s age and condition. There are similarities between the views expressed in our case report and those described in a previously published epidemiological and final results database study covering 1984 to 2015[12]. In other words, the above factors affect the overall survival outcomes of patients. In addition, age over 60 years, Eastern Cooperative Oncology Group (ECOG) performance greater than 2, elevated LDH, clinical stage III/IV, and more than 1 extranodal involvement have been found to reduce overall survival times. Some immune markers confer a favorable prognosis, such as CD19, CD20, CD22, and CD45. Very rarely, these tumors can express CD5, which has a poor prognosis[13]
In primary diffuse renal large B-cell lymphoma, the main challenge faced by many doctors is differentiating renal lymphoma from other renal masses, especially in the case of unilateral lesions. Clinical, physical, laboratory, imaging, morphology and immunophenotyping analyses are used to diagnose the patients and monitor the treatment effects. The standard treatment is radical nephrectomy, and systemic chemotherapy using the R-CHOP regimen is the subsequent treatment of choice. When surgery is combined with chemotherapy, the overall survival rates and long-term progression outcomes are relatively better. However, some epidemiological factors and gene rearrangements result in highly aggressive clinical behaviors and extremely poor prognoses.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dalal N, India S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Eriksson M, Hardell L, Carlberg M, Akerman M. Pesticide exposure as risk factor for non-Hodgkin lymphoma including histopathological subgroup analysis. Int J Cancer. 2008;123:1657-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 2. | Erdoğmuş Ş, Aktürk S, Kendi Çelebi Z, Kiremitçi S, Kaygusuz G, Altınbaş NK, Üstüner E, Keven K. Diffuse Large B-Cell Lymphoma Presenting with Bilateral Renal Masses and Hematuria: A Case Report. Turk J Haematol. 2016;33:159-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Oda Y, Ishioka K, Ohtake T, Sato S, Tamai Y, Oki R, Matsui K, Mochida Y, Moriya H, Hidaka S, Kobayashi S. Intravascular lymphoma forming massive aortic tumors complicated with sarcoidosis and focal segmental glomerulosclerosis: a case report and literature review. BMC Nephrol. 2018;19:300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Liu Y, Barta SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019;94:604-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 5. | Ganeshan D, Iyer R, Devine C, Bhosale P, Paulson E. Imaging of primary and secondary renal lymphoma. AJR Am J Roentgenol. 2013;201:W712-W719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Orimo M, Kondo M, Takeyama K, Masui K, Tanaka J, Tagaya E. A case of lymphangioleiomyomatosis with diffuse large B-cell lymphoma: Usefulness of FDG-PET. Respir Med Case Rep. 2020;29:100999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Armitage JO. Staging non-Hodgkin lymphoma. CA Cancer J Clin. 2005;55:368-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 8. | Ansell SM. Non-Hodgkin Lymphoma: Diagnosis and Treatment. Mayo Clin Proc. 2015;90:1152-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Di M, Huntington SF, Olszewski AJ. Challenges and Opportunities in the Management of Diffuse Large B-Cell Lymphoma in Older Patients. Oncologist. 2021;26:120-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Li C, Sun Y, Wang J, Tang L, Jiang H, Guo T, Liu L, Wu Y, Ai L, Xia L, Wu J, Lin Z, Qian Q, Hu Y, Mei H. PiggyBac-Generated CAR19-T Cells Plus Lenalidomide Cause Durable Complete Remission of Triple-Hit Refractory/Relapsed DLBCL: A Case Report. Front Immunol. 2021;12:599493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Papageorgiou SG, Thomopoulos TP, Katagas I, Bouchla A, Pappa V. Prognostic molecular biomarkers in diffuse large B-cell lymphoma in the rituximab era and their therapeutic implications. Ther Adv Hematol. 2021;12:20406207211013987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Taneja A, Kumar V, Chandra AB. Primary renal lymphoma: A population-based analysis using the SEER program (1973-2015). Eur J Haematol. 2020;104:390-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Hu S, Xu-Monette ZY, Balasubramanyam A, Manyam GC, Visco C, Tzankov A, Liu WM, Miranda RN, Zhang L, Montes-Moreno S, Dybkær K, Chiu A, Orazi A, Zu Y, Bhagat G, Richards KL, Hsi ED, Choi WW, Han van Krieken J, Huang Q, Huh J, Ai W, Ponzoni M, Ferreri AJ, Zhao X, Winter JN, Zhang M, Li L, Møller MB, Piris MA, Li Y, Go RS, Wu L, Medeiros LJ, Young KH. CD30 expression defines a novel subgroup of diffuse large B-cell lymphoma with favorable prognosis and distinct gene expression signature: a report from the International DLBCL Rituximab-CHOP Consortium Program Study. Blood. 2013;121:2715-2724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |