Published online Jun 6, 2022. doi: 10.12998/wjcc.v10.i16.5196
Peer-review started: July 12, 2021
First decision: July 26, 2021
Revised: August 15, 2021
Accepted: April 9, 2022
Article in press: April 9, 2022
Published online: June 6, 2022
Processing time: 324 Days and 23.6 Hours
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is a rare but impor
To better understand anti-NMDAR encephalitis through literature review and patients enrolled in our hospital.
The six patients enrolled in the study were those diagnosed with anti-NMDAR encephalitis. Their history, clinical manifestations, and medications were recorded and optimum treatment provided in addition to maintaining a record of the follow-ups. In addition, we also extensively surveyed the literature and provide summarized data from 155 published cases of anti-NMDAR encephalitis from 130 case reports. PubMed and Scopus were the sources of these publications and the time period covered was 6 years ranging from January 2014 through December 2019.
The six patients enrolled for this study presented with typical symptoms resulting in a diagnosis of ovarian teratoma induced anti-NMDAR encephalitis. Appropriate interventions led to a positive outcome in all the patients, with five of six patients reporting full recovery and the sixth patient recovering with a few deficits. No death was recorded. The literature survey comprising of 155 patients cases across 130 case reports of anti-NMDAR encephalitis clearly indicated an upward trend in the reports/diagnosis in China, particularly in the surveyed time from 2014 through 2019. The majority of patients (150/155) underwent surgical intervention resulting in positive outcome. No treatment intervention was mentioned for one case while the four patients who were not surgically operated succumbed to the disease.
Suspected anti-NMDAR encephalitis should be quickly evaluated for anti-NMDAR antibodies since early diagnosis is important. In case of a tumor, its earliest and complete removal is recommended. Finally, early use of corticosteroids and IgG-depleting strategies (intravenous immunoglobulin or plasma exchange) may improve outcome.
Core Tip: We describe our findings from six cases from our own hospital and, in addition, we review 155 cases reported in 130 case reports. We describe the symptoms, diagnosis, and treatment, as well as prognosis. Our results indicate the importance of an early and quick intervention through surgery and immunotherapy, failing which the condition can be fatal.
- Citation: Li SJ, Yu MH, Cheng J, Bai WX, Di W. Ovarian teratoma related anti-N-methyl-D-aspartate receptor encephalitis: A case series and review of the literature. World J Clin Cases 2022; 10(16): 5196-5207
- URL: https://www.wjgnet.com/2307-8960/full/v10/i16/5196.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i16.5196
Encephalitis is a complex neurological syndrome that is caused by inflammation of the brain parenchyma[1]. The main causes of encephalitis include a range of infectivity and autoimmunity. Viruses are the most commonly identified pathogenic factors[1]. Autoimmune encephalitis (AE) has two major subtypes: (1) Classic paraneoplastic limbic encephalitis marked by well-characterized onconeural autoantibodies against intracellular neuronal antigens; and (2) new-type AE characterized by autoantibodies against neuronal surface or synaptic antigens[2]. N-methyl-D-aspartate receptor (NMDAR) encephalitis is a new-type AE[3,4], wherein antibodies attack N-methyl-D-aspartate (NMDA)-type glutamate receptors at central neuronal synapses[5]. Affected patients develop prominent psychiatric and behavioral symptoms, rapid memory loss, seizures, abnormal movements (dyskinesias), hypoventilation, and autonomic instability[6-8].
The first case of paraneoplastic encephalitis related to ovarian teratoma was described in 1997[9,10]. In 2005, a syndrome marked by psychiatric symptoms, memory deficits, hypoventilation, and decreased consciousness was reported in four young women with ovarian teratomas[11,12]. A severe form of encephalitis associated with antibodies against NR1–NR2 heteromers of the NMDAR was identified by Dalmau et al[6] in 2007. The target antigen was identified as the NMDAR, and the disorder named “anti-NMDAR encephalitis”; specific autoantibodies to the NMDAR were soon detected in these and eight other patients with similar neurological symptoms, seven of whom also had ovarian teratomas. Iizuka et al[8] confirmed the presence of NMDAR antibodies in four young women with ovarian teratoma and described the clinical course progression through five phases of anti-NMDAR encephalitis: Prodromal, psychotic, unresponsive, hyperkinetic, and gradual recovery.
Herpes simplex encephalitis (HSE) plays a vital role in triggering the synthesis of anti-NMDAR antibodies[13,14]. A study of 501 patients with anti-NMDAR encephalitis found that 38% of patients had concomitant tumors, most commonly ovarian teratomas. Other relatively rare neoplasms include extra-ovarian teratomas, testicular germ-cell tumors, small-cell lung cancer, and Hodgkin’s lymphoma[15]. From a cohort study of 577 patients with anti-NMDAR encephalitis, 220 patients (38%) had an underlying neoplasm, among which 207 tumors (94%) were ovarian teratomas[4]. A review of 432 cases of anti-NMDAR encephalitis revealed that of the 293 female patients, 68 (23%) had ovarian teratoma[16].
The first case of anti-NMDAR encephalitis with ovarian teratoma was reported in China in 2010[17]. A single-center prospective study that included patients with anti-NMDAR encephalitis with ovarian teratoma from 2011 to 2016 admitted to Peking Union Medical College Hospital, Beijing, discussed the clinical characteristics, treatment, and prognosis of the disease[18]. The association between ovarian teratoma and anti-NMDAR encephalitis is relatively unknown and most of the present studies on anti-NMDAR encephalitis with ovarian teratoma are case reports and systematic reviews. Here, we illustrate six cases of ovarian teratoma-related anti-NMDA receptor encephalitis, and also present the results of a systematic review and analysis of cases reported after 2013.
Between July 2012 and December 2019, six patients with ovarian teratoma-associated anti-N-methyl-D-aspartate receptor encephalitis were enrolled in Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University. All patients’ data like clinical characteristics, treatment, and follow-up were reviewed. The study was approved by the Ethic Committee of Shanghai Jiao Tong University. Informed consent was obtained from all patients for participation in this study and the publication of results.
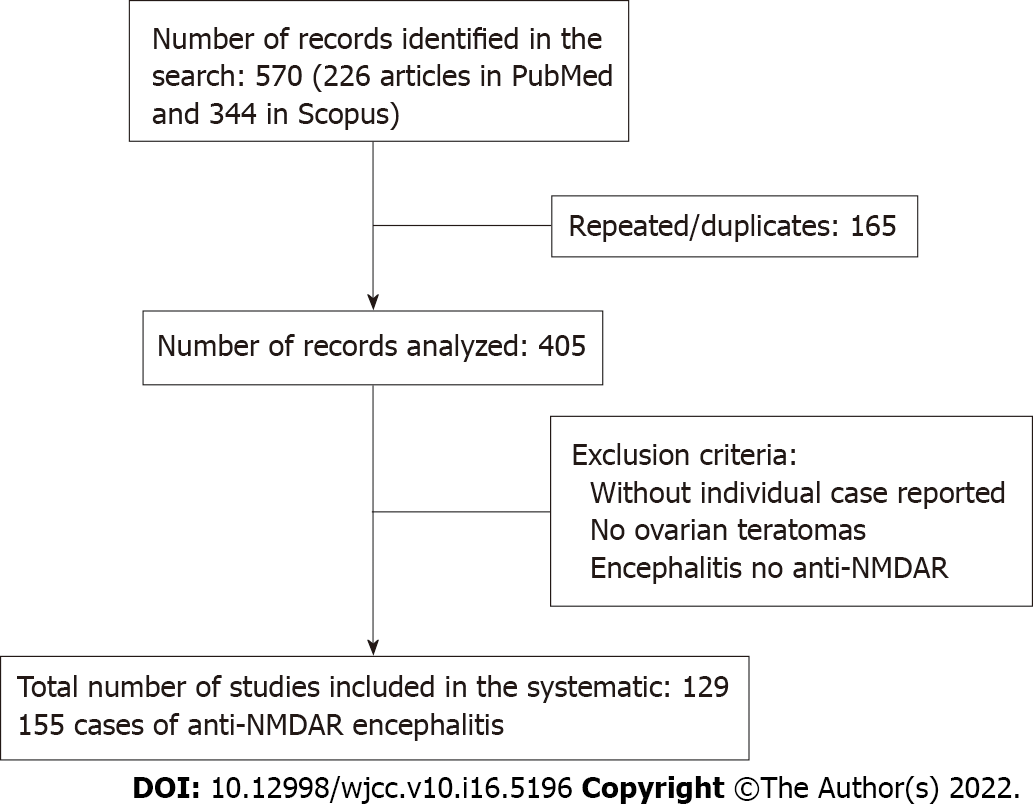
Sources: A comprehensive search of PubMed and Scopus was performed for all studies published prior from January 2014 to December 2019, using the search terms “encephalitis” and “teratoma”, which yielded 226 articles in PubMed and 344 in Scopus (Figure 1). A systematic review of these papers was performed, and after removal of repeated 165 articles from both searches, the full text of all articles were evaluated to determine whether case reports with ovarian teratoma were included. There were no language restrictions.
Data extraction, collection, and analysis: In the selected articles, a comprehensive data set was collected via a form designed for the present study. The form consisted of an Excel spreadsheet (Microsoft, Redmond, WA, United States), where each column captured a unique piece of information. When data were inadequate or insufficient for a definite piece of information, we recorded it as ‘not available’. Data of the individual patients were then pooled and analysed via the spreadsheet. A Microsoft Word document transposition of the form is provided as Supplementary material (Supplementary Tables 1-4).
GraphPad Prism 8 was used for statistical analyses.
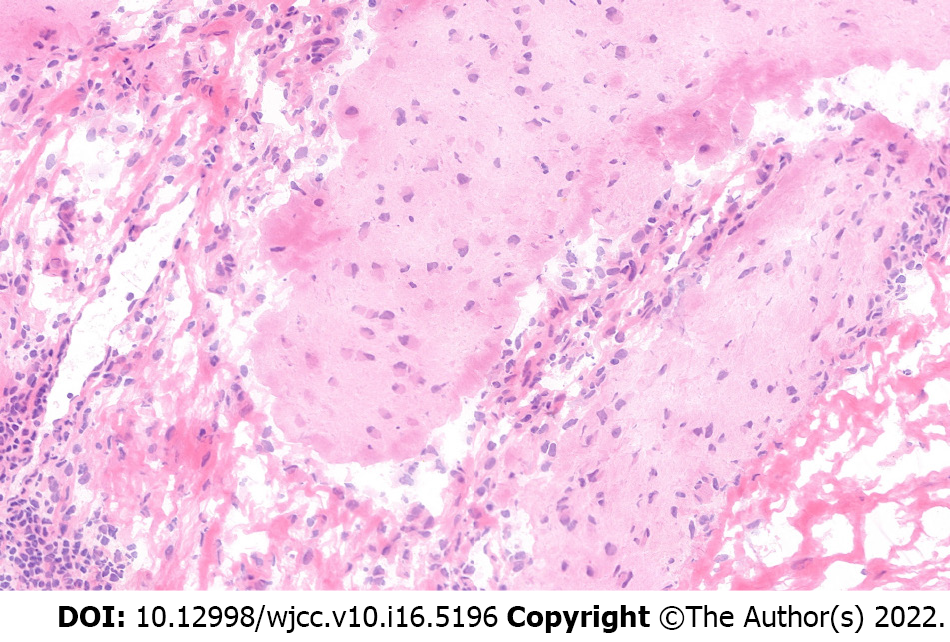
Typical psychotic symptoms, and memory and consciousness disorders accompanied by seizures were observed in all six patients from this study. All patients showed positive signals in serum and cerebrospinal fluid samples for NMDAR and received operation and immunotherapy. Three patients underwent unilateral oophorocystectomy and the other three underwent unilateral oophorectomy through minimally invasive surgeries, including laparoscopic and single port laparoscopic surgeries. So far, no deaths have occurred. Two patients had recurrent psychotic symptoms while the remaining four had no mental symptoms or tumor recurrence during postoperative follow-up (Supplementary Table 1). A representative brain tissue pathology from one of the patients is shown in Figure 2.
In this paper, 155 cases in 130 case reports of anti-NMDAR encephalitis caused by ovarian teratoma were studied and analyzed (Supplementary Table 1).
The number of papers and case reports has progressively increased since the 2007 publication of Hughes et al[19] (Table 1). In 2014, a systematic review of 173 cases of ovarian teratoma-associated anti-NMDAR encephalitis was published. Most articles containing case reports have been published in neurology or psychiatry journals. This is consistent with the results of the 2014 article[19].
| Year of publication and country of study | |
| Year of publication (No. of papers) | n (%) |
| 2014 (17) | 22 (14.2) |
| 2015 (19) | 20 (12.9) |
| 2016 (28) | 31 (20.0) |
| 2017 (32) | 38 (24.5) |
| 2018 (17) | 18 (11.6) |
| 2019 (17) | 26 (16.8) |
| Total (130) | 155 (100.0) |
| Country of birth or study | n (%) |
| Asia: Japan-28; China-26; Korea-5; India-5; Taiwan-3; Thailand-2; Malaysia-1; Saudi Arabia-1; Israel-1 | 72 (46.5) |
| Australia: 5 | 5 (3.2) |
| Europe: Spain-12; United Kingdom-6; Italy-3; Netherlands-3; Poland-2; Russia-2; Germany-1; France-1; Hungary-1; Ireland-1; Croatia-1; Turkey-1; Espana-1; Romania-1; Osterreich-1 | 37 (23.9) |
| North-America: United States-36; Canada-3; Mexico-1 | 40 (25.8) |
| South-America: Ecuador-1 | 1 (0.6) |
| Total | 155 (100) |
As would be expected, there is a global disparity in terms of the available data from individual countries. The differences in national wealth/resources and the differences in health care play a major role in this disparity. It is further evident in the fact that there is not even a single report from Africa, to the best of the knowledge of authors. Further, there has been major advancements in the last few years as there was just a single case reported from China prior to the year 2014 whereas 27 cases have been reported after 2014 (Table 1)[19]. Thus, clearly the number of cases in China has increased significantly recently. Mean age of the patients was 25.0 ± 8.0 years with a median age of 25 years. The tumors were predominantly reported to be located on the right ovary. Immature teratomas or mature teratomas with immature foci were larger than dermoid cysts (Table 2).
| Variable | Histological type of ovarian teratoma | Total cases with teratoma | |||
| Mature | Immature | Mature and immature | Not specified | ||
| Age (n) | 130 | 14 | 2 | 9 | 155 |
| mean ± SD | 25.1 ± 8.3 | 24.8 ± 7.1 | 23.0 ± 0.0 | 25.2 ± 8.0 | 25.0 ± 8.0 |
| Median (min-max) | 25 (7-73) | 25 (9-38) | 23 (23-23) | 25 (14-36) | 25 (7-73) |
| Tumor location | |||||
| Right ovary | 56 (88.9) | 6 (9.5) | 0 | 1 (1.6) | 63 |
| Left ovary | 38 (86.4) | 6 (13.6) | 0 | 0 | 44 |
| Bilateral | 18 (85.7) | 0 | 2 (9.5) | 1 (4.8) | 21 |
| Not marked | 18 (66.7) | 2 (7.4.) | 0 | 7 (25.9) | 27 |
| Total | |||||
| Teratoma size (cm) | |||||
| mean ± SD (n) | 3.3 ± 2.4 (65) | 10.2 ± 3.9 (10) | 3.5 ± 1.3 (2) | - | 4.1 ± 3.3 (77) |
| Median (min-max) | 3.4 (0.4-13) | 10.2 (2.5-17) | 3.5 (2.4-4.9) | - | 5.7 (0.4-17) |
It is interesting to report that the clinical presentation of our six cases is very consistent with those reported earlier. Common symptoms were viral-like prodrome, including fever, headache or dizziness, nausea or vomiting, and general discomfort, along with abdominal pain, high blood pressure, and decreased sleep. Patients also reported severe psychiatric symptoms, speech dyskinesias, memory loss, seizures, reduced consciousness and sometimes orofacial dyskinesias, and progression to autonomic and respiratory instability.
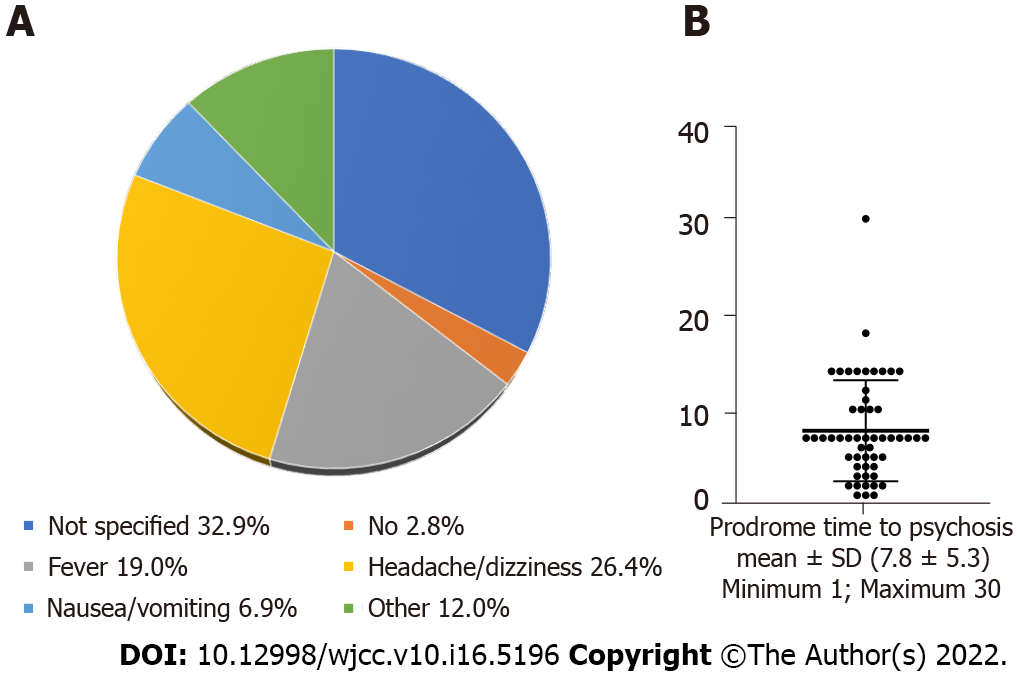
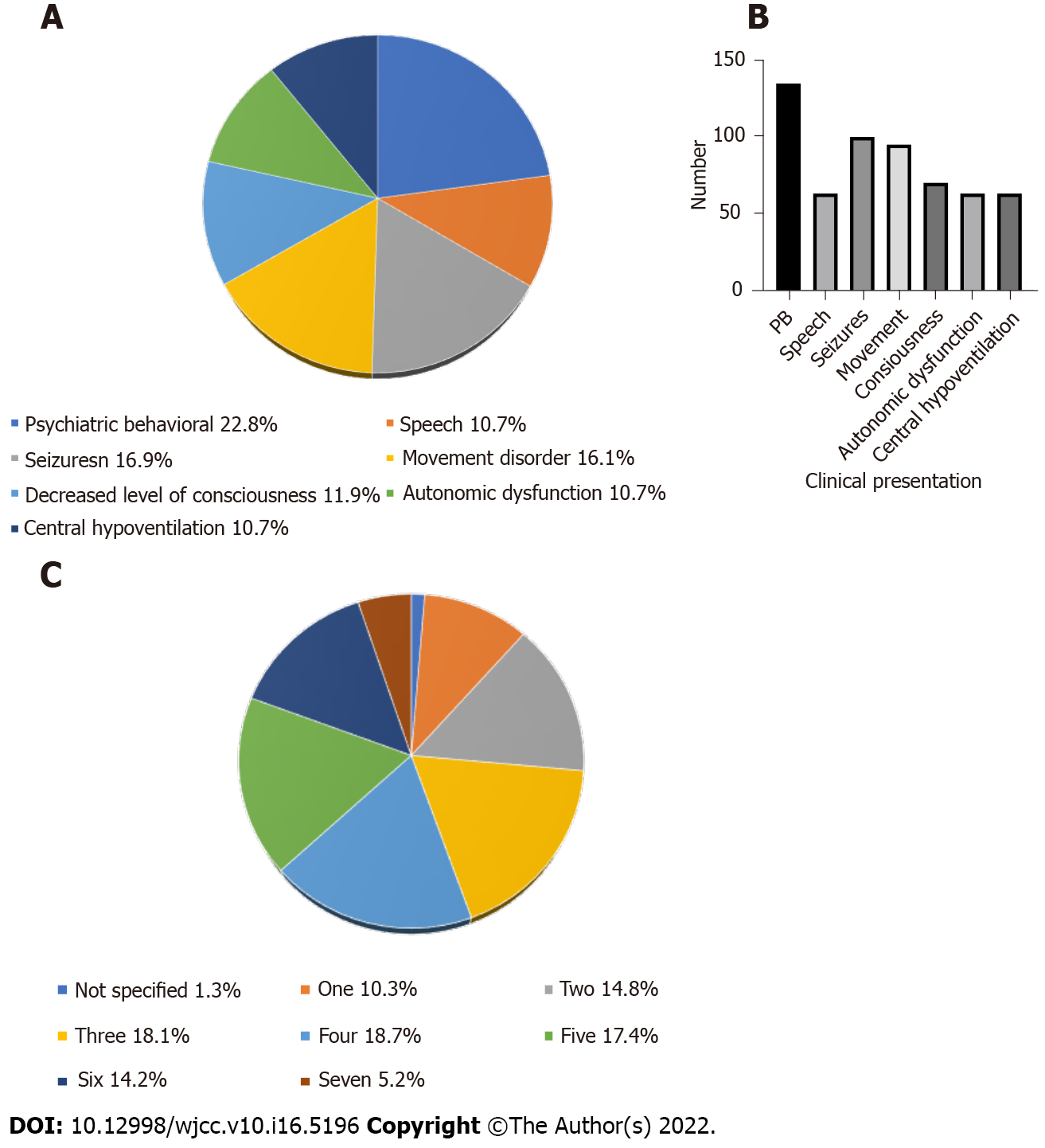
This pooled study showed that prodrome with fever occurred in 19.0% of the patients, headache or dizziness in 26.4%, nausea or vomiting in 6.9%, other in 12.0%, no prodrome in 2.8%, and not specified in 32.9% (Figure 3A). The mean time from prodrome to psychosis was about 1 wk (Figure 3B). The clinical symptoms were psychiatric behavioral (22.8%), speech dyskinesias (pressured speech, verbal reduction, and mutism) (10.7%), seizures (16.9%), movement disorder (16.1%), decreased level of consciousness (11.9%), autonomic dysfunction (10.7%), and central hypoventilation (10.7%) (Figure 4A). Among them, psychosomatic behavioral symptoms were the most common (Figure 4B) and more than half of the patients presented with three to five clinical symptoms (Figure 4C).
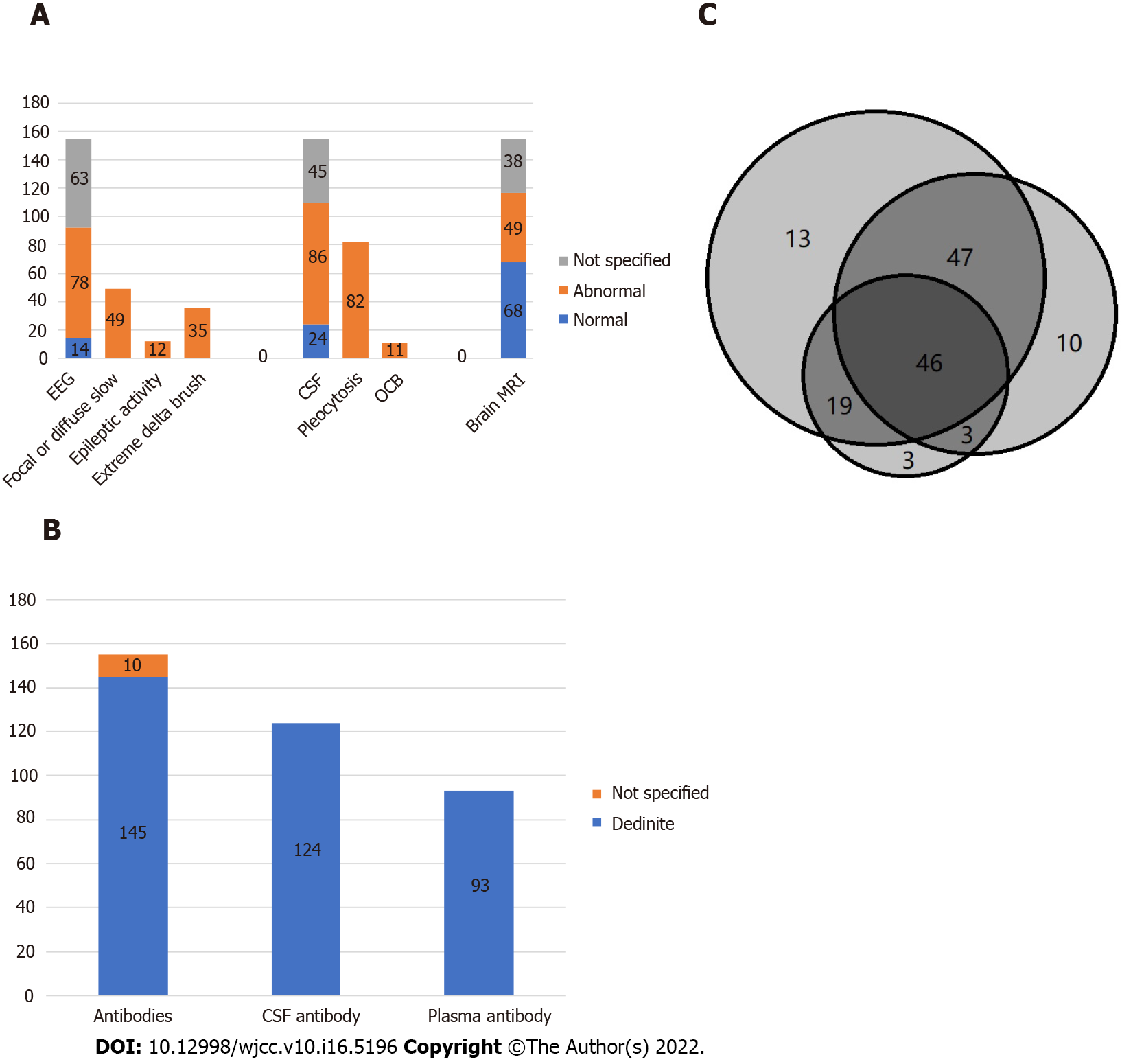
Examinations included electroencephalography (EEG), colony-stimulating factor (CSF), brain magnetic resonance imaging (MRI), and IgG anti-GluN1 antibodies. More than half of the patients showed abnormal electroencephalogram, common for focal or diffuse slow or disorganized activity, epileptic activity, and extreme delta brush. More than half of the patients also showed abnormal cerebrospinal fluid, common for pleocytosis (> 5 white blood cells/mm3), and oligoclonal bands. About a third of the cases had brain MRI abnormalities (Figure 5A). Among 155 cases, 145 were positive for antibody test, and 10 were not specified (Figure 5B).
Among the 150 surgical patients, 99 had full recovery and mild deficits, two had severe deficits, three died (including deep vein thrombosis and while receiving anticoagulation development of gastrointestinal bleeding in 1; severe septicemia in 2), and 47 had no prognosis data.
Treatment includes surgery and immunomodulation treatment. In 155 cases, 150 underwent surgical treatment, four did not undergo surgery, and one had no data on whether there was surgical treatment (Table 3). One patient with no information about surgical intervention, was in coma after 2 mo of follow-up. All four patients who did not undergo surgery died.
| Outcome type | Tumor excision | Oomph | Surgery type not specified | No surgery | Not specified |
| Full recovery | 37 | 27 | 7 | ||
| Mild deficits | 18 | 9 | 1 | ||
| Severe deficits | 1 | 1 | 1 | ||
| Death | 1 | 2 | 4 | ||
| Not specified | 11 | 29 | 6 |
For the immunomodulation treatment, the combination of corticosteroid and intravenous immunoglobulin (IV Ig) and the combination of corticosteroid and IV Ig and plasmapheresis are common (Figure 5C).
We report here a series of six cases of ovarian teratoma-associated anti-NMDAR encephalitis from our hospital. Further, in the systematic review, we detailed cases of ovarian teratoma-associated anti-NMDAR encephalitis from the published literature. The pathogenesis of ovarian teratoma-associated anti-NMDAR encephalitis remains unclear. NMDARs originate from heteromers of NR1 and NR2 subunits. The NR1 subunit is known to bind to glycine while the NR2 subunit is known to bind to glutamate[20,21]. Antibodies in anti-NMDAR encephalitis patients cause a reversible titer-dependent loss of NMDARs[22], and they target an epitope on the NR1 subunit that resides in the hippocampal and frontotemporal regions[23,24]. Anti-NMDAR antibody production is related to the presence of tumors, mostly teratomas.
There seems to be a connection between the prodromal flu-like symptoms and the antibodies against NMDAR. Some researchers have emphasized the connection between viral (e.g., HSV) infection and injury of the blood–brain barrier. Therefore, analysis of CSF for the presence of NMDA receptor antibodies is important in patients with relapsing symptoms after HSE[25,26]. Human immunodeficiency virus and other neurotropic viruses (e.g., HSV) might also be a trigger for anti-NMDAR encephalitis[27]. Meningitis can induce transient blood-brain barrier disruption, which facilitates transmission of NMDAR autoantibodies to the CNS[28].
In a typical presentation of anti-NMDA receptor encephalitis, there is reported development of severe psychiatric symptoms, seizures, memory loss, and reduced consciousness. Often, there are additional manifestations such as orofacial dyskinesias and progression to autonomic and respiratory instability. Anti-NMDAR encephalitis is known to progress through five characteristic phases. The advanced stage is typically hallmarked by extreme autonomic instability with hyperthermia alternating with hypothermia, hypoventilation, fluctuating blood pressures, tachycardia, and even bradycardia as severe as asystole. Dysautonomia, sinus pauses, and asystole are likely caused by disruption in the balance between parasympathetic and sympathetic activity. Up to 90% of rhythm disturbances originate from sinus node abnormalities. Life-threatening cardiac dysrhythmia and cardiac arrest require urgent management[29,30]. Temporary pacing is occasionally required, but permanent pacing appears to be unnecessary[31].
In a case series of 100 anti-NMDA-R encephalitis patients, 69% of the patients were reported with autonomic instability and these patients required an average of 2 mo of ventilator support, with around 37% of the patients diagnosed with cardiac arrhythmias and four reported to be requiring pacemakers[7]. On the other hand, in a report that included 360 patients, two died because of sudden cardiac death while the reason for the death of one other patient was not fully determined[30].
A number of diagnostic tools are now available. These include serum analysis and CSF antibody against the NMDA receptor, EEG, analysis of CSF, and brain MRI. Additionally, the diagnosis of the tumor requires further tests such as transvaginal ultrasound, CT/MRI, and the evaluation of blood tumor markers. Pelvic ultrasound has also been employed to detect ovarian teratomas. CT scans can help identify calcification within the mass; however, MRI is much more accurate when making diagnoses for ovarian teratoma or the Mullerian duct anomalies. Further, whole-body PET/CT have consistently proven to be highly accurate when it comes to staging this disease. The diagnosis of anti-NMDA receptor encephalitis typically involves exclusion of other causes of encephalopathy. In case that the cause of a patient’s encephalopathy is not evident, attending physician needs to rule out anti-NMDA receptor encephalitis, particularly in patients with no reported psychiatric history. For diagnosis, lumbar puncture is required to collect and analyze CSF and then test for NR1 and NR2 using specific antibodies. A confirmed diagnosis is made if the anti- NMDAR antibodies are found in the CSF or in the serum. The diagnostic criteria have been described in the literature[27]. Based on the presence of clinical features consistent with the probable criteria, finding an adnexal mass, most likely a teratoma, upon finding anti-NMDAR antibodies in the CSF or serum, and after the exclusion of other possible etiologies, a diagnosis of anti-NMDA receptor encephalitis can be established.
Anti-NMDAR encephalitis had initially been described in young women with ovarian teratoma, but it is also common in women without tumor, in men, or in children[32]. Although not all patients presenting with NMDAR encephalitis are females with ovarian teratomas, the frequency of these patients mandates screening of females to rule out a causative tumor. The mainstays of treatment include immunomodulation and neoplasm removal targeting both symptomatic and causal factors[24]. Tumor removal is an effective treatment for anti-NMDAR encephalitis. Tumor removal in those with identifiable lesions leads to rapid clinical improvement. Even if none of the investigations are indicative of an ovarian teratoma, there still may be an occult ovarian teratoma[33]. In some cases, the teratoma is microscopic and only found following oophorectomy[34]. Tumor search and diagnosis are extremely important, and oophorocystectomy and oophorectomy are justified. Whether empiric exploratory laparotomy or laparoscopy and blind oophorectomies should be performed in patients with anti-NMDA receptor encephalitis without clinical evidence of a tumor is debatable. Because a laparoscopic examination for determining ovarian teratoma is less-invasive than laparotomy, trial laparoscopy may be acceptable for a treatment strategy if an ovarian tumor cannot be detected by various imaging tests.
Immunomodulation treatment consists of a first line therapy and a second line therapy. The first line therapy includes corticosteroid, IV Ig, and plasmapheresis used alone or in combination. Steroids, IV Ig, and plasmapheresis help reduce antibody titers. The second line therapy includes rituximab and cyclophosphamide, whether alone or in combination. Benzodiazepines and antipsychotics round out the pharmacotherapies employed in the treatment of seizures, psychosis, and behavioral dysfunction[31]. When the patient does not have a tumor, first-line therapy with IV Ig, methylprednisolone, and plasma exchange can be used in sequence or in combinations[35]. The second-line therapy with rituximab (against CD-20 B-lymphocytes) or cyclophosphamide can also be used[36]. It has been reported that almost half of the patients show significant improvement within a month of first line treatment and tumor removal. Further, second line therapy has been reported to be effective in up to two thirds of the patients who progressed after the first line of treatment. Thus, the prognosis of patients is generally very good once they have been administered either the first or, if needed, second line therapy[7].
In a systemic review of 100 cases of anti-NMDAR encephalitis, it was revealed that a better neurological outcome is achieved if surgical removal of the teratoma was performed quickly upon the onset of symptoms. This ensures much reduced probability of relapse and significantly improved recovery time[37]. Further, a systematic review of 174 cases of anti-NMDAR encephalitis revealed that even the small teratomas that contain nervous tissues, can result in severe complications which can be secondary to anti-NMDAR encephalitis[19].
Prognosis is generally poor for the patients, particularly those who are not attended to early in the disease. Generally, patients have a slow and incomplete recovery of neuropsychiatric sequelae in up to 3/4 of patients within an average of about 7 mo. It has been reported that a positive prognosis is linked to decreased anti-body titers. The one alarming statistic is that almost a quarter of patients are reported to relapse. The relapse is mostly reported within the first 2 years and there have been reports where relapse was associated with ovarian teratoma recurrence. On a bright side, relapses are often less severe. According to some estimates, 5%-7% of patients succumb to the disease within an average of 3.5 mo[7,30].
Despite the emerging evidence, an association between ovarian teratoma and anti-NMDAR encephalitis is not fully realized. Anti-NMDAR encephalitis, a rare complication of ovarian teratoma, can be fatal. Therefore, its further understanding cannot be underestimated. Behavioral changes, acute psychiatric symptoms accompanied by seizures, and memory and consciousness disorders should be recognized, the possibility of anti-NMDAR encephalitis should be considered, and examinations for anti-NMDAR antibodies need to be completed to confirm the diagnosis as early as possible. Tumor location should be prioritized, once diagnosis is defined, and the tumor search should focus on the ovaries. If a tumor is detected (even with a benign appearance), it is recommended to remove the tumor as soon as possible. Choice of surgical procedure should be decided considering pathology, age, fertility desire, and patients’ requirements, and it should be ensured that tumors are completely removed during operation. Early use of corticosteroids and IgG-depleting strategies (IVIg or plasma exchange) may improve outcome. Postoperative follow-up is particularly important in case of recurrence.
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is a rare complication of ovarian teratoma that remains poorly understood.
Anti-NMDAR encephalitis can be fatal and the pathogenesis involving association with ovarian teratoma needs to be better understood in order to improve diagnosis as well as patient outcome.
We aimed to better understand anti-NMDAR encephalitis through a thorough examination of six patients enrolled in our hospital in addition to survey of the literature.
We evaluated six patients enrolled in our hospital and, additionally, surveyed PubMed and Scopus to evaluate 155 cases of anti-NMDAR encephalitis in 130 reports. Focus was on diagnosis, treatments, and patient outcomes.
In our patient cohort, five of six patients fully recovered while the 6th patient recovered with deficits. In the surveyed literature, the majority of patients, particularly those with surgical intervention, had positive outcome.
Our evaluations revealed that surgical outcomes are favorable and early removal of tumor is critical. The importance of postoperative follow-up cannot be over-estimated.
Early use of corticosteroids and IgG-depleting strategies may improve outcome. Postoperative follow-up is particularly important in case of recurrence.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Solanki SL, India S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Yan JP
| 1. | Britton PN, Eastwood K, Paterson B, Durrheim DN, Dale RC, Cheng AC, Kenedi C, Brew BJ, Burrow J, Nagree Y, Leman P, Smith DW, Read K, Booy R, Jones CA; Australasian Society of Infectious Diseases (ASID); Australasian College of Emergency Medicine (ACEM); Australian and New Zealand Association of Neurologists (ANZAN); Public Health Association of Australia (PHAA). Consensus guidelines for the investigation and management of encephalitis in adults and children in Australia and New Zealand. Intern Med J. 2015;45:563-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338:94-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 3. | Guan HZ, Ren HT, Cui LY. Autoimmune Encephalitis: An Expanding Frontier of Neuroimmunology. Chin Med J (Engl). 2016;129:1122-1127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2136] [Article Influence: 178.0] [Reference Citation Analysis (0)] |
| 5. | Kayser MS, Dalmau J. Anti-NMDA Receptor Encephalitis in Psychiatry. Curr Psychiatry Rev. 2011;7:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 6. | Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2002] [Cited by in RCA: 1726] [Article Influence: 95.9] [Reference Citation Analysis (0)] |
| 7. | Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch DR. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2443] [Cited by in RCA: 2158] [Article Influence: 126.9] [Reference Citation Analysis (0)] |
| 8. | Iizuka T, Sakai F, Ide T, Monzen T, Yoshii S, Iigaya M, Suzuki K, Lynch DR, Suzuki N, Hata T, Dalmau J. Anti-NMDA receptor encephalitis in Japan: long-term outcome without tumor removal. Neurology. 2008;70:504-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 419] [Cited by in RCA: 356] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 9. | Okamura H, Oomori N, Uchitomi Y. An acutely confused 15-year-old girl. Lancet. 1997;350:488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Nokura K, Yamamoto H, Okawara Y, Koga H, Osawa H, Sakai K. Reversible limbic encephalitis caused by ovarian teratoma. Acta Neurol Scand. 1997;95:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 73] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Iizuka T. A distinct syndrome of encephalitis presenting as acute onset of psychosis followed by unresponsiveness, hypoventilation, and intractable orofacial-limb hyperkinetic movements (Supple 1). Neurology. 2005;64. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Vitaliani R, Mason W, Ances B, Zwerdling T, Jiang Z, Dalmau J. Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 464] [Cited by in RCA: 386] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 13. | Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, Llufriu S, Muchart J, Erro ME, Abraira L, Moris G, Monros-Giménez L, Corral-Corral Í, Montejo C, Toledo M, Bataller L, Secondi G, Ariño H, Martínez-Hernández E, Juan M, Marcos MA, Alsina L, Saiz A, Rosenfeld MR, Graus F, Dalmau J; Spanish Herpes Simplex Encephalitis Study Group. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 420] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 14. | Armangue T, Moris G, Cantarín-Extremera V, Conde CE, Rostasy K, Erro ME, Portilla-Cuenca JC, Turón-Viñas E, Málaga I, Muñoz-Cabello B, Torres-Torres C, Llufriu S, González-Gutiérrez-Solana L, González G, Casado-Naranjo I, Rosenfeld M, Graus F, Dalmau J; Spanish Prospective Multicentric Study of Autoimmunity in Herpes Simplex Encephalitis. Autoimmune post-herpes simplex encephalitis of adults and teenagers. Neurology. 2015;85:1736-1743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 193] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Zandi MS, Irani SR, Follows G, Moody AM, Molyneux P, Vincent A. Limbic encephalitis associated with antibodies to the NMDA receptor in Hodgkin lymphoma. Neurology. 2009;73:2039-2040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Zhang L, Wu MQ, Hao ZL, Chiang SM, Shuang K, Lin MT, Chi XS, Fang JJ, Zhou D, Li JM. Clinical characteristics, treatments, and outcomes of patients with anti-N-methyl-d-aspartate receptor encephalitis: A systematic review of reported cases. Epilepsy Behav. 2017;68:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 17. | Xu CL, Zhao WQ, Li JM, Wang JW, Wang SH, Wang DX, Liu MY, Qiao SS, Jin JY, He ZP, Ji XJ. Anti-N-methyl-D-aspartate receptor encephaliits:an adolescent with ovarian teratoma. Zhonghua Shenjingke Zazhi. 2010;43:781-783. [DOI] [Full Text] |
| 18. | Dai Y, Zhang J, Ren H, Zhou X, Chen J, Cui L, Lang J, Guan H, Sun D. Surgical outcomes in patients with anti-N-methyl D-aspartate receptor encephalitis with ovarian teratoma. Am J Obstet Gynecol. 2019;221:485.e1-485.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 19. | Acién P, Acién M, Ruiz-Maciá E, Martín-Estefanía C. Ovarian teratoma-associated anti-NMDAR encephalitis: a systematic review of reported cases. Orphanet J Rare Dis. 2014;9:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Kendrick SJ, Lynch DR, Pritchett DB. Characterization of glutamate binding sites in receptors assembled from transfected NMDA receptor subunits. J Neurochem. 1996;67:608-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Laube B, Hirai H, Sturgess M, Betz H, Kuhse J. Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit. Neuron. 1997;18:493-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 351] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 22. | Hughes EG, Peng X, Gleichman AJ, Lai M, Zhou L, Tsou R, Parsons TD, Lynch DR, Dalmau J, Balice-Gordon RJ. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866-5875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 928] [Cited by in RCA: 842] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 23. | Moscato EH, Jain A, Peng X, Hughes EG, Dalmau J, Balice-Gordon RJ. Mechanisms underlying autoimmune synaptic encephalitis leading to disorders of memory, behavior and cognition: insights from molecular, cellular and synaptic studies. Eur J Neurosci. 2010;32:298-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Barry H, Byrne S, Barrett E, Murphy KC, Cotter DR. Anti-N-methyl-d-aspartate receptor encephalitis: review of clinical presentation, diagnosis and treatment. BJPsych Bull. 2015;39:19-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 25. | Prüss H, Finke C, Höltje M, Hofmann J, Klingbeil C, Probst C, Borowski K, Ahnert-Hilger G, Harms L, Schwab JM, Ploner CJ, Komorowski L, Stoecker W, Dalmau J, Wandinger KP. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. 2012;72:902-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 305] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 26. | Armangue T, Leypoldt F, Málaga I, Raspall-Chaure M, Marti I, Nichter C, Pugh J, Vicente-Rasoamalala M, Lafuente-Hidalgo M, Macaya A, Ke M, Titulaer MJ, Höftberger R, Sheriff H, Glaser C, Dalmau J. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol. 2014;75:317-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 312] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 27. | Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2680] [Article Influence: 297.8] [Reference Citation Analysis (0)] |
| 28. | Hammer C, Stepniak B, Schneider A, Papiol S, Tantra M, Begemann M, Sirén AL, Pardo LA, Sperling S, Mohd Jofrry S, Gurvich A, Jensen N, Ostmeier K, Lühder F, Probst C, Martens H, Gillis M, Saher G, Assogna F, Spalletta G, Stöcker W, Schulz TF, Nave KA, Ehrenreich H. Neuropsychiatric disease relevance of circulating anti-NMDA receptor autoantibodies depends on blood-brain barrier integrity. Mol Psychiatry. 2014;19:1143-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 270] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 29. | Inayat F, Hung Pinto WA, Ahmad S, Hussain A, Ullah W. Anti-N-methyl-D-aspartate Receptor Encephalitis Associated with Ictal Torsades de Pointes and Cardiac Arrest. Cureus. 2019;11:e4837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Nazif TM, Vázquez J, Honig LS, Dizon JM. Anti-N-methyl-D-aspartate receptor encephalitis: an emerging cause of centrally mediated sinus node dysfunction. Europace. 2012;14:1188-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10: 63-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1879] [Cited by in RCA: 1652] [Article Influence: 118.0] [Reference Citation Analysis (0)] |
| 32. | Prüss H, Dalmau J, Arolt V, Wandinger KP. [Anti-NMDA-receptor encephalitis. An interdisciplinary clinical picture]. Nervenarzt. 2010;81:396, 398, 400, passim. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Boeck AL, Logemann F, Krauß T, Hussein K, Bültmann E, Trebst C, Stangel M. Ovarectomy despite Negative Imaging in Anti-NMDA Receptor Encephalitis: Effective Even Late. Case Rep Neurol Med. 2013;2013:843192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Raynor G, Bader C, Srikanth M, Kroll D, Gutheil T, Berkowitz A. Psychosis Secondary to Anti-N-methyl-D-Aspartate Receptor Encephalitis. Harv Rev Psychiatry. 2016;24:229-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 35. | Pham HP, Daniel-Johnson JA, Stotler BA, Stephens H, Schwartz J. Therapeutic plasma exchange for the treatment of anti-NMDA receptor encephalitis. J Clin Apher. 2011;26:320-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Kashyape P, Taylor E, Ng J, Krishnakumar D, Kirkham F, Whitney A. Successful treatment of two paediatric cases of anti-NMDA receptor encephalitis with cyclophosphamide: the need for early aggressive immunotherapy in tumour negative paediatric patients. Eur J Paediatr Neurol. 2012;16:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 37. | Tumbi A, Gilani A, Scarff JR, Kaur G, Lippmann S. Anti-N-methyl-D encephalitis. Innov Clin Neurosci. 2011;8:24-25. [PubMed] |