Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.5103
Peer-review started: December 22, 2021
First decision: February 8, 2022
Revised: February 17, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 26, 2022
Processing time: 153 Days and 5.6 Hours
Synovial sarcoma (SS) is an uncommon and highly malignant soft tissue sarcoma in the clinic, with primary pulmonary SS (PPSS) being extremely rare. Here, we describe the clinical characteristics, diagnosis, and treatment of a solitary PPSS case confirmed via surgical resection and fluorescence in situ hybridization (FISH).
A 33-year-old man was admitted because of intermittent coughing and hemoptysis for one month, with lung shadows observed for two years. Whole-body positron emission tomography-computed tomography (PET-CT) revealed a solitary mass in the upper lobe of the right lung, with uneven radioactivity uptake and a maximum standardized uptake value of 5.6. The greyish-yellow specimen obtained following thoracoscopic resection was covered with small multi-nodulated structures and consisted of soft tissue. Hematoxylin and eosin staining revealed spindle-shaped malignant tumor cells. Immunohistochemistry indicated these tumor cells were CD99 and BCL-2-positive. Furthermore, the FISH test revealed synovial sarcoma translocation genetic reassortment, which confirmed the diagnosis of SS.
PPSS is extremely rare and tends to be misdiagnosed as many primary pulmonary diseases. PET-CT, histologic analysis, and FISH tests can be used to differentiate PPSS from other diseases. Surgical resection is regularly recommended for the treatment of solitary PPSS and is helpful for improving the prognosis.
Core Tip: Synovial sarcoma (SS) is an uncommon and highly malignant soft tissue sarcoma in the clinic, with primary pulmonary SS (PPSS) being extremely rare. It tends to be misdiagnosed as many primary pulmonary diseases. Here, we described the clinical characteristics, diagnosis, and treatment of a solitary PPSS case. Positron emission tomography-computed tomography, histologic analysis, and fluorescence in situ hybridization tests can be used to differentiate PPSS from other diseases. Surgical resection is regularly recommended for the treatment of solitary PPSS and is helpful for improving the prognosis.
- Citation: He WW, Huang ZX, Wang WJ, Li YL, Xia QY, Qiu YB, Shi Y, Sun HM. Solitary primary pulmonary synovial sarcoma: A case report. World J Clin Cases 2022; 10(15): 5103-5110
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/5103.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.5103
Synovial sarcoma (SS) is an uncommon and malignant soft tissue tumor that accounts for around 10% of soft tissue sarcomas[1]. Although it often arises in the limbs, particularly near the knee joints, SS has the potential to occur in any part of the body, including the head and neck, lungs, kidneys, prostate, skin, vulva, blood vessels, and nerve tissue[2]. The remarkable genetic feature of SS is the pathognomonic t(X;18)(p11;Q11) translocations, resulting in fusion of the SS18 gene, known as SYT, on chromosome 18 with one of the three highly homologous SSX genes (SSX1, SSX2, or SSX4), which are all on the X-chromosome[3,4].
While most of the synovial sarcoma in the lung is metastatic, primary pulmonary SS (PPSS) is extremely rare, especially solitary PPSS[5]. Because of a lack of specific characteristics, PPSS tends to be confused with other benign or malignant lung lesions, such as teratoma, lymphoma, spherical tuberculosis, and pulmonary cryptococcosis. In this report, we describe a case of solitary PPSS that was confirmed via positron emission tomography-computed tomography (PET-CT), histological analysis, immunohistochemistry (IHC), and fluorescence in situ hybridization (FISH) genetic testing. Additionally, we conduct a review of the clinical manifestations, diagnosis, and treatment of this type of disease using pertinent literature.
In August 2020, a 33-year-old male was admitted due to two years of discovered lung shadows, and intermittent coughing and hemoptysis for one month.
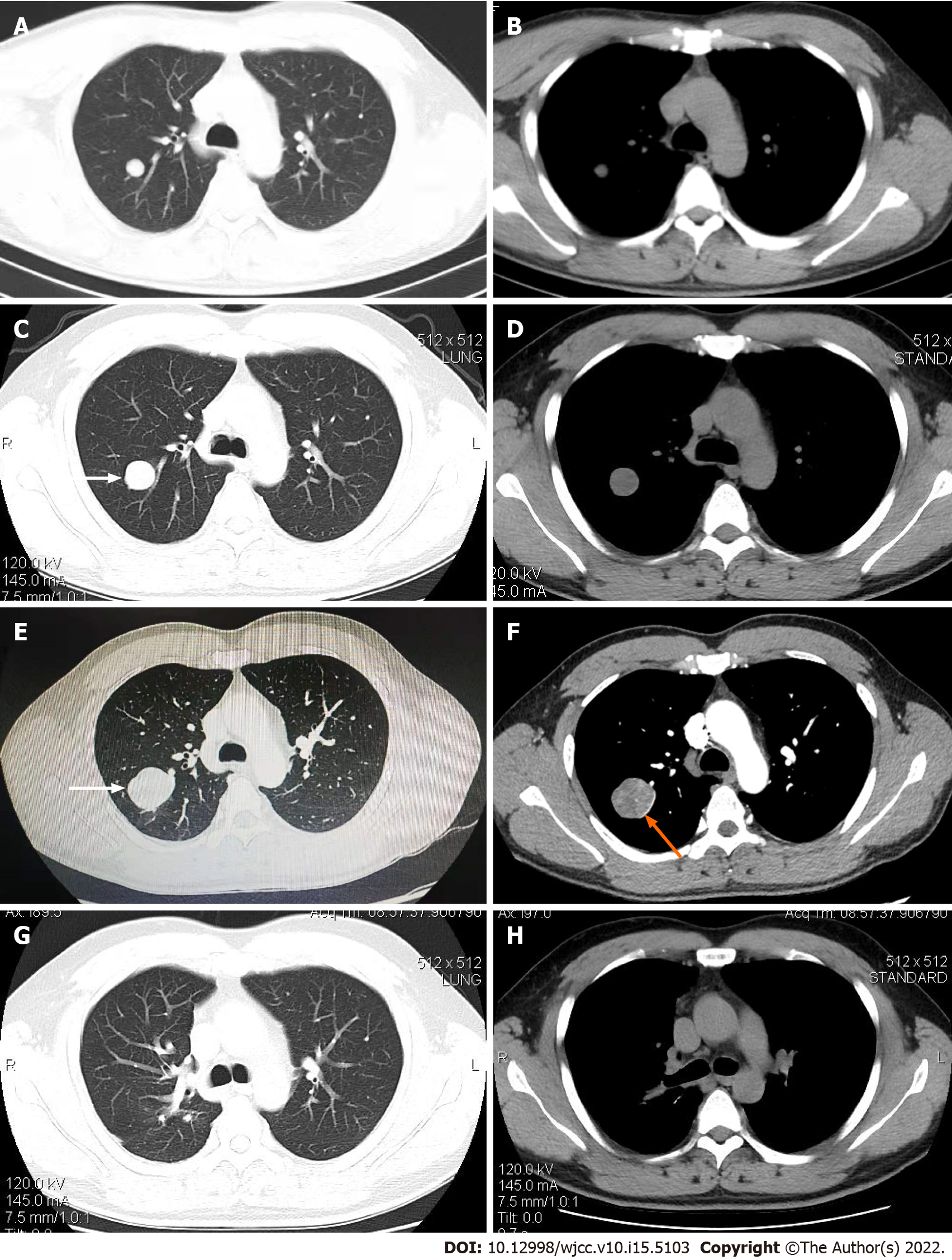
The patient had undergone a routine chest computed tomography (CT) examination in July 2018 due to trauma, which had shown a solitary roundish nodule with a maximum diameter of approximately 14 mm and smooth edges in the upper lobe of the right lung (Figure 1A and B). The nodule was initially diagnosed as a benign lung tumor and was left untreated. In November 2019, a chest CT re-examination revealed that the lesion had grown bigger significantly, reaching approximately 30 mm in diameter, with a smooth edge and visible shallow lobes. The patient declined the suggestion of a further diagnosis by biopsy (Figure 1C and D). From July 2020, the patient exhibited intermittent coughing with white sputum and hemoptysis. The amount of hemoptysis varied from 50 to 100 mL, without accompanying chills, fever, chest tightness, wheezing, chest pain, weight loss, or night sweats. The hemoptysis became severe two days before admission, occurring dozens of times per day.
The patient had not noticed any remarkable medical conditions in the past.
The patient has no known family history of genetic diseases.
Upon admission to the hospital, the patient’s initial physical examination were all within normal limits.
The patient’s major laboratory tests, including hematological test, blood biochemical test, infection related, tumor biomarker tests and immune related were all within normal limits.
The enhanced chest CT revealed a significantly enlarged round mass of approximately 20 mm × 34 mm × 42 mm in the posterior segment of the right upper lobe with uneven density, distinct edge boundary, spot-like calcification, and a slightly shallow profile. Additionally, the enhanced chest CT scan revealed an uneven level of enhancement of the mass, and the mass periphery had an enhanced shadow in the shape of a ring (Figure 1E and F).
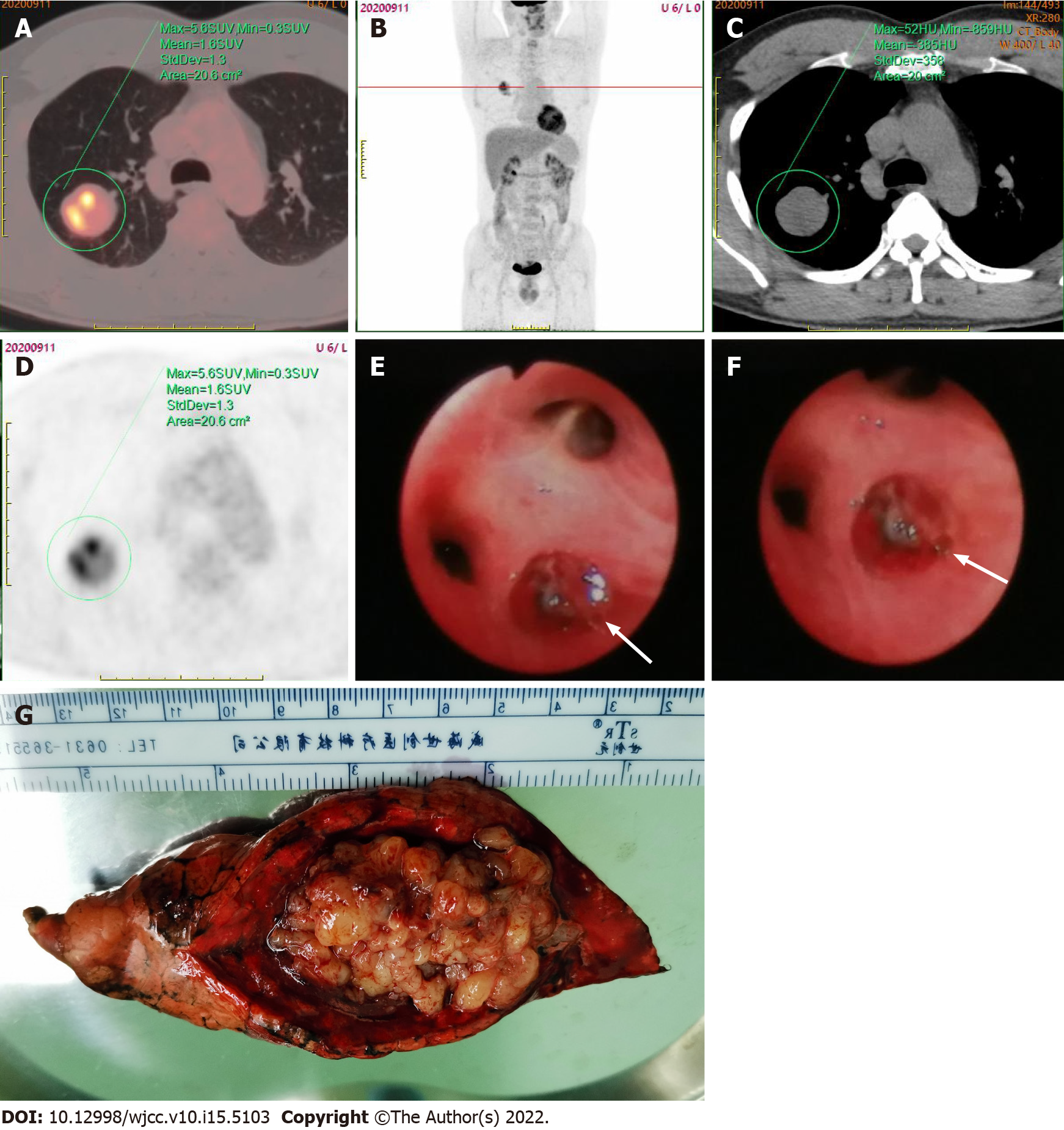
Later, the bronchoscopy procedure revealed a neoplasm at the posterior opening of the right upper lobe surrounded by purulent secretions and a small amount of bleeding. Clamp biopsy was performed due to the mass completely obstructing the airway and preventing the lens body from passing through (Figure 2E and F). H&E staining of the biopsy showed spindle-shaped malignant tumor cells, and IHC showed CKpan (-), CK8/18 (-), CK7 (-), Syn (-), TTF1 (-), P40 (-), P63 (-), CK5/6 (-), dcsmin (-), SMA (-), S-100 (-), and Ki-67 (30% +). The subsequent PET-CT whole-body scan revealed only a mass in the upper lobe of the right lung, with uneven radioactivity uptake and a standardized uptake value (SUV) max of 5.6 (Figure 2A-D).
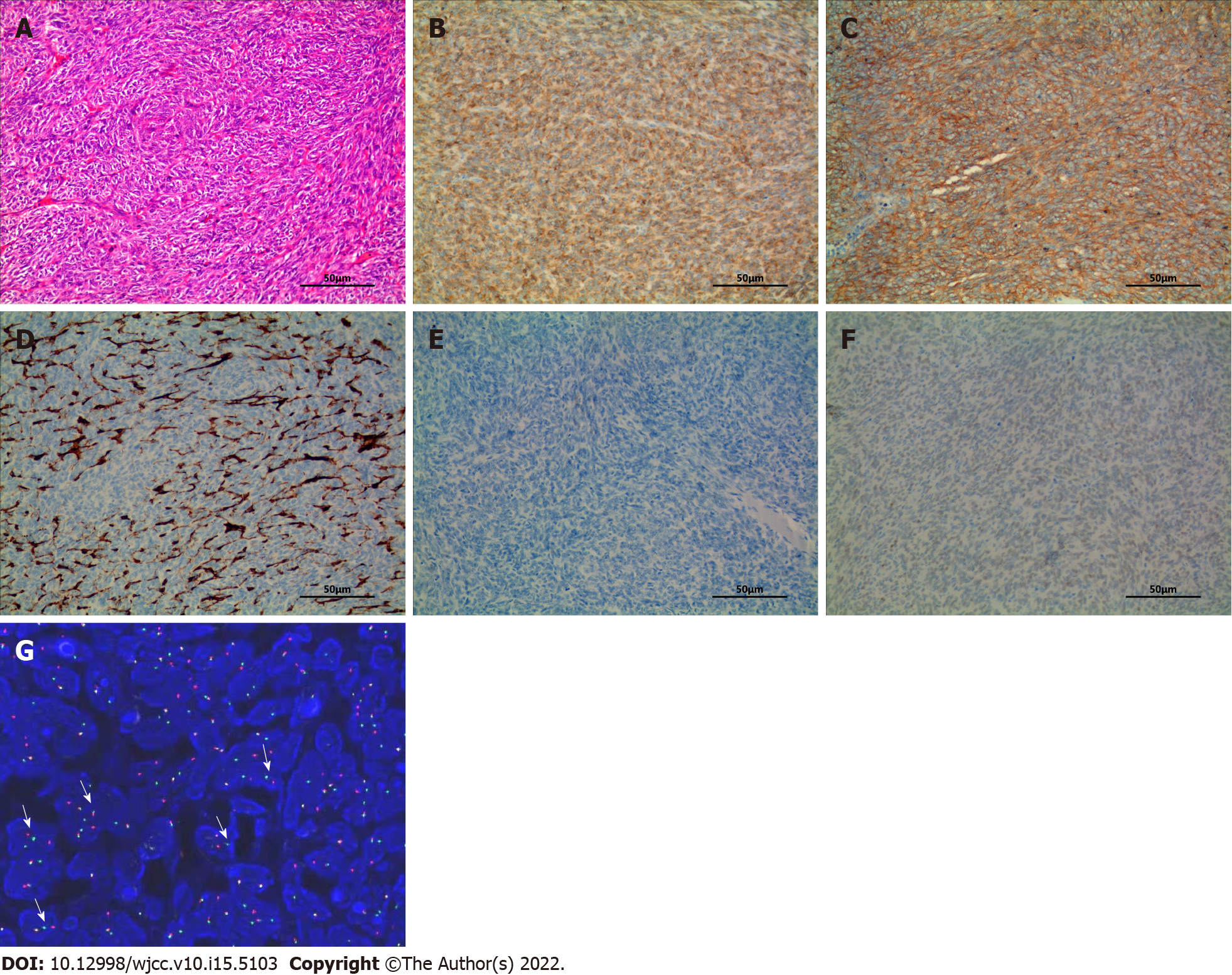
Under general anesthesia, the patient underwent surgical thoracoscopic resection of the posterior segment of the right upper lobe of the lung. The specimen was grayish-yellow in color, covered with small multi-nodulated structures, and composed of soft tissue (Figure 2G). H&E staining of the excised specimen showed a large number of spindle-shaped malignant tumor cells (Figure 3A), and IHC indicated CD99 (3+), BCL-2 (2+), EMA (-), CD34 (-), and TLE1 (-) (Figure 3B-F). In addition, the FISH test showed the splitting of SS18, which furthermore confirmed the diagnosis of SS, pathologically classified as monophasic fibrous or spindle-cell type.
The final diagnosis of the presented case is PPSS.
Under general anesthesia, the patient underwent surgical thoracoscopic resection of the posterior segment of the right upper lobe of the lung. After the operation, the patient recovered and was discharged after declining chemotherapy.
The patient recovered after the operation, and six months after the operation, the follow-up chest CT examination revealed no obvious abnormality (Figure 1G and H).
PPSS is more prevalent in young and middle-aged adults, with an average onset age of 36.5-58 years[6-10] and a male-to-female incidence ratio of 2:1[6,11]. Cough, chest pain, dyspnea, and hemoptysis are all common symptoms. In this case, the patient's primary clinical manifestations were intermittent cough and severe hemoptysis. We ruled out the potential of metastatic lesions from an extrapulmonary primary SS using a PET-CT whole-body scan. Additional H&E staining, IHC, and FISH detection of surgically resected specimens confirmed the diagnosis of PPSS.
PPSS is characterized by roundish parenchymal masses with smooth edges and inconspicuous lobes on imaging. The lesions may be accompanied by necrosis, liquefaction, calcification without cavitation, burrs, and bronchial traction. In addition, enhanced CT frequently reveals an uneven enhancement of the mass or a thick-walled ring, often associated with ipsilateral pleural effusion[12]. In this case, the chest enhanced CT revealed typical peripheral pattern changes of PPSS, including smooth edges, uneven lesion density, calcification, and ring enhancement.
Metastatic lesions of primary SS often occur in the lungs and share similar lung imaging characteristics as PPSS. Thus, it is necessary to use an approach capable of distinguishing these two types of lesions for the determination of further treatment[13]. In this case, using the whole body PET-CT scan ruled out the possibility of extrapulmonary primary SS. PET-CT examination frequently reveals a significant increase in fluorodeoxyglucose (FDG) uptake in lung lesions, with an SUVmax ranging from 2.2 to 17.6[14]. In this case, the SUVmax value of the lesion was 5.6, which indicated that hypermetabolic activity occurred within the tumor cells.
As with SS, PPSS exhibits bidirectional differentiation into mesenchymal and epithelial tissues. Monophasic fibrous and (or) epithelial cells are intertwined under the microscope to form dense tumor cell bundles, some of which are mucous. IHC helps in identifying the pathological type of SS by detecting epithelial and mesenchymal biomarkers. Machen et al[15] reported that BCL-2 was expressed diffusely in 98% of SS cases and CD99 was expressed focally or diffusely in approximately 60% of cases. Consistent with these earlier studies, IHC analysis of the PPSS tissue in this case revealed increased expression of BCL-2 and CD99, as well as a significantly increased Ki-67 index of 30%.
FISH using an SS18 break-apart probe is currently the most widely used approach to demonstrate the presumptive presence of one of the SS18-SSX fusions. If the probe breaks, it suggests that the SS18 gene and SSX gene are fused[16]. It has been reported that the sensitivity and specificity of FISH detection using the SS18 two-color fracture separation probe in the diagnosis of synovial sarcoma are 83% and 100%, respectively[17,18]. In this study, SS18 two-color break separation probe FISH technology was used to specifically detect whether the SS18 gene was broken. The use of isolated probes can cover different fusion subtypes and determine the fusion genes more comprehensively. In this case, the breaking apart of the SYT gene was detected by FISH, which confirmed the final diagnosis of SS.
PPSS has a poor overall prognosis, with a 5-year survival rate of approximately 50% to 80%. Due to the rarity of PPSS, there are no guidelines for its optimal treatment. Therefore, the treatment recom
PPSS is an extremely rare disease with atypical clinical manifestations and imaging changes, raising the risk of diagnostic confusion with other lung diseases, which should attract attention from clinicians. Here, we excluded the potential of SS lung metastasis through systemic examination and diagnosed this case as solitary PPSS based on pathological classification using IHC measurements and the SYT gene break-apart characteristics determined with a FISH test. Later, the tumor was removed by surgical resection, which will benefit the patient’s survival and prognosis.
The authors would like to thank Dr. Yan-Wen Yao and Dr. Wei-Quan Zhu for their constructive comments on this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brat K, Czech Republic; Ishida H, Japan S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Mankin HJ, Hornicek FJ. Diagnosis, classification, and management of soft tissue sarcomas. Cancer Control. 2005;12:5-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Panigrahi MK, Pradhan G, Sahoo N, Mishra P, Patra S, Mohapatra PR. Primary pulmonary synovial sarcoma: A reappraisal. J Cancer Res Ther. 2018;14:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Zaborowski M, Vargas AC, Pulvers J, Clarkson A, de Guzman D, Sioson L, Maclean F, Chou A, Gill AJ. When used together SS18-SSX fusion-specific and SSX C-terminus immunohistochemistry are highly specific and sensitive for the diagnosis of synovial sarcoma and can replace FISH or molecular testing in most cases. Histopathology. 2020;77:588-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Suurmeijer AJH, de Bruijn D, Geurts van Kessel A, Miettinen MM. Synovial sarcoma. In: Fletcher CDM, Bridge JA, Hogendoor P, Mertens F, editing. WHO classification of soft tissue and bone tumors. Fourth edition. Lyon: IARC. 2013: 213-215. |
| 5. | Etienne-Mastroianni B, Falchero L, Chalabreysse L, Loire R, Ranchère D, Souquet PJ, Cordier JF. Primary sarcomas of the lung: a clinicopathologic study of 12 cases. Lung Cancer. 2002;38:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Lan T, Chen H, Xiong B, Zhou T, Peng R, Chen M, Ye F, Yao J, He X, Wang Y, Zhang H. Primary pleuropulmonary and mediastinal synovial sarcoma: a clinicopathologic and molecular study of 26 genetically confirmed cases in the largest institution of southwest China. Diagn Pathol. 2016;11:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Zeren H, Moran CA, Suster S, Fishback NF, Koss MN. Primary pulmonary sarcomas with features of monophasic synovial sarcoma: a clinicopathological, immunohistochemical, and ultrastructural study of 25 cases. Hum Pathol. 1995;26:474-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 116] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Bégueret H, Galateau-Salle F, Guillou L, Chetaille B, Brambilla E, Vignaud JM, Terrier P, Groussard O, Coindre JM. Primary intrathoracic synovial sarcoma: a clinicopathologic study of 40 t(X;18)-positive cases from the French Sarcoma Group and the Mesopath Group. Am J Surg Pathol. 2005;29:339-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Essary LR, Vargas SO, Fletcher CD. Primary pleuropulmonary synovial sarcoma: reappraisal of a recently described anatomic subset. Cancer. 2002;94:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Okamoto S, Hisaoka M, Daa T, Hatakeyama K, Iwamasa T, Hashimoto H. Primary pulmonary synovial sarcoma: a clinicopathologic, immunohistochemical, and molecular study of 11 cases. Hum Pathol. 2004;35:850-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Suster S, Moran CA. Primary synovial sarcomas of the mediastinum: a clinicopathologic, immunohistochemical, and ultrastructural study of 15 cases. Am J Surg Pathol. 2005;29:569-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Hartel PH, Fanburg-Smith JC, Frazier AA, Galvin JR, Lichy JH, Shilo K, Franks TJ. Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series. Mod Pathol. 2007;20:760-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Frazier AA, Franks TJ, Pugatch RD, Galvin JR. From the archives of the AFIP: Pleuropulmonary synovial sarcoma. Radiographics. 2006;26:923-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Kim GH, Kim MY, Koo HJ, Song JS, Choi CM. Primary Pulmonary Synovial Sarcoma in a Tertiary Referral Center: Clinical Characteristics, CT, and 18F-FDG PET Findings, With Pathologic Correlations. Medicine (Baltimore). 2015;94:e1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Machen SK, Fisher C, Gautam RS, Tubbs RR, Goldblum JR. Utility of cytokeratin subsets for distinguishing poorly differentiated synovial sarcoma from peripheral primitive neuroectodermal tumour. Histopathology. 1998;33:501-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Terry J, Barry TS, Horsman DE, Hsu FD, Gown AM, Huntsman DG, Nielsen TO. Fluorescence in situ hybridization for the detection of t(X;18)(p11.2;q11.2) in a synovial sarcoma tissue microarray using a breakapart-style probe. Diagn Mol Pathol. 2005;14:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Sun B, Sun Y, Wang J, Zhao X, Zhang S, Liu Y, Li X, Feng Y, Zhou H, Hao X. The diagnostic value of SYT-SSX detected by reverse transcriptase-polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization (FISH) for synovial sarcoma: a review and prospective study of 255 cases. Cancer Sci. 2008;99:1355-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Vargas AC, Selinger C, Satgunaseelan L, Cooper WA, Gupta R, Stalley P, Brown W, Soper J, Schatz J, Boyle R, Thomas DM, Tattersall MHN, Bhadri V, Maclean F, Bonar SF, Scolyer RA, Karim RZ, McCarthy SW, Mahar A, O'Toole SA. FISH analysis of selected soft tissue tumors: Diagnostic experience in a tertiary center. Asia Pac J Clin Oncol. 2019;15:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Spurrell EL, Fisher C, Thomas JM, Judson IR. Prognostic factors in advanced synovial sarcoma: an analysis of 104 patients treated at the Royal Marsden Hospital. Ann Oncol. 2005;16:437-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 164] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Sleijfer S, Ouali M, van Glabbeke M, Krarup-Hansen A, Rodenhuis S, Le Cesne A, Hogendoorn PC, Verweij J, Blay JY. Prognostic and predictive factors for outcome to first-line ifosfamide-containing chemotherapy for adult patients with advanced soft tissue sarcomas: an exploratory, retrospective analysis on large series from the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group (EORTC-STBSG). Eur J Cancer. 2010;46:72-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 206] [Article Influence: 13.7] [Reference Citation Analysis (0)] |