Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.5051
Peer-review started: December 2, 2021
First decision: December 27, 2021
Revised: February 10, 2022
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: May 26, 2022
Processing time: 172 Days and 21.9 Hours
Carotid blowout syndrome (CBS) refers to rupture of the extracranial carotid artery and its branches; as a severe complication, it usually occurs after surgery or radiotherapy for malignant tumours of the head and neck. We present a case of CBS caused by chronic infection of the external carotid artery (ECA). In this case, we did not find any evidence of head and neck tumours.
A 42-year-old man was referred to the Emergency Department with a complaint of a lump found on the left side of his neck with pain and fever for 4 d. We diagnosed the condition as neck infection with abscess formation based on physical examination, routine blood examination, ultrasound examination and plain computed tomography (CT) and decided to perform emergency surgery. During the operation, 30 mL of grey and smelly pus was drained from the deep surface of the sternocleidomastoid muscle. The second day after the operation, the patient suddenly exhibited a large amount of haemoptysis and incision bleeding. The enhanced CT showed distal occlusion of the left ECA and irregular thickening of the broken ends of the artery encased in an uneven enhancement of soft tissue density. Infected ECA occlusion and rupture were considered. The patient was transferred to a vascular unit for transcatheter ECA embolization and recovered well.
Surgeons need to pay attention to vascular lesions caused by chronic infection that may develop into acute CBS.
Core Tip: Carotid blowout syndrome (CBS) refers to rupture of the extracranial carotid artery and its branches; as a severe complication, it usually occurs after surgery or radiotherapy for malignant tumours of the head and neck. We present a case of CBS caused by chronic infection of the external carotid artery. In this case, we did not find any evidence of head and neck tumours. This suggested that surgeons need to pay attention to vascular lesions caused by chronic infection that may develop into acute CBS.
- Citation: Xie TH, Zhao WJ, Li XL, Hou Y, Wang X, Zhang J, An XH, Liu LT. Carotid blowout syndrome caused by chronic infection: A case report. World J Clin Cases 2022; 10(15): 5051-5056
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/5051.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.5051
Carotid blowout syndrome (CBS) refers to rupture of the extracranial carotid artery and its branches; as a severe complication, it usually occurs after surgery or radiotherapy for malignant tumours of the head and neck. CBS can lead to severe haemorrhage and life-threatening neurological complications of up to 60% and mortality of up to 40%[1]. We present a case of CBS caused by chronic infection of the external carotid artery (ECA). In this case, we did not find any evidence of head and neck tumours.
A 42-year-old man was referred to the Emergency Department in July 2019 with a complaint of a lump found on the left side of his neck with pain and fever for 4 d.
He had a slight sore throat and difficulty swallowing.
He had no history of diabetes, hypertension, smoking or other underlying infectious source such as bad tooth, foreign body, etc.
He had no obvious medical history, irradiation history or family history with regard to tumours in the head and neck. And he had no history of head and neck trauma.
A physical examination revealed a solid and hemispherical lump 6 cm in diameter located on the left side of the neck, and the lump was tender and fixed the underlying tissues. The boundary between the lump and surrounding tissue is unclear. The skin on the surface of the lump was swelling and red and had a high temperature. No jugular venous distention or abnormal carotid arterial pulse was observed.
The routine blood examination was essentially normal except for elevations in white blood cell count, haemoglobin levels, neutrophil count and eutrophilic granulocyte percentage: 11.78 × 109/L (reference limits, 3.50 × 109/L to 9.50 × 109/L), 120 g/L (130 g/L to 175 g/L), 9.81 × 109/L (1.80 × 109/L to 6.30 × 109/L) and 83.2% (40.0% to 75.0%). The remaining laboratory blood tests, including assessments of tumour markers (alpha fetoprotein, carcinoembryonic antigen, carbohydrate antigen (CA) 125, CA15-3, CA19-9, and CA72-4), and coagulation function, did not reveal abnormalities.
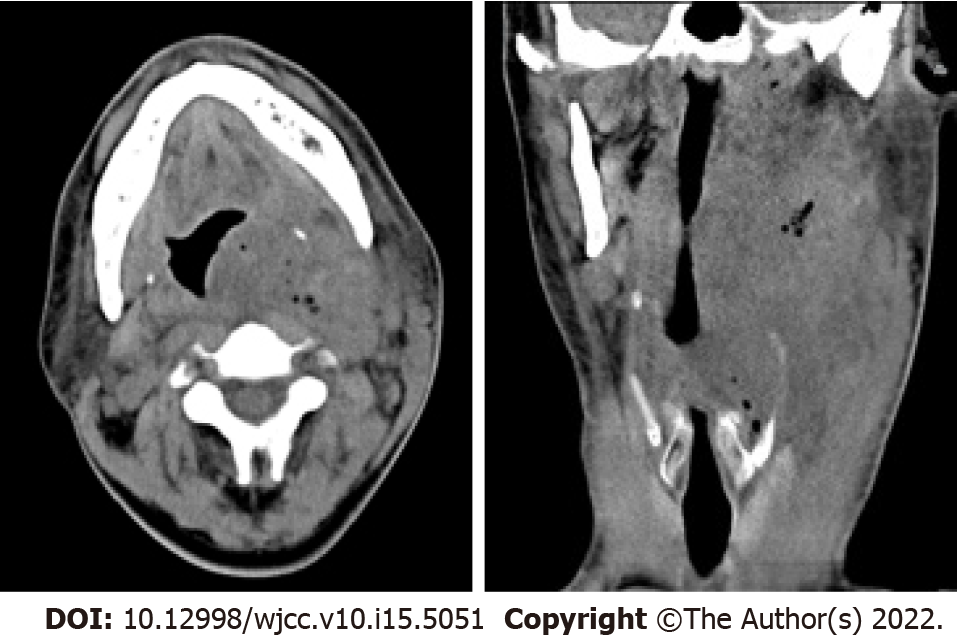
Ultrasound examination showed an irregular hypoechoic mass (dimensions: 10.6 cm × 6.3 cm) in the left neck, which was considered an infectious lesion. Abundant blood flow signals were detected around and within the mass through colour Doppler flow imaging. The size and shape of the parotid, thyroid and submandibular glands were normal, and the echo of the parenchyma was uniform. Plain computed tomography (CT) revealed a soft tissue mass containing scattered air (dimensions: 9.9 cm × 7.3 cm × 4.6 cm; CT value: 22-34 HU) that had a vague boundary and an irregular shape on the left poststyloid parapharyngeal space, and the oropharynx and laryngeal left lateral wall were thickened (Figure 1).
Disease development: We diagnosed the condition as neck infection with abscess formation based on physical examination, routine blood examination, ultrasound examination and plain CT and decided to perform emergency surgery. During the operation, 30 mL of grey and smelly pus was drained from the deep surface of the sternocleidomastoid muscle. There was not any main artery was exposed in the operation field, and no suture or ligation. After the purulent cavity was cleared, iodophor gauze was stuffed into the space. The incision was not sutured. The bacterial culture of the pus was Gram-positive cocci.
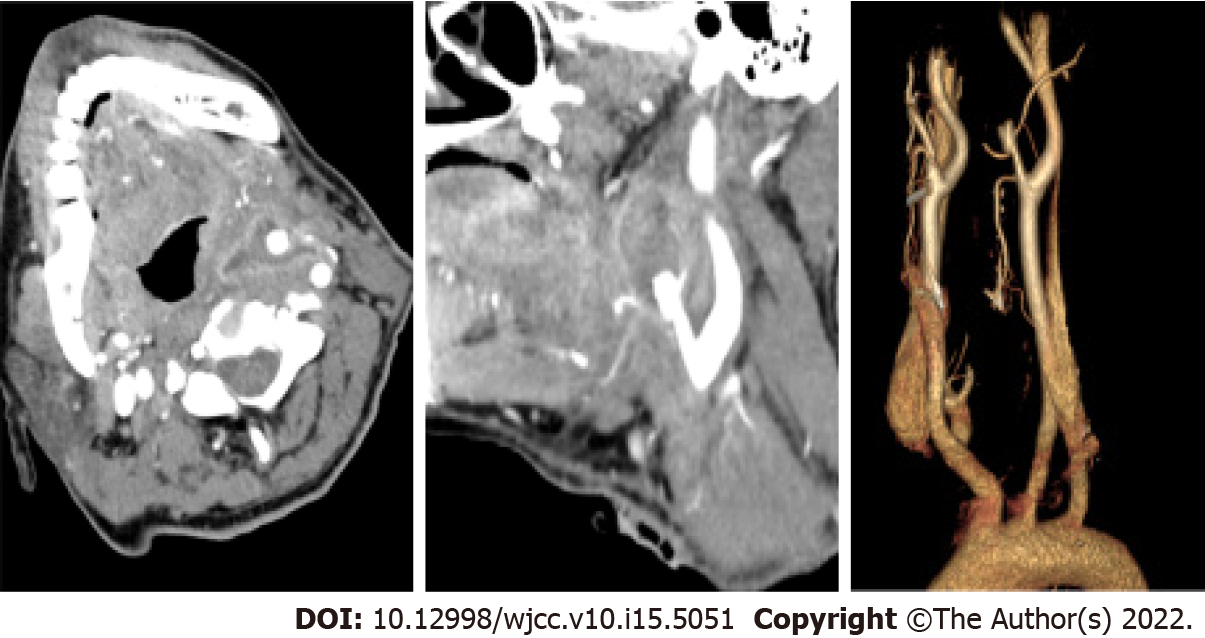
The second day after the operation, the patient suddenly exhibited a large amount of haemoptysis and incision bleeding. After symptomatic treatment, the patient underwent enhanced CT examination of the neck. The enhanced CT showed distal occlusion of the left ECA and irregular thickening of the broken ends of the artery encased in an uneven enhancement of soft tissue density. Infected ECA occlusion and rupture were considered (Figure 2).
CBS caused by chronic infection of the left ECA was considered.
The patient was transferred to a vascular unit for transcatheter ECA embolization. Under general anesthesia, the left ECA angiography indicated the significantly localized expanded ECA. Then, a Guglielmi detachable coil was used to occlude the initial part of ECA completely. Postoperative immediate angiography confirmed the complete occlusion of the left ECA. Bleeding stopped after the procedure without any complications.
The neck incision healed 3 wk after anti-infection treatment and dressing changes. No relapse of haemoptysis or stroke was found at the 1-year follow-up.
CBS can be defined as a rupture of the extracranial carotid arteries or one of their branches and occurs in 6% to 10% of advanced head and neck cancer cases[2,3]. Risk factors for CBS include radiotherapy, necrosis of the skin flap, wound infection, fistula formation, radical neck dissection, and tumour recurrence[4]. However, some patients often have multiple risk factors. In this case, the patient had no history of neck radiation or surgery and no evidence supporting head and neck tumours. We concluded that there was no significant relationship between the carotid lesion and the abscess drainage procedure. Instead, we determined that the initial lesion was an infection of the pharynx, followed by chronic inflammation spreading around the carotid sheath. Chronic inflammation corroded the ECA, leading to its distal occlusion, which may be why the patient did not have neurological complications. When the infection progressed into an acute phase, the broken end of the ECA was eroded and ruptured.
When CBS occurs, blood flow to the airway can cause asphyxia, and haemorrhage can lead to hypovolemic shock, which is a major risk factor for stroke. If the patient is not rescued in time, his condition will deteriorate rapidly. The basic principles for the treatment of CBS are local pressure for haemostasis, such as pushing the common carotid artery backward to the transverse process of the 6th cervical vertebra or compressing the wound with gauze filling for haemostasis, ensuring airway patency, and resuscitating using liquid or blood products[5]. Tracheal intubation is important for some cases because an inflatable aerocyst can effectively prevent blood flow to the airway and keep the airway open. After the patient's condition is stabilised, the next step of treatment should be carried out as soon as possible, including endovascular interventional therapy or surgical treatment.
The traditional treatment for CBS is vessel ligation, which has been widely used in the past few decades as an effective method to prevent rebleeding. Upile et al[6] reported that there was no significant change in the incidence of major complications after ligation of the ECA, but the incidence of major complications after ligation of the common carotid artery or internal carotid artery increased to 60%. This result may be related to the presence of many vascular anastomotic branches in the head and neck. Among them, the circle of Willis is considered to be the most important. If the circle of Willis is intact and supported by a sufficient bloodstream, the incidence of cerebrovascular complications after carotid artery ligation is relatively low.
Endovascular interventional therapy, including endovascular embolization and endovascular stent implantation, is another effective treatment for CBS[7]. The technique can reduce the risk of surgery by reducing damage to surrounding tissue and bleeding. Coil embolization is considered to be a safe and effective alternative to vascular ligation, and it has been reported in the literature that coil embolization can control life-threatening haemorrhage caused by trauma, postoperative infection and radiotherapy[8]. However, if the cause is infection easily leading to delayed wound healing, tissue necrosis, and vascular erosion, clinicians should be aware that interventional therapy may be delayed[2]. The key point in this case was that the patient’s ECA had been completely blocked due to chronic infection, and the risk of complications in regard to interventional embolizationwas reduced after abscess incision and drainage.
CBS can be divided into three subtypes according to its clinical severity: (1) Threatened CBS, in which the carotid artery is exposed to the external circumstances and physical or radiologic examination are prompt messages for inevitable carotid haemorrhage in the immediate future if no treatment is carried out; (2) Upcoming CBS manifesting as sentinel haemorrhage that resolves spontaneously or with packing; and (3) Acute CBS presenting as a nonself-limited massive haemorrhage[5]. In this case, this patient was in the second phase when he came to the hospital. According to plain CT, neck infection with abscess formation was diagnosed, and abscess incision and drainage were performed. Because the patient had no signs of bleeding or neurological symptoms, we did not perform radiologic examinationofblood vessels until a large amount of haemoptysis and incision bleeding suddenly appeared on the second day after the operation, which signalled the third phase.
Acute CBS is life-threatening and requires urgent intervention. Because chronic head and neck infections develop into acute episodes, we need to pay attention to vascular lesions to avoid CBS.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Peripheral vascular disease
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bendari M, Morocco; Malekzadegan A, Iran S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Lesley WS, Chaloupka JC, Weigele JB, Mangla S, Dogar MA. Preliminary experience with endovascular reconstruction for the management of carotid blowout syndrome. AJNR Am J Neuroradiol. 2003;24:975-981. [PubMed] |
| 2. | Roh JL, Suh DC, Kim MR, Lee JH, Choi JW, Choi SH, Nam SY, Kim SY. Endovascular management of carotid blowout syndrome in patients with head and neck cancers. Oral Oncol. 2008;44:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Lu HJ, Chen KW, Chen MH, Chu PY, Tai SK, Wang LW, Chang PM, Yang MH. Predisposing factors, management, and prognostic evaluation of acute carotid blowout syndrome. J Vasc Surg. 2013;58:1226-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Pyun HW, Lee DH, Yoo HM, Lee JH, Choi CG, Kim SJ, Suh DC. Placement of covered stents for carotid blowout in patients with head and neck cancer: follow-up results after rescue treatments. AJNR Am J Neuroradiol. 2007;28:1594-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Cohen J, Rad I. Contemporary management of carotid blowout. Curr Opin Otolaryngol Head Neck Surg. 2004;12:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Upile T, Triaridis S, Kirkland P, Archer D, Searle A, Irving C, Rhys Evans P. The management of carotid artery rupture. Eur Arch Otorhinolaryngol. 2005;262:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Jong MA, Candanedo C, Gross M, Cohen JE. Intervening in the Acute Phase of Postradiation Carotid Blowout Syndrome. Int Arch Otorhinolaryngol. 2019;23:172-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Hertz JA, Valentino J, Kwolek CJ, Endean ED. Carotid blowout with infection: management with endovascular and open vascular approaches--a case report. Vasc Endovascular Surg. 2004;38:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |