Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.5042
Peer-review started: December 2, 2021
First decision: January 8, 2022
Revised: January 22, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 26, 2022
Processing time: 173 Days and 7.3 Hours
Coronavirus disease-2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is characterized by systemic inflammatory response syndrome and vasculopathy. SARS-CoV-2 associated mortality ranges from 2% to 6%. Liver dysfunction was observed in 14%-53% of COVID-19 cases, especially in moderate severe cases. However, no cases of spontaneous hepatic rupture in pregnant women with SARS-CoV-2 have been reported.
A 32-year-old pregnant patient (gestational age: 32 wk + 4 d) without any remarkable medical history or long-term medication presented with epigastralgia. Infectious, non-infectious, and pregnancy-related hepatopathies were excluded. Sudden onset of right subcostal pain with D-dimer and liver enzyme elevation was followed by shock with thrombocytopenia. While performing an emergency cesarean section, hemoperitoneum was observed, and the patient delivered a stillbirth. A 6-cm liver rupture at the edges of segments V and VI had occurred, which was sutured and drained. SARS-CoV-2 positivity on reverse transcription-polymerase chain reaction was confirmed. Further revisions for intrahepatic hematoma with hemorrhagic shock and abdominal compartment syndrome were performed. Subsequently, the patient developed hemoptysis, which was treated using bronchoscopic therapy and non-invasive ventilation. Liver tissue biopsy revealed hemorrhagic foci and necrosis with an irregular centrilobular distribution. Antiphospholipid syndrome and autoimmune hepatitis were also ruled out. Fetal death was caused by acute intrauterine asphyxia.
This case reveals that pregnant women with SARS-CoV-2 infection may be predisposed to liver parenchyma disease with liver rupture.
Core Tip: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may contribute to the worsening of hepatopathy during pregnancy, because of its effect on the endothelium in the systemic inflammatory response syndrome microenvironment. Liver rupture causes high mortality in both the mother and fetus. Such a life-threatening scenario requires close collaboration between the obstetrician and the surgeon with an urgent indication for cesarean section, preferably by midline laparotomy with meticulous control of the liver and treatment of any injury. The presence of SARS-CoV-2 in pregnant women and its association with the development of severe hepatopathy in pregnancy requires further research.
- Citation: Ambrož R, Stašek M, Molnár J, Špička P, Klos D, Hambálek J, Skanderová D. Spontaneous liver rupture following SARS-CoV-2 infection in late pregnancy: A case report. World J Clin Cases 2022; 10(15): 5042-5050
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/5042.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.5042
Coronavirus disease-2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is characterized by systemic inflammatory response syndrome (SIRS), vascular damage, microangiopathy, angiogenesis, and thrombosis[1]. High mortality rates are associated with SARS-CoV-2 (2%-6%), especially among the elderly and individuals with underlying comorbidities such as diabetes, hypertension, and heart disease[2]. In the current pandemic, hepatic dysfunction was observed in 14%-53% of SARS-CoV-2-infection cases, especially in severe cases. Moreover, acute liver injury has a higher mortality rate[2]. Studies related to liver disease and involvements during pregnancy seem to be limited.
The mechanisms causing potential liver damage include direct viral cytotoxicity[3], immune-related damage due to SIRS[4], respiratory failure-related hypoxic changes, pro-coagulation intravascular state with endotheliitis, drug-induced liver injury and in severe cases of cardiac congestion. The exacerbation of hidden/pre-existing liver disease should also be considered.
In direct hepatic damage, angiotensin-converting enzyme 2 (ACE2) appears to be the key receptor for viral entry into the cell[5]. The virus attaches to the host cell via the viral S protein connection to the host transmembrane serine protease 2[6]. Subsequently, the virus is incorporated into the cell by endocytosis and the viral genome is released from the endosome[7,8]. The viral genome is replicated in the replicase-transcriptase complex[9,10]. The assembly of mature SARS-CoV-2 virions occurs within the endoplasmic reticulum-to-Golgi intermediate compartment[11,12] with subsequent exocytosis[13].
Cases of spontaneous liver rupture during pregnancy with SARS-CoV-2 infection have not been reported yet.
A 32-year-old woman with a gestational age of 32 wk + 4 d experienced epigastric pain. She was asymptomatic for COVID-19.
She first experienced pain in the epigastrium 2 h before the initial examination, with a gradual worsening of her condition.
The patient had no comorbidities and was not taking any chronic medication.
There was no significant personal or family history.
Physical examination revealed epigastric pain, with a body temperature of 36.5 °C, blood pressure of 17, 6/11 kPa, and oxygen saturation of 97%. Electrocardiogram (ECG) and cardiotocographic findings were normal. We initially suspected her to have hemolysis, elevated liver enzymes, and low platelet count syndrome (HELLP) and performed fetal lung maturity induction while administering 12 mg betamethasone intravenously for 24 h.
Blood tests results revealed elevated liver enzymes [alanine aminotransferase (ALT): 272.4 IU/L, aspartate transaminase (AST): 159.6 IU/L, and alkaline phosphatase (ALP): 172.8 IU/L], without any findings of viral hepatitis (the samples were negative for hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis E virus, Epstein-Barr virus (EBV), human cytomegalovirus (HCMV). Her coagulation parameters were normal; hence other pregnancy-related liver pathologies were excluded. The hepatologist suggested that her condition was most likely related to hepatopathy of unknown etiology. SARS-CoV-2 positivity on reverse transcription-polymerase chain reaction was confirmed, although the patient was asymptomatic.
The initial ultrasound examination revealed a live fetus without any abnormal findings.
Over a week, the liver enzymes and platelet count returned to normal levels. On the fourth day of hospitalization, pain developed in the right subcostal region and physical examination revealed a positive Murphy’s sign, while her ECG was normal, D-dimers concentration was above 10000 µg/L, and liver enzymes were slightly elevated (ALT: 372 IU/L; AST: 258 IU/L). Therefore, we ruled out acute coronary syndrome. Abdominal ultrasound examination confirmed no abnormalities in the abdominal organs, a viable fetus, and no free fluid in the abdominal cavity. We administered anticoagulant therapy (0.3 mL low molecular weight heparin every 12 h). The correlation between the laboratory findings and clinical findings was unclear. Within 2 h, the patient developed a collapsed state without trauma and her blood test showed a marked increase in ALT (1789.8 IU/L) and AST (1747.2 IU/L). In addition, lactate dehydrogenase (LD) levels (1671 IU/L) and platelets (88 × 109/L) were highly elevated. Therefore, an emergency cesarean section was planned.
According to the laboratory and clinical findings, the diagnosis was most likely spontaneous liver rupture associated with SARS-CoV-2 infection.
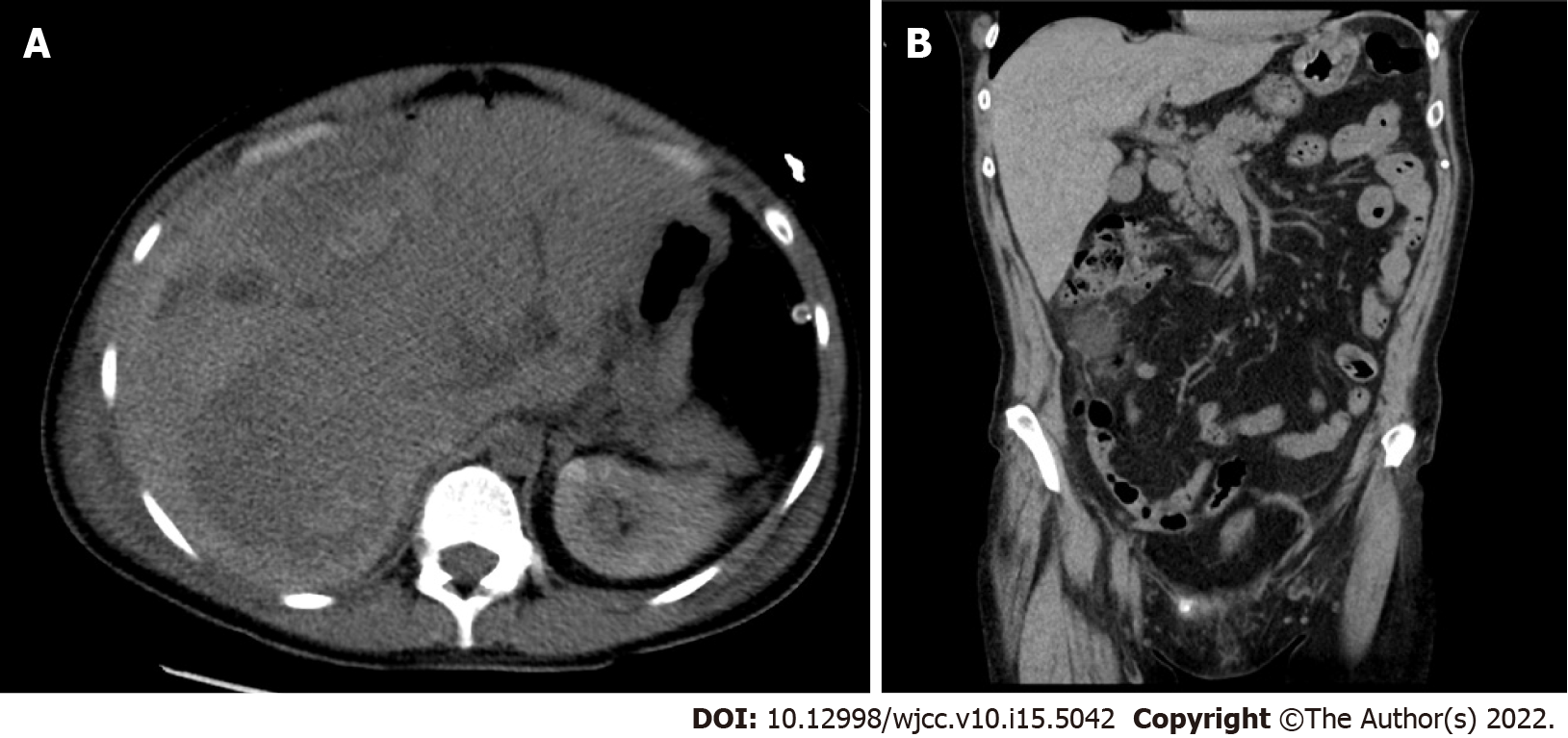
While performing the emergency cesarean section, we observed the findings of hemoperitoneum, and an apparent 6-cm liver rupture on the edges of segments V and VI of the right hepatic lobe. Suture and drainage were performed. The patient delivered a baby without signs of vitality, and lost 800 mL of blood. The postoperative condition was accompanied by a deficient response to supplementation with blood derivatives. The patient underwent computed tomography (CT) of the abdomen with intravenous contrast (Figure 1, Figure 2A and B), which revealed a massive subcapsular hematoma in the right hepatic lobe with active bleeding from the branches of the hepatic artery. Thereafter, embolization was performed as we suspected limited viability of the right hepatic lobe and proceeded with more conservative management. Hemodynamic decompensation continued, while paralytic ileus developed with an increase in the abdominal volume and dark bloody secretion was drained. There were signs of abdominal compartment syndrome and anemia (hemoglobin, 107-71 g/L). We investigated the abdominal cavity for bowel insufflation and performed drainage of a bulky subcapsular hematoma (involving the V, VI, VII, and VIII segments and good vitality of segments IV, II, and III). Liver packing was performed to control the damage. Postoperatively, the patient was maintained in a stable condition with mechanical ventilation and tested positive for SARS-CoV-2 infection. A repeat laparotomy was performed after two days when the liver packing was discontinued and no liver hemorrhages were observed. Furthermore, her subcapsular hematoma did not progress, and the liver parenchyma was stable. Thereafter, we gradually adjusted the liver parameters without coagulopathy or thrombocytopenia and extubated or spontaneously ventilated. A postoperative CT scan of the liver revealed no free fluid or progression of hepatic hematoma, and the drains were removed. Chest CT revealed ground-glass opacity in both lungs with right pleural effusion, which was resolved by puncture, followed by massive hemoptysis conservatively treated with bronchoscopy (adrenaline) and non-invasive ventilation with high-flow air.
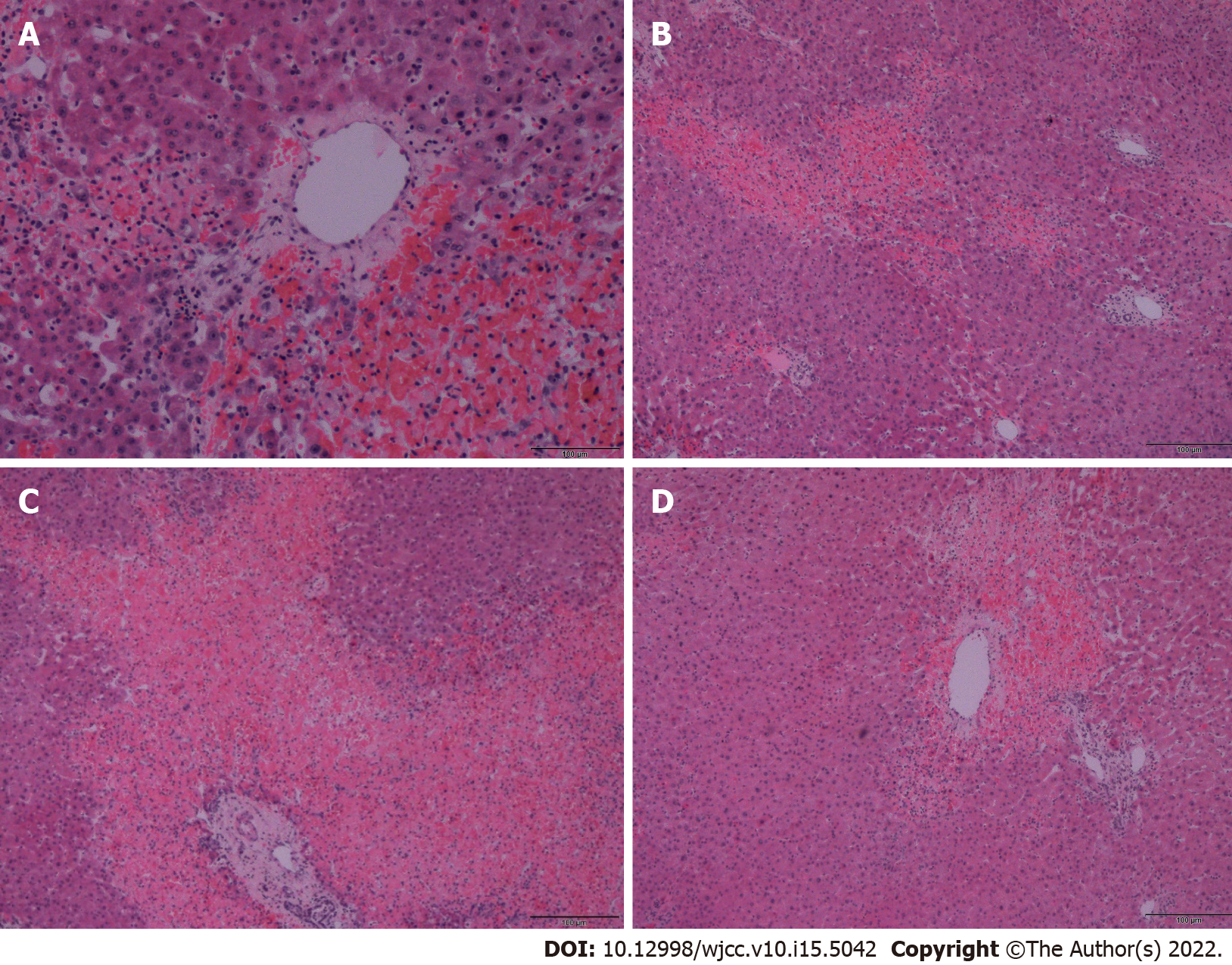
A biopsy of the liver tissue showed foci of hemorrhage and necrosis with an irregular centrilobular distribution (Figure 2), no hepatocyte steatosis and no cholestasis. Fibrin thrombi or inflammatory infiltrations were not detected. Thus, patients with HELLP syndrome were excluded. Pathological dissection of the placenta and fetus showed that the chorionic plate and decidua basalis did not show inflammation, thrombosis, or vasculitis. Both chorionic and stem villi were small with regard to the gestational age, suggesting an acceleration of maturation, and were vascularly altered with more abundant Hofbauer macrophages. We also noted irregular arborization with globular appearance of distal villous hypoplasia. Multifocally, a significant fibrinoid increase was clear, which occurred intravillously; fibrinoid sites completely filled individual villi but also intervillously and subchorially; however, this finding did not exceed 30% of the tissue volume. Escalation was excessive knotting of the hyperplastic syncytiotrophoblasts that appeared similar to Tenney-Parker changes. Cytotrophoblasts were discontinuous. Agglutination and infarcts were present below the decidua, chorion, and centrally in the placental parenchyma; however, they were within 15% of the parenchyma. Thus, it was shown that fetal death occurred because of acute intrauterine asphyxia during acute ischemia of the placenta under hemorrhagic shock conditions in the mother. The subsequent course was no longer complicated, and the patient was followed up at our department and the pulmonary clinic, and antiphospholipid syndrome was ruled out. A follow-up CT scan revealed significant regression of the pulmonary changes with fluidothorax regression on the right. According to the control spirometry findings, ventilation was within normal limits.
Pregnancy-specific liver diseases can be categorized as early and late pregnancy diseases; the HELLP syndrome is a late pregnancy-related liver disease along with liver rupture/infarction[14]. Other late pregnancy-related liver diseases include intrahepatic cholestasis in pregnancy, acute fatty liver of pregnancy (AFLP), and nonspecific elevated liver enzymes. Liver rupture occurs in approximately 1 per 200000 pregnancies and is largely associated with AFLP or HELLP syndrome, rarely without associated liver disease[15]. Running a panel of blood tests is the standard protocol for diagnosing hepatic pathology: ALT, AST, ALP, gamma-glutamyl transferase, bilirubin, Alpha-fetoprotein, LD, hepatitis antibodies (A, B, C, E, HCMV, EBV, ceruloplasmin, copper, iron, ferritin, dot liver 7, coagulation parameters, schistocyte smear). The differential diagnoses of the causes of liver rupture in pregnancy include HELLP syndrome, severe pre-eclampsia, and hemangioma rupture.
According to our differential diagnosis, HELLP syndrome appeared to be the most likely diagnosis, which occurs in 1 per 1000 pregnancies and is associated with a high mortality rate (up to 40%) due to the possible occurrence of disseminated intravascular coagulopathy[16] and spontaneous liver rupture[17,18]. The only causal therapy is to terminate the pregnancy immediately after confirming the diagnosis and evaluating the clinical condition of the pregnant woman and the gestational age of the fetus. In the present case, we observed the angiogenic markers, sharp rise in liver transaminases over a short period, inconclusive clinical signs, negative schistocytes, and the coincidence with lung involvement (Table 1), and concluded that they were unrelated to HELLP. Likewise, the histological findings of the liver biopsy specimens did not confirm this diagnosis. Pre-eclampsia, a multisystem disease associated with de novo hypertension occurring after the 20th week of pregnancy with proteinuria, was considered. One component of the clinical picture is characterized by the dysfunction of other maternal organs, such as renal insufficiency, hepatic involvement, neurological or hematological complications, and uteroplacental dysfunction or fetal growth restriction[19]. Risk factors for the occurrence of pre-eclampsia include antiphospholipid syndrome, previous pre-eclampsia, chronic hypertension, pre-gestational diabetes, pre-pregnancy body mass index > 30 kg/m2, and the use of assisted reproductive technology[20]. Pre-eclampsia commonly presents with headache, epigastric pain, visual disturbances, nausea, and vomiting[21]. The association between pre-eclampsia and HELLP syndrome requires sufficient explanation.
| Hypertension | Hemolysis/changes in coagulation/endothelial lesions | Leu/Tc | Liver function tests | Symptomatics | Histology | Proteinuria/Creatinine | |
| HELLP | Hypertension SBP ≥ 18, 6 kpa or DBP ≥ 11, 9 kpa | Hemolysis (2 of the criteria listed): Peripheral blood smear with schistocytes and echinocytes; SBR ≥ 20,52 μmol/L; Low SHP ≤ 2210,5 μmol/L or LDH ≥ 2 times higher than normal level; severe anemia without blood loss | Tc: < 100 × 109/L | Elevation of LFT: AST or ALT ≥ 2 times higher than the normal level | Severe pain in the right upper abdominal quadrant; headache; nausea and vomiting; swelling of the extremities | Placental: Small placenta to gestational age, decidual vasculopathy, infarcts in the central portion, retroplacental hematoma, intravillous thrombosis; hepatic: Periportal hepatocellular necrosis, sharply demarcated hemorrhage with extended fibrin distribution from surrounding liver parenchyma, leukostasis in hepatic sinusoids | Proteinuria |
| PRE-ECLAMPSIA mild form | Hypertension SBP ≥ 18, 6 kpa or DBP ≥ 11, 9 kpa, measured on at least two occasions 4 h apart in previously normotensive women | NS | NS | NS | NS | NS | Proteinuria: ≥ 0, 3 g / 24 h, but < 5 g/24 h |
| PRE-ECLAMPSIASevere form | Hypertension: SBP ≥ 21, 3 kpa or DBP ≥ 14, 6 kpa, measured on at least two occasions 4 h apart | Schistocytes on peripheral blood smear; DIC | Tc: < 100 × 109/L | Elevation of LFT | Severe pain in the right or middle epigastrium; newly developed cerebral/visual symptoms; pulmonary oedema | Placental: No significant differences from HELLP | Scr > 97.262 µmol/L or doubling of scr level in the absence of other kidney disease |
| Spontaneous liver rupture in pregnancy | NS | Endothelial dysfunction; fibrin thrombus production | NS | Elevation of LFT | Abdominal pain; nausea; vomiting | NS | NS |
| Patient | Normotension | Negative schistocytes on peripheral smear | Leu: 13 × 109/L; Tc in normal range | SBR in normal range; LDH in normal range; ALT: 272,4 IU/L; AST: 159 IU/L; ALP: 172,8 IU/L | Epigastric and right hypochondrium pain | Hepatic: With foci of hemorrhage and necrosis centrilobularly; placental: Without signs of uterine vasculopathy | Sflt-1/plgf = 151 |
Regarding the inconclusive diagnostic modalities associated with lung involvement, we considered the possibility of liver rupture due to damage caused by SARS-CoV-2 infection. The presumed mechanism of viral entry is via the host’s ACE2 receptors, which are abundantly present in alveolar type-2 cells. ACE2 receptors are expressed in the gastrointestinal tract, vascular endothelium, and liver cholangiocytes. Hepatic involvement may be directly related to the cytopathic effect of the virus, uncontrolled immune reaction, sepsis, or drug-induced liver damage[2]. Recent findings suggest that viral elements may be present in endothelial cells, with an accumulation of dead inflammatory endothelial cells. These findings indicate that SARS-CoV-2 infection facilitates the pathogenesis of endotheliitis in several organs as a direct consequence of viral involvement and the inflammatory response of the host[22].
Vascular effects of SARS-CoV-2 (endotheliitis, procoagulation, and thrombosis) seem to be important contributors to placental damage. Potential fetal complications are most likely linked to placental circulation damage. Vivanti et al[23] described a transplacental transmission of SARS-CoV-2, where the placenta showed diffuse perivillous fibrin deposits with infarction and acute and chronic intervillous lesions. Immunohistochemistry may prove a seropositive status of the villous trophoblast for SARS-CoV-2 nucleocapsid protein[23]. Linenhan et al[24] hypothesized that trophoblast necrosis is directly related to either viral damage or the host’s subsequent inflammatory response. Thus, it appears as a form of placental disease that is specifically associated with direct infection of the placental villous trophoblast by SARS-CoV-2, which represents true SARS-CoV-2 placentitis. During hepatic hemorrhage, apart from offering conservative therapy with hemoptysis, vasopressor support, and hemotherapy, the possibility of performing embolization of the branches of the hepatic artery should be considered along with surgical management. The success of embolization depends on the visualization of the hemorrhagic focus, embolization technique, and availability of the artery. This is indicated in hemodynamically stable patients[25].
Surgical intervention is indicated for hemodynamic instability, usually involving an approach by a midline laparotomy, also accounting for the possibility of association with the cesarean section; when it is technically impossible to carry out a primary liver suture, it is suitable to apply the principles of damage control surgery (packing, ligation of the portal vein branch, followed by stabilization, and a repeat laparotomy with a definitive control of hemorrhage and a liver tissue biopsy).
Liver transplantation can be considered an ultimum refugium in cases of hepatic failure. However, in cases of SARS-CoV-2 infection, liver transplantation is a high-risk procedure, despite reports of successful cases[26]. Hence, the prognosis of the patient depends on the degree of SIRS development, multi-organ involvement during SARS-CoV-2 infection, and the early indication of interventional therapy.
The limitation of histology is the absence of direct detection of viral particles in the liver parenchyma, and the examination was not available at the time of the histological evaluation. The influence of viral infection can be considered a primary and secondary cause. It can be assumed that SARS-CoV-2 infection may have contributed to the development of SIRS and endotheliitis, with an effect on the occurrence of microvascular and macrovascular thromboses to aggravate pre-existing hepatopathy. However, this premise needs to be further investigated and evaluated in a larger cohort of patients.
SARS-CoV-2 infection may contribute to the worsening of hepatopathy during pregnancy, possibly by affecting the endothelium in the SIRS microenvironment that may contribute to liver rupture developing in the framework of severe hepatopathy. Spontaneous liver rupture is associated with massive infant and maternal mortality rates as high as 42%[27] and 39%[17], respectively. The management of such clinical case scenarios requires close collaboration between the obstetrician and the surgeon with an urgent indication for cesarean section, preferably approaching through midline laparotomy after a thorough liver examination for any liver injury and its treatment. Nevertheless, the complications induced by the presence of SARS-CoV-2 in the development of severe hepatopathy during pregnancy require further research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Česká lékařská komora, No. 1150408180.
Specialty type: Surgery
Country/Territory of origin: Czech Republic
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Diab R, Iran; Zandi M, Iran S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Stasi C, Fallani S, Voller F, Silvestri C. Treatment for COVID-19: An overview. Eur J Pharmacol. 2020;889:173644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 2. | Jothimani D, Venugopal R, Abedin MF, Kaliamoorthy I, Rela M. COVID-19 and the liver. J Hepatol. 2020;73:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (1)] |
| 3. | Wang Y, Liu S, Liu H, Li W, Lin F, Jiang L, Li X, Xu P, Zhang L, Zhao L, Cao Y, Kang J, Yang J, Li L, Liu X, Li Y, Nie R, Mu J, Lu F, Zhao S, Lu J, Zhao J. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J Hepatol. 2020;73:807-816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 457] [Article Influence: 91.4] [Reference Citation Analysis (0)] |
| 4. | Kucharski AJ, Russell TW, Diamond C, Liu Y, Edmunds J, Funk S, Eggo RM; Centre for Mathematical Modelling of Infectious Diseases COVID-19 working group. Early dynamics of transmission and control of COVID-19: a mathematical modelling study. Lancet Infect Dis. 2020;20:553-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1646] [Cited by in RCA: 1366] [Article Influence: 273.2] [Reference Citation Analysis (0)] |
| 5. | Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15248] [Cited by in RCA: 14131] [Article Influence: 2826.2] [Reference Citation Analysis (1)] |
| 6. | Glowacka I, Bertram S, Müller MA, Allen P, Soilleux E, Pfefferle S, Steffen I, Tsegaye TS, He Y, Gnirss K, Niemeyer D, Schneider H, Drosten C, Pöhlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol. 2011;85:4122-4134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 840] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 7. | Perlman S, Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat Rev Microbiol. 2009;7:439-450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1309] [Cited by in RCA: 1163] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 8. | Wang H, Yang P, Liu K, Guo F, Zhang Y, Zhang G, Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18:290-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 478] [Cited by in RCA: 532] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 9. | Snijder EJ, van der Meer Y, Zevenhoven-Dobbe J, Onderwater JJ, van der Meulen J, Koerten HK, Mommaas AM. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J Virol. 2006;80:5927-5940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 407] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 10. | Knoops K, Kikkert M, Worm SH, Zevenhoven-Dobbe JC, van der Meer Y, Koster AJ, Mommaas AM, Snijder EJ. SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 2008;6:e226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 716] [Cited by in RCA: 808] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 11. | V'kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19:155-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2164] [Cited by in RCA: 2018] [Article Influence: 504.5] [Reference Citation Analysis (0)] |
| 12. | Cortese M, Lee JY, Cerikan B, Neufeldt CJ, Oorschot VMJ, Köhrer S, Hennies J, Schieber NL, Ronchi P, Mizzon G, Romero-Brey I, Santarella-Mellwig R, Schorb M, Boermel M, Mocaer K, Beckwith MS, Templin RM, Gross V, Pape C, Tischer C, Frankish J, Horvat NK, Laketa V, Stanifer M, Boulant S, Ruggieri A, Chatel-Chaix L, Schwab Y, Bartenschlager R. Integrative Imaging Reveals SARS-CoV-2-Induced Reshaping of Subcellular Morphologies. Cell Host Microbe. 2020;28:853-866.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 13. | Wolff G, Melia CE, Snijder EJ, Bárcena M. Double-Membrane Vesicles as Platforms for Viral Replication. Trends Microbiol. 2020;28:1022-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 14. | Westbrook RH, Dusheiko G, Williamson C. Pregnancy and liver disease. J Hepatol. 2016;64:933-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 144] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Dhillon A, Steadman RH. Liver Diseases. In: Fleisher LA. Anesthesia and Uncommon Diseases. 6th ed. Philadelphia: W.B. Saunders, 2012: 162-214. [DOI] [Full Text] |
| 16. | Haram K, Svendsen E, Abildgaard U. The HELLP syndrome: clinical issues and management. A Review. BMC Pregnancy Childbirth. 2009;9:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 17. | Reck T, Bussenius-Kammerer M, Ott R, Müller V, Beinder E, Hohenberger W. Surgical treatment of HELLP syndrome-associated liver rupture -- an update. Eur J Obstet Gynecol Reprod Biol. 2001;99:57-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Zhou X, Zhang M, Liu Z, Duan M, Dong L. A rare case of spontaneous hepatic rupture in a pregnant woman. BMC Pregnancy Childbirth. 2018;18:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Tranquilli AL, Dekker G, Magee L, Roberts J, Sibai BM, Steyn W, Zeeman GG, Brown MA. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertens. 2014;4:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 881] [Cited by in RCA: 988] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 20. | Bartsch E, Medcalf KE, Park AL, Ray JG; High Risk of Pre-eclampsia Identification Group. Clinical risk factors for pre-eclampsia determined in early pregnancy: systematic review and meta-analysis of large cohort studies. BMJ. 2016;353:i1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 665] [Cited by in RCA: 590] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 21. | Mol BWJ, Roberts CT, Thangaratinam S, Magee LA, de Groot CJM, Hofmeyr GJ. Pre-eclampsia. Lancet. 2016;387:999-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 1207] [Article Influence: 134.1] [Reference Citation Analysis (0)] |
| 22. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4583] [Article Influence: 916.6] [Reference Citation Analysis (0)] |
| 23. | Vivanti AJ, Vauloup-Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 595] [Cited by in RCA: 737] [Article Influence: 147.4] [Reference Citation Analysis (0)] |
| 24. | Linehan L, O'Donoghue K, Dineen S, White J, Higgins JR, Fitzgerald B. SARS-CoV-2 placentitis: An uncommon complication of maternal COVID-19. Placenta. 2021;104:261-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 25. | Xu H, Jie L, Kejian S, Xiaojun H, Chengli L, Hongyi Z, Yalin K. Selective Angiographic Embolization of Blunt Hepatic Trauma Reduces Failure Rate of Nonoperative Therapy and Incidence of Post-Traumatic Complications. Med Sci Monit. 2017;23:5522-5533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Manzia TM, Gazia C, Lenci I, Angelico R, Toti L, Monaco A, Anselmo A, Baiocchi L, Grossi P, Tisone G. Liver transplantation performed in a SARS-CoV-2 positive hospitalized recipient using a SARS-CoV-2 infected donor. Am J Transplant. 2021;21:2600-2604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 27. | Marsh FA, Kaufmann SJ, Bhabra K. Surviving hepatic rupture in pregnancy--a literature review with an illustrative case report. J Obstet Gynaecol. 2003;23:109-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |