Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.5012
Peer-review started: November 19, 2021
First decision: January 11, 2022
Revised: January 18, 2022
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: May 26, 2022
Processing time: 186 Days and 1.2 Hours
Recently reported cases of pyogenic liver abscess associated with colonic cancer in the absence of underlying disease, have included a small number of cases of gastric heterotopia (GHT). GHT is a congenital anomaly composed of ectopic gastric mucosa and can occur anywhere in the gastrointestinal tract but is more frequently encountered in the cervical esophagus. However, it is rarely observed in colon. Furthermore, most reported cases of GHT of the colon involved the rectum, and GHT involving the colon proximal to the rectum is rare.
An 83-year-old male patient presented with fever and a diagnosis of pyogenic liver abscess. Colonoscopy was performed for colon cancer workup and revealed a 1.0 cm sized polyp at the transverse colon. The polyp was removed by endoscopic mucosal resection by monopolar electrocauterization using a snare. Pathological examination revealed GHT. After administering intravenous antibiotics, the patient recovered well.
GHT in the colon could affect the development of pyogenic liver abscess by enabling hematogenous propagation of Klebsiella pneumoniae through mucosal damage. However, more study is needed due to the lack of cases.
Core Tip: Gastric heterotopia in the colon could affect the development of pyogenic liver abscess by enabling hematogenous propagation of Klebsiella pneumoniae through mucosal damage. However, more study is needed due to the lack of cases. Colonoscopy should be performed to the patients with a pyogenic liver abscess.
- Citation: Park JG, Suh JI, Kim YU. Gastric heterotopia of colon found cancer workup in liver abscess: A case report. World J Clin Cases 2022; 10(15): 5012-5017
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/5012.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.5012
Gastric heterotopia (GHT) is defined as the presence of gastric mucosal tissue in a non-physiological site. Several hypotheses have been offered to explain its occurrence, but the mechanism involved has not been elucidated. GHT is usually asymptomatic and can occur anywhere in the gastrointestinal (GI) tract, but is rarely encountered in the colon. Most cases of GHT involve the rectum and the most common symptom is chronic bleeding[1]. GHT involving the colon proximal to the rectum is rare, and to our knowledge, only 11 such cases have been reported[2]. Pyogenic liver abscess is usually associated with hepatobiliary tract disease or intra-abdominal infections such as cholecystitis, cholangitis, pylephlebitis, appendicitis, diverticulitis, or peritonitis[3-5]. However, colorectal cancer without underlying infection is considered a rare cause of liver abscess[6-9]. Although the mechanism is unclear, disruption of the mucosal barrier by colorectal cancer and bacterial translocation into the portal venous system may, in part, be responsible for the pathogenesis of liver abscess[9]. To the best of our knowledge, no case report has been issued on the co-occurrence of pyogenic liver abscess and GHT. Here, we report a case in which gastric heterotopic mucosa in colon was discovered after a diagnosis of pyogenic liver abscess. We suggest that diagnostic colonoscopy be performed in patients with a pyogenic liver abscess.
An 83-year-old male patient presented at our emergency department due to fever.
The patient also complained of epigastric pain.
The patient had no medical history of cardiovascular disease, diabetes, or chronic liver disease.
The patient had no relevant family history but had been drinking for 50 years.
At presentation, his blood pressure was 150/90 mmHg, heart rate 100 bpm, respiratory rate 20 breaths per minute, and temperature 39.5 °C. Physical examination revealed right quadrant tenderness.
Laboratory examinations performed at admission revealed a white blood cell count of 14,150/μL (neutrophils 91.1%, lymphocytes 4.7%, monocytes 3.0%, eosinophils 0.7%), hemoglobin 12.9 g/dL, platelet count 125,000 /μL, and hematocrit 38.2%. Serum blood urea nitrogen was 13 mg/dL, creatinine 0.87 mg/dL, AST 33 IU/L, ALT 47 IU/L, total bilirubin 0.76 mg/dL, sodium 137 mEq/L, potassium 3.3 mEq/L, and chloride 102 mEq/L. Serum prothrombin time was 14.8 sec (INR of 1.34), protein 6.6 g/dL, albumin 4.0 g/dL, γ-GTP 138 U/L, ALP 69 U/L. Hepatitis viral markers were HAV Ab IgM negative, HBsAg negative, anti-HBsAb positive, and anti-HCV Ab negative. Tumor markers were AFP 2.7 ng/mL, CA19-9 7.96 U/mL, and CEA 1.4 ng/mL. Fecal occult blood test (FOBT) was negative. Parasite specific antibodies were negative. Pus and blood cultures were positive for Klebsiella pneumoniae (K. pneumoniae).
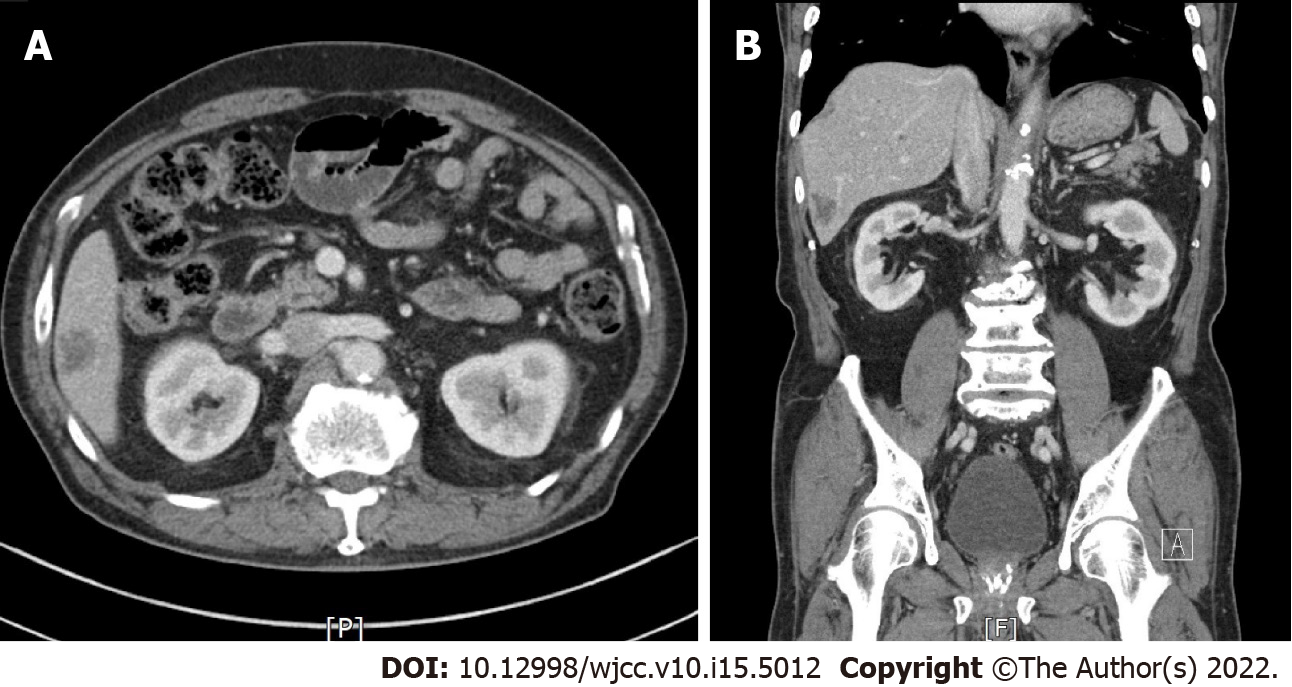
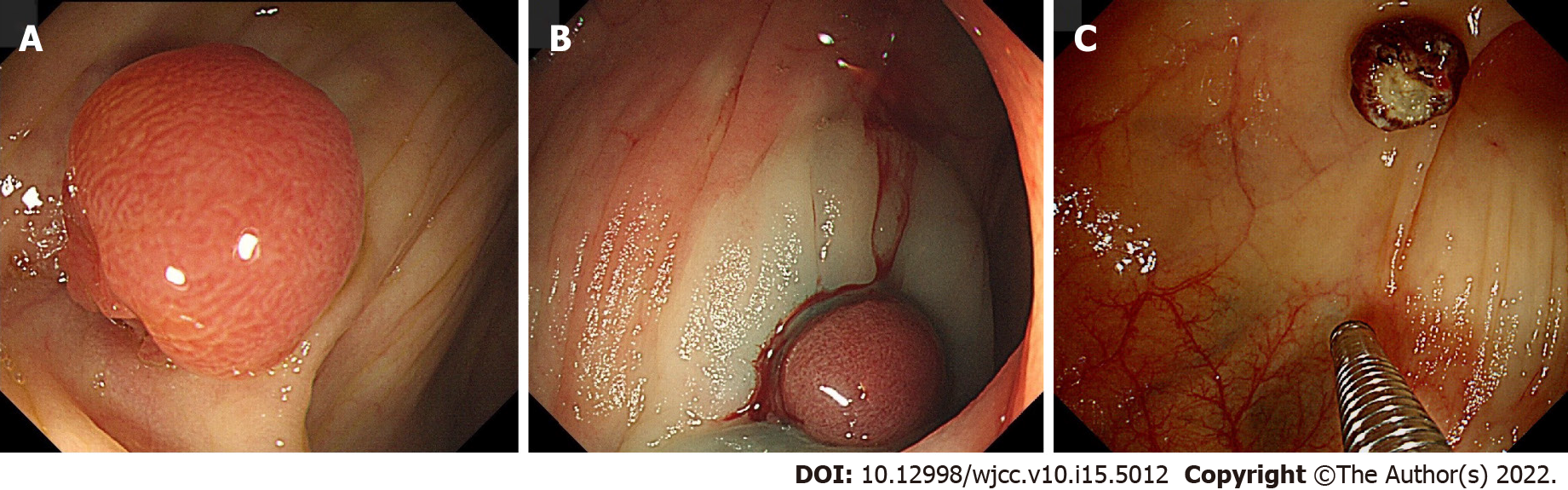
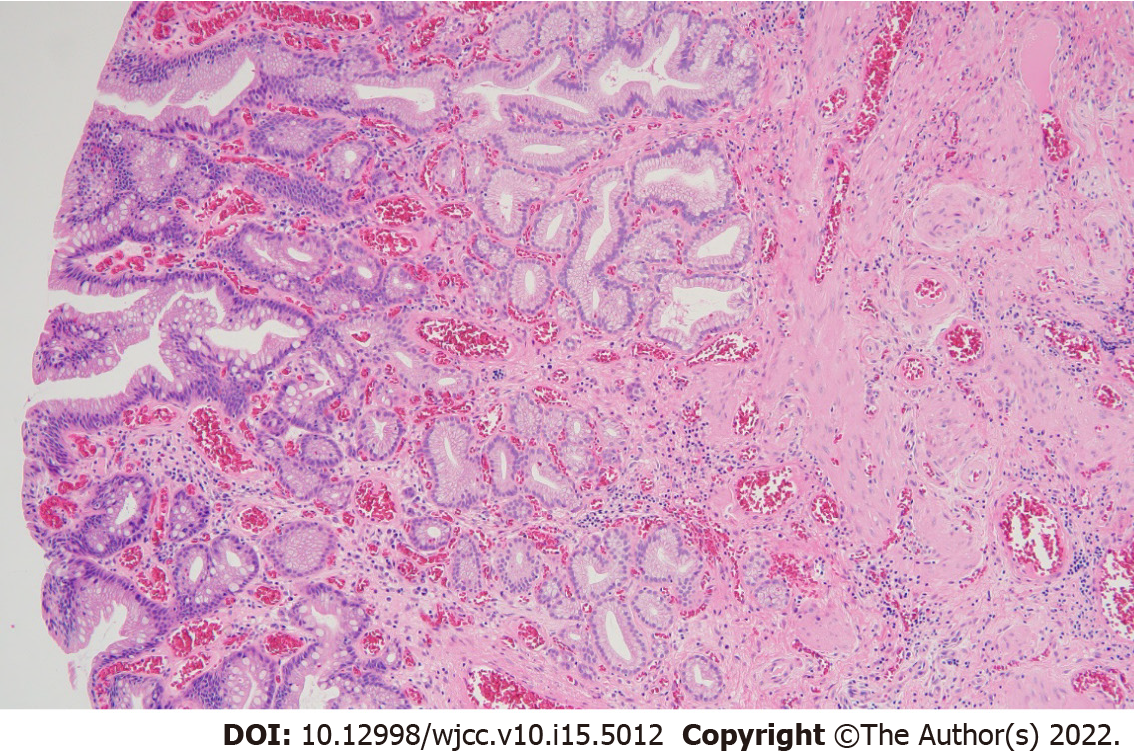
Abdominal computed tomography revealed a 2 cm × 2.5 cm sized, ill-defined, low attenuation nodular lesion in liver segment 6 (Figure 1). Colonoscopy was performed for colon cancer workup and revealed a 1.0 cm sized semi-pedunculated polyp at the transverse colon (Figure 2). The polyp was removed by endoscopic mucosal resection by monopolar electrocauterization using a snare. After endoscopic polypectomy, pathological examination revealed a gastric gland consistent with heterotopic gastric tissue, intestinal metaplasia, fibrosis, and vascular and nervous tissue proliferation (Figure 3).
The final diagnosis was pyogenic liver abscess and GHT of the colon.
Based on abdominal CT findings, the liver lesion was initially considered to be a pyogenic liver abscess, and thus, intravenous antibiotic therapy with ceftriaxone 2g QD plus metronidazole 500 mg TID was administered. On the second day of hospitalization, the patient was asymptomatic, and his body temperature had dropped to 36.5℃. Antibiotic administration was continued for 4 wk.
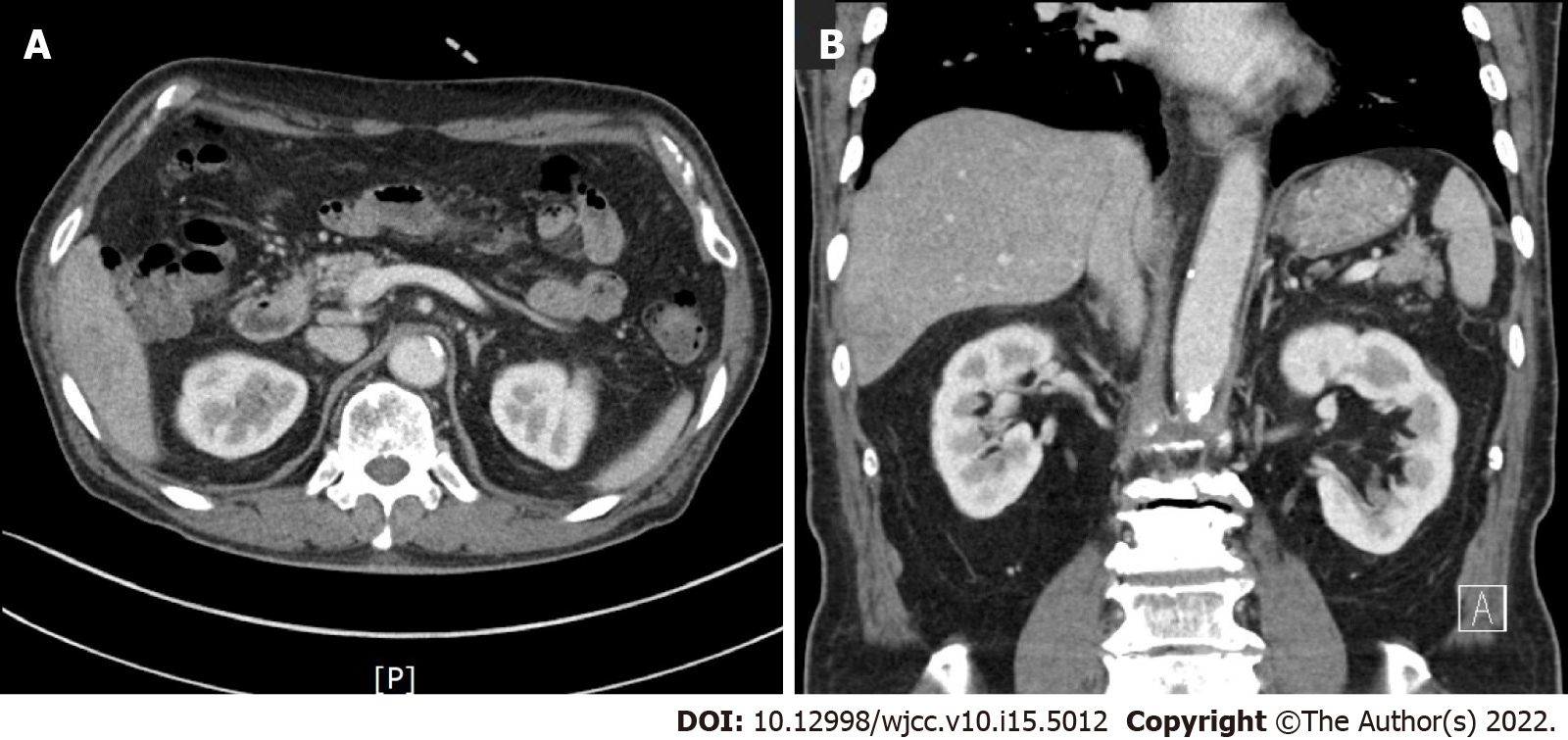
On hospital day 33, follow-up abdominal computed tomography showed the lesion had completely resolved (Figure 4).
Liver abscess is an infectious disease in which an abscess forms within liver parenchyma, and those usually encountered are pyogenic or amoebic. The most common pathogen of pyogenic liver abscess is K. pneumoniae[10], and the main pathogenic pathways are via hepatobiliary tract disease or intra-abdominal infections such as cholecystitis, cholangitis, pylephlebitis, appendicitis, diverticulitis, or peritonitis[3-5]. However, 20%-55% of pyogenic abscess cases are idiopathic[11]. Furthermore, the detection rate of colon cancer is high in patients with a pyogenic liver abscess of unknown origin[12].
GHT is defined as the presence of normal gastric mucosal tissue in a non-physiological site. GHT is an uncommon condition, and differs from metaplasia, which involves the transformation of one type of fully differentiated tissue into another. Thus, heterotopia is a developmental malformation, whereas metaplasia is an acquired condition[13]. GHT may be found in various locations in the GI tract, from the oral cavity to the anus[14], and can occur in the biliary tract, gallbladder[15,16], or pancreas[17], but is usually encountered in the upper GI tract. GHT in colon is rare, and in most cases, it involves the rectum[18,19]. To the best of our knowledge, only 11 cases of GHT in the colon proximal to rectum have been reported[2].
Several hypotheses have been suggested to explain the presence of GHT in the GI tract. The congenital theory proposes it results from a genetic error during embryogenesis[20], whereas the acquired theory posits it is due to abnormal regeneration after intestinal mucosal injury[21]. The stem cell theory suggests mispositioning of endodermal stem cells during organogenesis or erroneous differentiation of pluripotent endodermal stem cells in the GI tract[22]. On the other hand, it is also possible that local injury and inflammation trigger the dysregulations and reactivations of genes and cause gastric differentiation in the colon[23].
Many GHT patients are asymptomatic, and the condition is usually detected during evaluations of other bowel ailments. Symptoms are mainly caused by acid secretion from heterotopic tissue. The most common symptom is hematochezia followed by anal pain, tenesmus, burning, or anal pruritus[14,20,24]. Incidental diagnoses of GHT in colon over the past decade are largely attributed to the expanded use of colonoscopy for colorectal cancer screening, irritable bowel, or indigestion[14]. Notably, colonoscopy has not been identified as the cause of pyogenic liver abscess in any reported case. Resection is the main treatment modality for GHT of the colon[14,25]. In our case, the tumor was successfully removed by endoscopic mucosal resection, though in other reported cases, tumors were removed by endoscopic submucosal dissection[14]. For medical treatment, H2 blockers and proton pump inhibitors may be used[23,26,27].
The association between GHT and colon cancer has not been studied. However, considering that mucosal damage by colon cancer is one of the mechanisms of pyogenic liver abscess[9], GHT may be associated with pyogenic liver abscess. GHT in colon can cause mucosal damage by secreting gastric acid, and GHT formation might cause mucosal barrier damage. This suggests that GHT in colon could affect the development of pyogenic liver abscess by enabling the hematogenous propagation of K. pneumoniae through mucosal damage. However, more study is needed due to the lack of cases. Nonetheless, colonoscopy should be performed to determine the cause of GHT in patients with a pyogenic liver abscess. We describe a rare case of ascending colonic GHT found during colonoscopy performed for the differential diagnosis of pyogenic liver abscess of unknown cause and provide a review of the literature.
GHT in the colon could affect the development of pyogenic liver abscess by enabling hematogenous propagation of K. pneumoniae through mucosal damage. However, more study is needed due to the lack of cases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anatomy and morphology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Deng K, China; Liu Z, China; Sulbaran MN, Brazil S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Murray FE, Lombard M, Dervan P, Fitzgerald RJ, Crowe J. Bleeding from multifocal heterotopic gastric mucosa in the colon controlled by an H2 antagonist. Gut. 1988;29:848-851. [PubMed] [DOI] [Full Text] |
| 2. | Mannan AASR, Vieth M, Khararjian A, Khandakar B, Lam-Himlin D, Heydt D, Bhaijee F, Venbrux HJ, Byrnes K, Voltaggio L, Barker N, Yuan S, Montgomery EA. The outlet patch: gastric heterotopia of the colorectum and anus. Histopathology. 2018;73:220-229. [PubMed] [DOI] [Full Text] |
| 3. | SHERMAN JD, ROBBINS SL. Changing trends in the casuistics of hepatic abscess. Am J Med. 1960;28:943-950. [PubMed] [DOI] [Full Text] |
| 4. | Miedema BW, Dineen P. The diagnosis and treatment of pyogenic liver abscesses. Ann Surg. 1984;200:328-335. [PubMed] [DOI] [Full Text] |
| 5. | Stain SC, Yellin AE, Donovan AJ, Brien HW. Pyogenic liver abscess. Modern treatment. Arch Surg. 1991;126:991-996. [PubMed] [DOI] [Full Text] |
| 6. | Cohen JL, Martin FM, Rossi RL, Schoetz DJ Jr. Liver abscess. The need for complete gastrointestinal evaluation. Arch Surg. 1989;124:561-564. [PubMed] [DOI] [Full Text] |
| 7. | Lonardo A, Grisendi A, Pulvirenti M, Della Casa G, Melini L, Di Gregorio C, Nasi G, Sarti M, Tamborrino E, Lonardo F. Right colon adenocarcinoma presenting as Bacteroides fragilis liver abscesses. J Clin Gastroenterol. 1992;14:335-338. [PubMed] [DOI] [Full Text] |
| 8. | Giuliani A, Caporale A, Demoro M, Scimò M, Galati F, Galati G. Silent colon carcinoma presenting as a hepatic abscess. Tumori. 2007;93:616-618. [PubMed] |
| 9. | Teitz S, Guidetti-Sharon A, Manor H, Halevy A. Pyogenic liver abscess: warning indicator of silent colonic cancer. Report of a case and review of the literature. Dis Colon Rectum. 1995;38:1220-1223. [PubMed] [DOI] [Full Text] |
| 10. | Jun JB. Klebsiella pneumoniae Liver Abscess. Infect Chemother. 2018;50:210-218. [PubMed] [DOI] [Full Text] |
| 11. | Zhu X, Wang S, Jacob R, Fan Z, Zhang F, Ji G. A 10-year retrospective analysis of clinical profiles, laboratory characteristics and management of pyogenic liver abscesses in a chinese hospital. Gut Liver. 2011;5:221-227. [PubMed] [DOI] [Full Text] |
| 12. | Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39:1654-1659. [PubMed] [DOI] [Full Text] |
| 13. | Omotosho PA, Varnholt H, Tirabassi MV, Prasad R, Moriarty KP. Giant polypoid gastric heterotopia of the jejunum presenting with intermittent intussusception. J Laparoendosc Adv Surg Tech A. 2007;17:249-251. [PubMed] [DOI] [Full Text] |
| 14. | Iacopini F, Gotoda T, Elisei W, Rigato P, Montagnese F, Saito Y, Costamagna G, Iacopini G. Heterotopic gastric mucosa in the anus and rectum: first case report of endoscopic submucosal dissection and systematic review. Gastroenterol Rep (Oxf). 2016;4:196-205. [PubMed] [DOI] [Full Text] |
| 15. | Hayama S, Suzuki Y, Takahashi M, Hazama K, Fujita M, Kondo S, Katoh H. Heterotopic gastric mucosa in the gallbladder: report of two cases. Surg Today. 2010;40:783-787. [PubMed] [DOI] [Full Text] |
| 16. | Kotsis T, Orend KH. Management of traumatic aortic rupture (Watanabe et al. Surg Today. 2013; 43: 1339-46). Surg Today. 2014;44:1789-1790. [PubMed] [DOI] [Full Text] |
| 17. | Jarry J Jr, Rault A, Sa Cuhna A, Collet D, Masson B. Acute recurrent pancreatitis by heterotopic fundic mucosa at the ampulla of vater. Pancreas. 2009;38:351-353. [PubMed] [DOI] [Full Text] |
| 18. | Yu L, Yang Y, Cui L, Peng L, Sun G. Heterotopic gastric mucosa of the gastrointestinal tract: prevalence, histologic features, and clinical characteristics. Scand J Gastroenterol. 2014;49:138-144. [PubMed] [DOI] [Full Text] |
| 19. | Terada T. Heterotopic gastric mucosa of the gastrointestinal tract: a histopathologic study of 158 cases. Pathol Res Pract. 2011;207:148-150. [PubMed] [DOI] [Full Text] |
| 20. | Ko H, Park SY, Cha EJ, Sohn JS. Colonic adenocarcinoma arising from gastric heterotopia: a case study. Korean J Pathol. 2013;47:289-292. [PubMed] [DOI] [Full Text] |
| 21. | Sauer CG, Bickston SJ, Borowitz SM. Gastric heterotopia of the rectum. J Pediatr Gastroenterol Nutr. 2010;50:329-333. [PubMed] [DOI] [Full Text] |
| 22. | Wolff M. Heterotopic gastric epithelium in the rectum: a report of three new cases with a review of 87 cases of gastric heterotopia in the alimentary canal. Am J Clin Pathol. 1971;55:604-616. [PubMed] [DOI] [Full Text] |
| 23. | Beck F, Chawengsaksophak K, Waring P, Playford RJ, Furness JB. Reprogramming of intestinal differentiation and intercalary regeneration in Cdx2 mutant mice. Proc Natl Acad Sci U S A. 1999;96:7318-7323. [PubMed] [DOI] [Full Text] |
| 24. | Rifat Mannan AA, Kahvic M, Bharadwaj S, Grover VK. Gastric heterotopia of the anus: report of two rare cases and review of the literature. Indian J Pathol Microbiol. 2008;51:240-241. [PubMed] [DOI] [Full Text] |
| 25. | Devereaux CE, Devereaux RG. Heterotopic gastric mucosa of the rectum with a review of the literature. J Clin Gastroenterol. 1994;19:41-45. [PubMed] [DOI] [Full Text] |
| 26. | Matsubara A, Ogawa R, Suzuki H, Oda I, Taniguchi H, Kanai Y, Kushima R, Sekine S. Activating GNAS and KRAS mutations in gastric foveolar metaplasia, gastric heterotopia, and adenocarcinoma of the duodenum. Br J Cancer. 2015;112:1398-1404. [PubMed] [DOI] [Full Text] |
| 27. | Srinivasan R, Loewenstine H, Mayle JE. Sessile polypoid gastric heterotopia of rectum: a report of 2 cases and review of the literature. Arch Pathol Lab Med. 1999;123:222-224. [PubMed] [DOI] [Full Text] |