Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.5005
Peer-review started: November 6, 2021
First decision: December 27, 2021
Revised: January 7, 2022
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 26, 2022
Processing time: 199 Days and 13.2 Hours
Surgical repair of complex abdominal aortic aneurysm is associated with a higher perioperative mortality and morbidity. The advent of endovascular aortic repair (EVAR) has reduced perioperative complications, although the utilization of such techniques is limited by lesion characteristics, such as involvement of the visceral or renal arteries (RA) and/or presence of a sealing zone.
A 60-year-old male presented with a Crawford type IV complex thoracoabdominal aortic aneurysm (CAAA) starting directly distal to the diaphragm extending to both common iliac arteries (CIAs). The CAAA consist of a proximal and distal aneurysmal sac separated by a 1 cm-healthy zone in the infrarenal level. Due to the poor performance of the patient and the expansive disease, we planned a stepwise-combined surgery and EVAR to minimize invasiveness. A branched graft was implanted after surgical debranching of the visceral and RA. Since the patient had renal and liver injury after surgery, the second stage EVAR was performed 10 mo later. The stent graft was implanted from the distal portion of surgical branched graft to both CIAs during EVAR. The patient has been uneventful for 5-years after discharge and is being followed in the outpatient clinic.
The current case demonstrates that the surgical graft can provide a landing zone for second stage EVAR to avoid aggressive surgery in patients with poor performance with a long hostile CAAA.
Core Tip: Complex thoracoabdominal aortic aneurysm (CAAA) is an aortic disease requiring aggressive surgery. Herein, we report a case where branched graft was implanted covering half of the aortic disease, followed by a stent graft inserted through endovascular aortic repair to minimize invasiveness in a patient with extensive CAAA.
- Citation: Jang AY, Oh PC, Kang JM, Park CH, Kang WC. Extensive complex thoracoabdominal aortic aneurysm salvaged by surgical graft providing landing zone for endovascular graft: A case report. World J Clin Cases 2022; 10(15): 5005-5011
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/5005.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.5005
Surgical repair of complex thoracoabdominal aortic aneurysm (CAAA) is associated with a higher perioperative mortality[1,2]. Endovascular aortic repair (EVAR) has been increasingly performed due to its minimal invasiveness that results in reduced periprocedural complications[3]. However, EVAR is a challenging procedure in CAAA without the infrarenal neck due to the lack of landing zone for the stent grafts (SGs) or cases involving the visceral or renal arteries (RA). Herein, we report a case of CAAA with concomitant involvement of the common iliac artery (CIA) treated by a stepwise-combined surgery and EVAR, in which the surgical graft of the proximal half of CAAA facilitated a sealing zone for EVAR in the distal half.
A 60-year-old male was referred to our clinic for a large AAA detected during ultrasound screening of the abdominal aorta during cardiovascular screening check-up.
He had a history of heavy smoking (50 pack years). The patient had developed mild shortness of breath for the past 3 wk. Coronary angiography revealed chronic total occlusion of the mid right coronary artery with good collateral blood supply from the left coronary artery. Percutaneous coronary intervention was deferred as symptoms subsided after medical therapy. The patient was then referred to our clinic after the screening abdominal ultrasound revealed a CAAA.
The patient had a history of hypertension and renal tuberculosis 5-years prior. His electrocorticography performance status was also dramatically decreased from 4 to 2 after his diagnosis of renal tuberculosis.
The patient had not any personal or family history.
A pulsating mass was palpable in the peri-umbilical area.
The baseline creatinine level was 1.1 mg/dL. Other laboratory findings were all within normal limit.
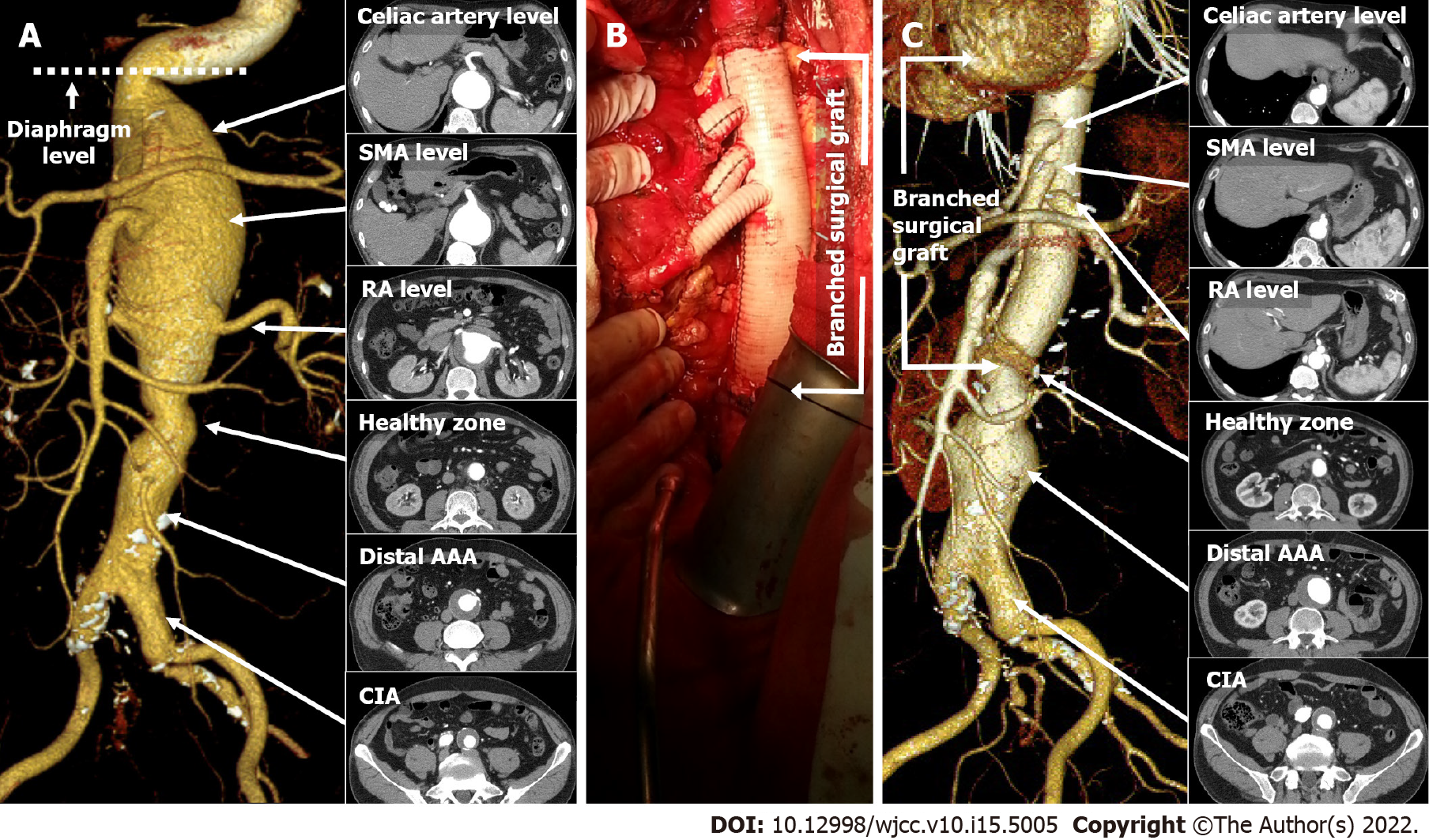
Computed tomographic aortography (CTA) was performed successfully. The diagnosis was made based on the serial axial images and its Three-dimensional reconstruction of the CTA. The patient had a Crawford type IV CAAA consisting of a proximal and distal aneurysmal sac separated by a 1 cm-healthy zone in the infrarenal level (Figure 1A). The proximal AAA was a fusiform juxtarenal aneurysm (max diameter: 79 mm), starting directly below a sharp kink at the diaphragm level and ending at approximately 4 cm distal to the RA (Figure 1A). The distal aneurysm (max diameter: 49 mm) was fusiform starting 5 cm distal to the RA and involving both the CIAs, where occlusion of the right internal iliac artery (IIA) was observed (Figure 1A).
Due to the poor performance of the patient, we planned a stepwise-combined surgery and EVAR (surgical debranching of the visceral/RA followed by a second stage EVAR) to minimize invasiveness.
The final diagnosis was a long CAAA involving visceral arteries, RAs, and both CIAs requiring extensive correction.
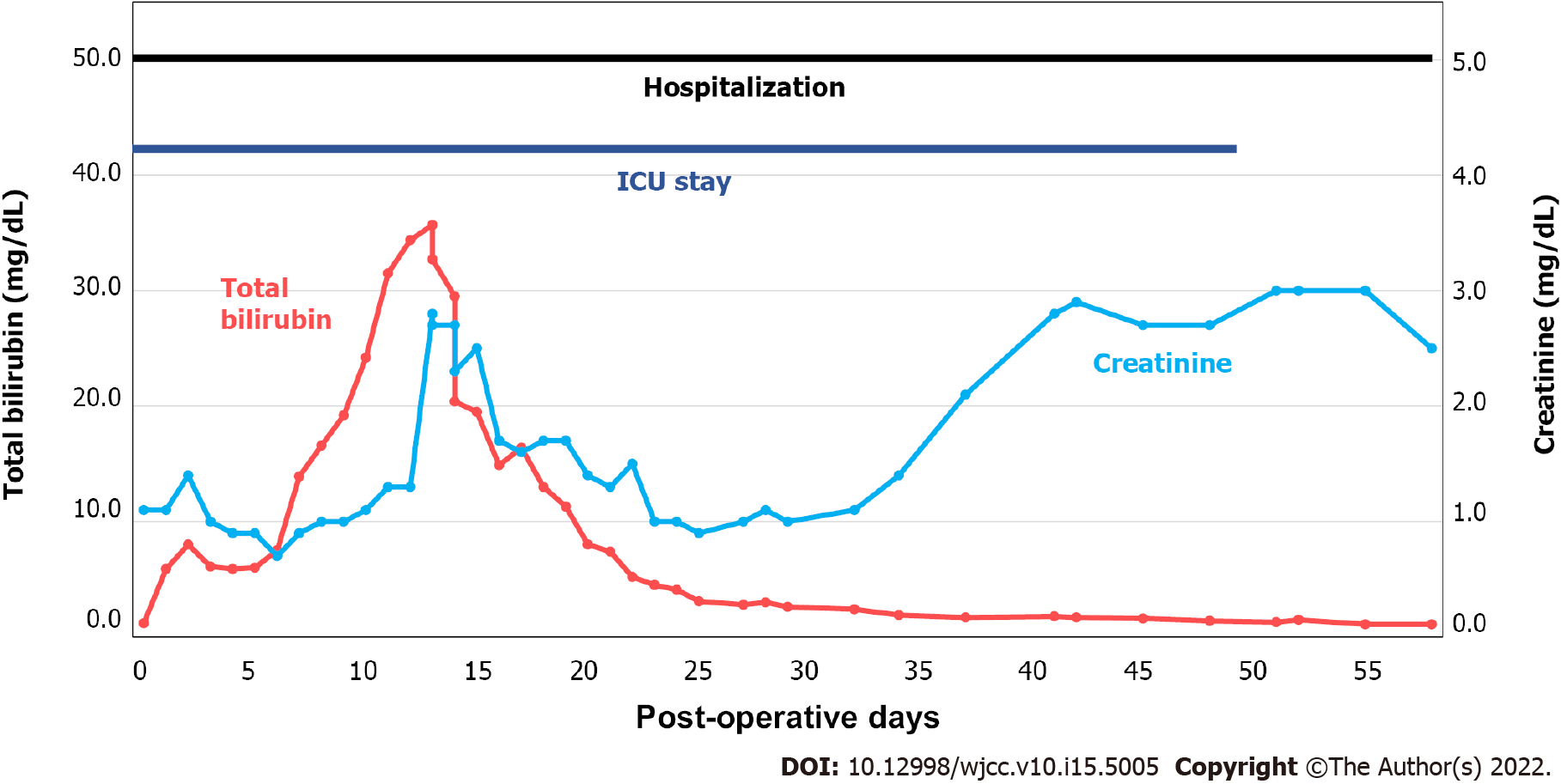
Open surgery was performed by a retroperitoneal approach. Supraceliac clamping was performed slightly distal to the diaphragm while distally clamping the infrarenal healthy zone of the aorta. Surgery was prolonged due to difficulty in locating the small-calibered calcified right RA. End-to-end anastomosis between the Coseli branched graft (Gelweave® Coselli Thoracoabdominal Graft, Vascuteck-Terumo, Renfrewshire Scotland, United Kingdom), proximal/distal aortic stump and visceral/RAs was performed, and reinforced by the remaining aortic wall (Figure 1B). The total duration of surgery was approximately 7 h from start to finish of anesthesia. After surgery, the patient remained in the intensive care unit (ICU) for approximately 50 d for liver failure, acute kidney injury (AKI), and pneumonia (Figure 2). The second stage EVAR was delayed until the patient was physically and mentally ready for the second procedure. He refused of undergoing any type of additional interventions, including surgery, due to the traumatic memories of staying in the ICU for almost 2 mo. The 10-mo post-surgical CTA showed no dysfunction of the surgical graft (Figure 1C). There was no change in diameter of the distal AAA (maximal diameter: 49 mm), although the diameter of the left CIA slightly increased from 27 mm to 30 mm, compared with previous CTA.
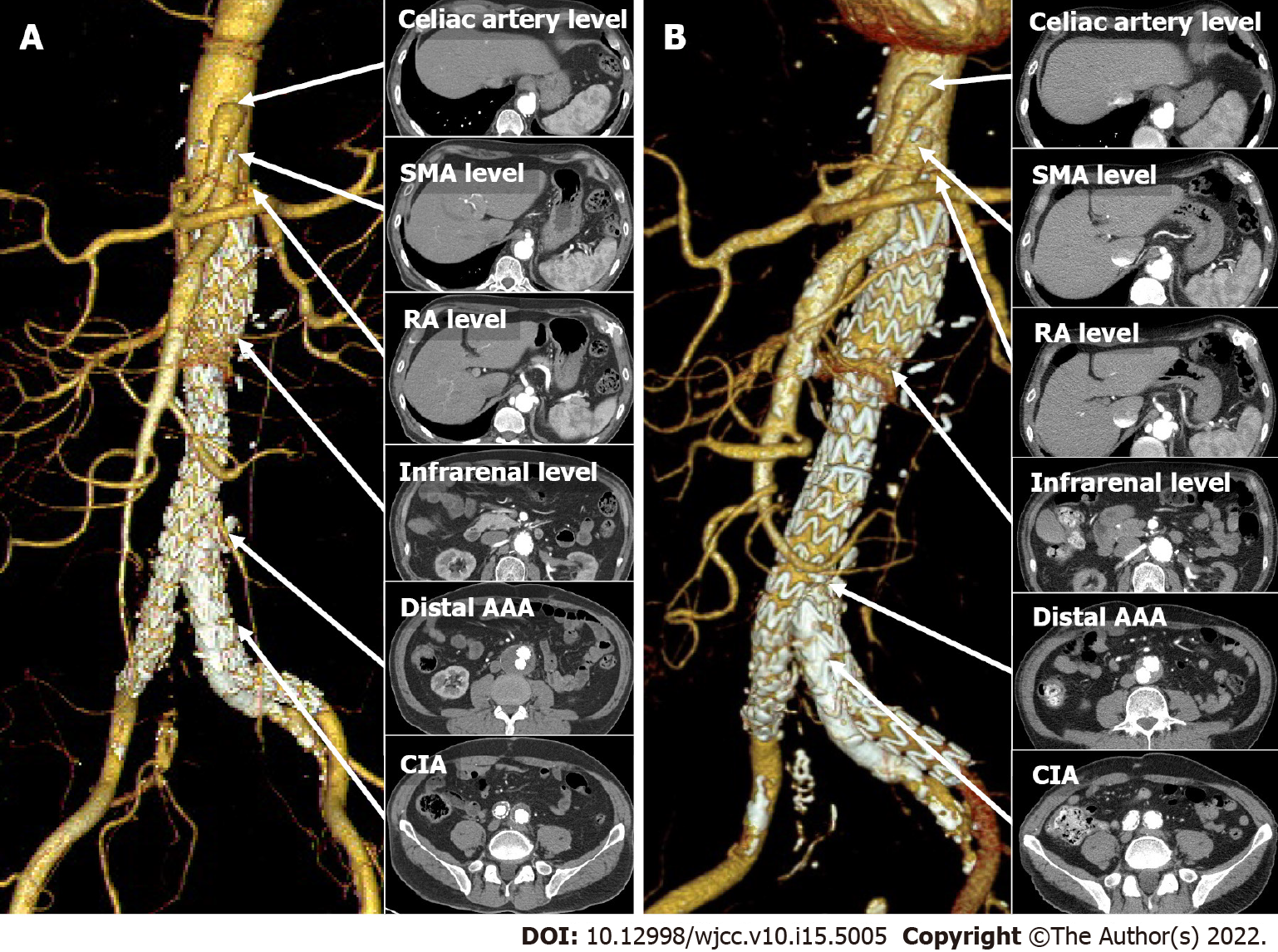
EVAR was performed 15 mo post-surgery. After implantation of the main SG (Endurant 32 × 103 mm, Medtronic, Minneapolis, MN, United States) on the surgical graft, a limb extension was deployed for the right CIA aneurysm (Endurant 16 × 156 mm). Another limb extension (Endurant 16 × 125 mm) for the left external iliac artery and an additional SG (GORE® VIABAHN® Endoprosthesis 8 × 100 mm, Gore, Flagstaff, AZ, United States) for the left IIA were implanted using the sandwich technique, to preserve the left IIA (Figure 3A). Follow-up angiography revealed a type Ib endoleak in the left IIA, which was sealed by a bare metal stent (Express™ LD 9 × 37 mm, Boston Scientific, Marlborough, MA, United States).
The patient was discharged in 3 d without any complications. CTA performed 5 mo post-EVAR showed no endoleaks or compromise of SGs (Figure 3A). CTA performed 5 years post-EVAR showed no endoleaks or graft failure (Figure 3B). Over time, the maximal diameter of the distal AAA (49 mm to 32 mm) and CIA (30 mm to 19 mm) were reduced. The patient has been uneventful without complaints for the following 5 years and is being followed up in the outpatient department.
Although extensive CAAAs are generally corrected by mega-aortic surgery, such invasive strategies are associated with a high-risk of mortality and morbidity. To our knowledge, this case is the first to demonstrate that Crawford type IV CAAA concurrently involving bilateral CIA can be successfully treated by coordinating the surgical graft to provide a landing zone for the infrarenal SG. This combined approach may be a considerable option for patients with extensive CAAA.
Crawford type IV AAA is associated with a high-risk of post-operative mortality and morbidity with mortality. Post-operative mortality is up to 7% with high rates of detrimental complications, such as spinal cord injury or AKI[1,2]. The preferred treatment strategy, surgical, EVAR, or combined, is debatable, although EVAR has become popularized for its less invasiveness. AAA with the CIA involvement is also associated with a high-risk surgical mortality and complications[3]. EVAR has not been proven to reduce mortality, but perioperative complication rates are less severe[4-7]. In this patient, open surgery of the whole extent of AAA was not considered because of the patient’s initial poor performance and high-risk. Additionally, EVAR for the juxtarenal aneurysm was not an option for the lack of infrarenal landing zone and angulated proximal neck. Fenestrated EVAR was also not available in South Korea at the time. However, combined surgery was feasible because there was an intact area of the infrarenal aorta without aneurysmal change or calcification, which enabled distal anastomosis of the surgical graft. The branched surgical graft also facilitated a sufficient landing zone for EVAR of the distal aneurysm and IIAs.
Although the surgery was successfully performed without any intra-operative complications, the patient exhibited life-threatening post-operative morbidities, such as AKI, liver failure, and pneumonia. Post-surgical AKI rate is as high as 39% in CAAA repair, while new onset dialysis incidence is up to 4%[1,2]. In patients with CAAA undergoing visceral vessel repair (VVR), the postoperative mortality was 8.9%, while chances for hemodialysis (HD) and pneumonia were 7.6% and 17.2%, respectively[8]. The independent predictors for mortality in patients undergoing VVR were poor functional status, baseline creatinine, the presence of diabetes and older age. It is likely that the patient’s poor initial performance status may have been associated with the life-threatening liver and renal injury after surgery. The most optimal way of reducing such liver or renal injuries is reducing ischemic time[9]. Alternative methods, such as selective renal perfusion with cold saline or crystalloids have been introduced[10]. Selective visceral perfusion to both the hepatic and renal artery have also shown promising results in reducing ischemic injury[11]. He developed AKI within 2 d of surgery and creatinine levels were not fully recovered until discharge (Figure 2). His creatinine level was 2.5 mg/dL at discharge. He gradually developed chronic kidney disease, which eventually led to HD in 3 years. His bilirubin level after initial surgery was also increased up to 35.7 mg/dL, while the exact cause of isolated bilirubinemia was not confirmed; abdominal ultrasound did not show obstruction of the biliary tract. The prolonged ischemic time may have caused impairment of the liver and kidney. The patient also developed hospital-acquired pneumonia 3-d after the surgery. He was unable to wean from ventilator support for 49 d, which prolonged his stay in the ICU. He was discharged 60 d after the surgery (Figure 2). These detrimental complications altogether suggest that the patient may have had a high chance of developing a debilitating complication had he received open mega surgery of the whole extent of CAAA, from the supraceliac to CIA level.
This case demonstrates that surgical graft can provide a sufficient landing zone for the EVAR in patients with extensive CAAA with the concomitant CIA involvement.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Dai X, China; Kharlamov AN, Netherlands; Lakusic N, Croatia; Yang L, China S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Latz CA, Boitano L, Schwartz S, Swerdlow N, Dansey K, Varkevisser RRB, Patel V, Schermerhorn ML. Editor's Choice - Mortality is High Following Elective Open Repair of Complex Abdominal Aortic Aneurysms. Eur J Vasc Endovasc Surg. 2021;61:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Swerdlow NJ, Wu WW, Schermerhorn ML. Open and Endovascular Management of Aortic Aneurysms. Circ Res. 2019;124:647-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 100] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 3. | Bannazadeh M, Jenkins C, Forsyth A, Kramer J, Aggarwal A, Somerset AE, Bove PG, Long GW. Outcomes for concomitant common iliac artery aneurysms after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2017;66:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Katsargyris A, Oikonomou K, Klonaris C, Bal A, Yanar F, Verhoeven EL. Common iliac and hypogastric aneurysms: open and endovascular repair. J Cardiovasc Surg (Torino). 2015;56:249-255. [PubMed] |
| 5. | Paravastu SC, Jayarajasingam R, Cottam R, Palfreyman SJ, Michaels JA, Thomas SM. Endovascular repair of abdominal aortic aneurysm. Cochrane Database Syst Rev. 2014;CD004178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 6. | Schermerhorn ML, O'Malley AJ, Jhaveri A, Cotterill P, Pomposelli F, Landon BE. Endovascular vs. open repair of abdominal aortic aneurysms in the Medicare population. N Engl J Med. 2008;358:464-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 552] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 7. | Stather PW, Sidloff D, Dattani N, Choke E, Bown MJ, Sayers RD. Systematic review and meta-analysis of the early and late outcomes of open and endovascular repair of abdominal aortic aneurysm. Br J Surg. 2013;100:863-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 252] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Patel VI, Lancaster RT, Conrad MF, Lamuraglia GM, Kwolek CJ, Brewster DC, Cambria RP. Comparable mortality with open repair of complex and infrarenal aortic aneurysm. J Vasc Surg. 2011;54:952-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Waked K, Schepens M. State-of the-art review on the renal and visceral protection during open thoracoabdominal aortic aneurysm repair. J Vis Surg. 2018;4:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Wynn MM, Acher C, Marks E, Engelbert T, Acher CW. Postoperative renal failure in thoracoabdominal aortic aneurysm repair with simple cross-clamp technique and 4°C renal perfusion. J Vasc Surg. 2015;61:611-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Fernández-Doblas J, Ortega-Loubon C, Pérez-Andreu J, Linés M, Fernández-Molina M, Abella RF. Selective visceral perfusion improves renal flow and hepatic function in neonatal aortic arch repair. Interact Cardiovasc Thorac Surg. 2018;27:395-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |