Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4949
Peer-review started: October 13, 2021
First decision: December 10, 2021
Revised: December 27, 2021
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: May 26, 2022
Processing time: 223 Days and 7.3 Hours
In most cases of yellow nail syndrome (YNS), the classic triad of yellow nails, lymphedema and respiratory manifestations rarely manifest simultaneously. Therefore, diagnosis is delayed or frequently missed.
We report a 62-year-old YNS patient presenting with bilateral pleural, pericardial and peritoneal effusions who, 2 mo later, developed minimal-change nephrotic syndrome. After treatment with vitamin E, clarithromycin and prednisone for 3 mo, effusions in the chest, pericardium and abdominal cavity decreased while urine protein levels returned to within normal ranges.
Clinicians should consider the possibility of YNS for patients presenting with multiple serous effusions and nephrotic syndromes.
Core Tip: Yellow nail syndrome (YNS) is a rare disease characterized by yellow nails, lymphedema and respiratory manifestations. However, few patients have a complete triad. We present a rare case of the classical triad in a patient with concomitant minimal-change nephrotic syndrome. And as far as we know, this is the first report about multiple effusions caused by YNS. This case highlights the variety of clinical manifestations, especially in comorbid cases, leading to delayed or missed diagnosis. Therefore, once YNS is considered, it is recommended to improve relevant examinations of the lymphatic system for further determinations.
- Citation: Zhang YN, Wang MH, Yu WC, Cheng W, Cong JP, Huang XP, Wang FF. Yellow nail syndrome accompanied by minimal-change nephrotic syndrome: A case report. World J Clin Cases 2022; 10(15): 4949-4956
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4949.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4949
Yellow nail syndrome (YNS) is a rare disorder characterized by yellow nails, lymphedema, and respiratory manifestations; two of which are sufficient for the diagnosis of YNS. The complete triad is present only in 27% to 60% of patients[1], and symptoms may occur years apart, which delays diagnosis. Adults aged > 50 years are highly susceptible to this disease, and a few pediatric cases have been reported[2,3]. Currently, the pathogenesis of YNS has not been fully elucidated. Some cases have previously been reported in families[4], with most members acquiring the disease. Lymphatic involvement and microvasculopathy are the most important pathological changes. In this report, we describe a case of YNS with bilateral pleural, pericardial and peritoneal effusions as well as a secondary nephrotic syndrome.
Dyspnea, cough, expectoration and abdominal distension for 1 mo.
A 62-year-old man presented with a 1-mo history of dyspnea, cough, expectoration and abdominal distension. Due to mild proteinuria, hypoalbuminemia and elevated blood creatinine levels, he was diagnosed with glomerulonephritis at the local hospital, and was treated with diuretics and drugs to lower urine protein levels. Despite treatment, dyspnea worsened. Therefore he was referred to our hospital for further treatment.
The patient reported no history of hepatitis, tuberculosis or heart disease before.
The patient reported no personal or family history.
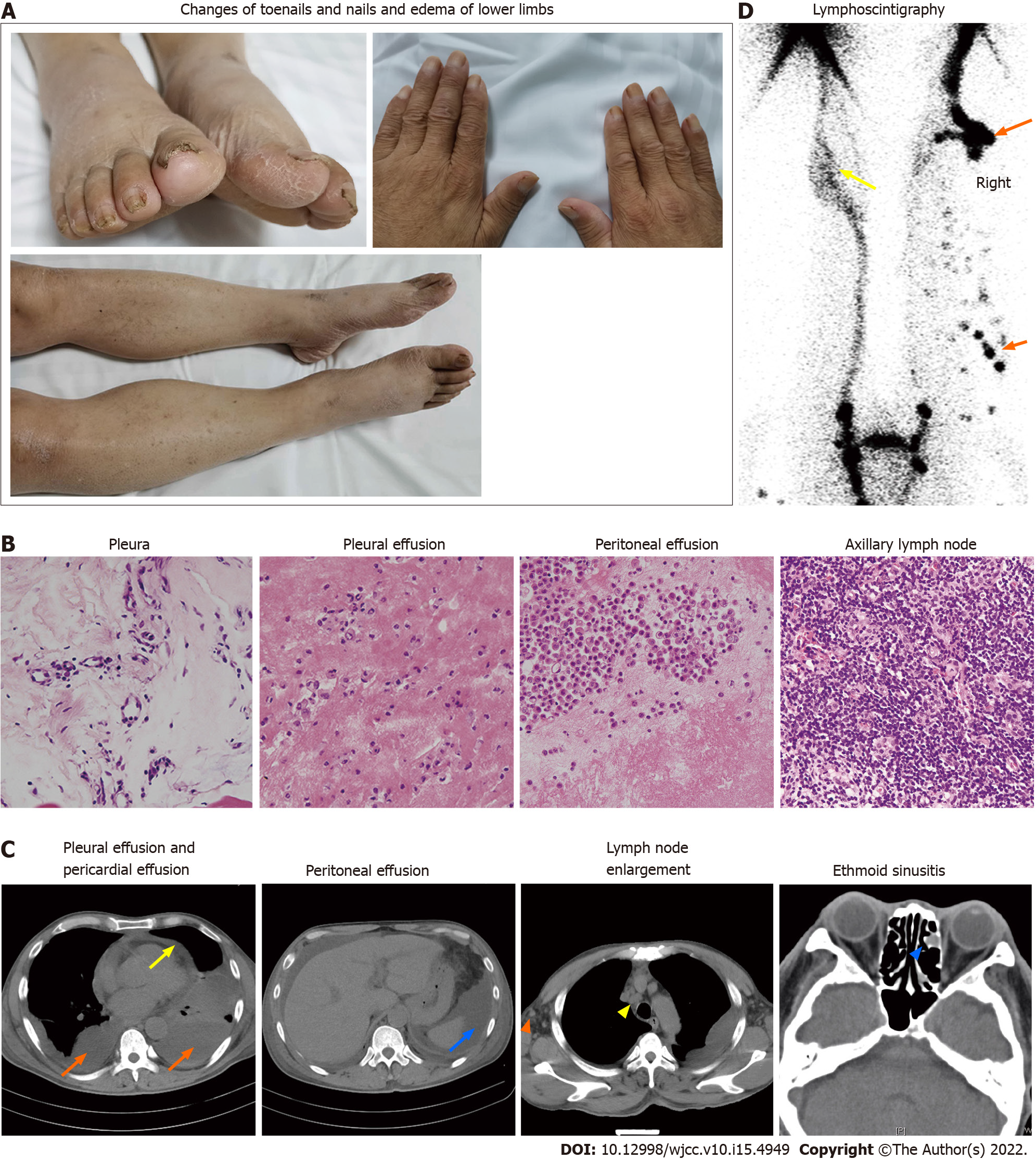
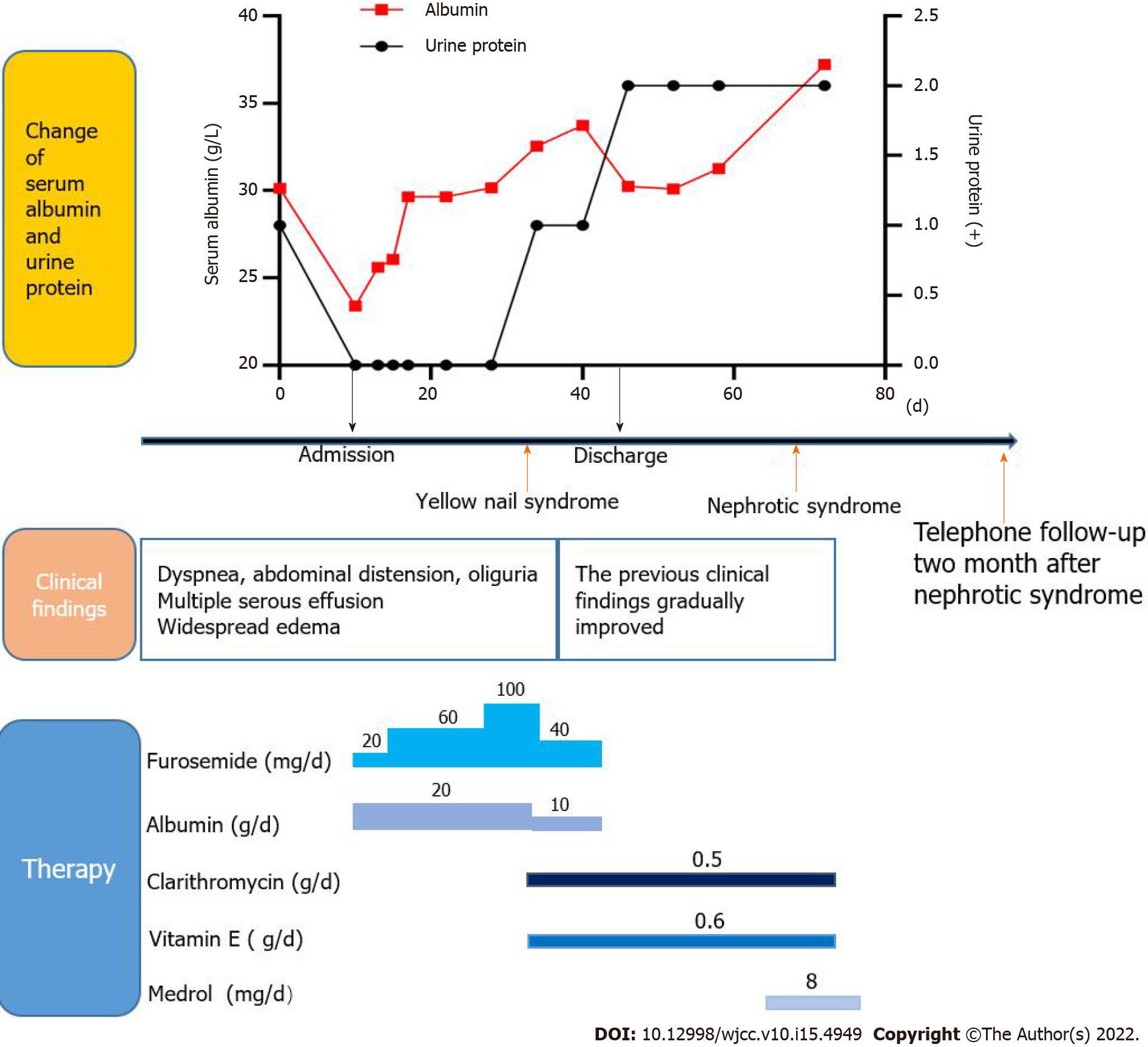
Physical examination revealed multiple effusions in the chest, pericardium and abdominal cavity, accompanied by nonpitting edema of the chest and abdominal walls, as well as pitting edema of both lower limbs (heavier in the right lower limb). The patient’s toenails were thickened and yellow, with increased curvature and slow growth (0.2 mm/wk), as were his nails (Figure 1A).
Clinical assessment revealed mild proteinuria (0.32 g/24 h), severe hypoalbuminemia (23.39 g/L), elevated serum creatinine levels (139 umol/L) and D-dimer (3270 pg/mL) levels. Even though white blood cell counts were elevated (11.33 × 109/L), the neutrophil/lymphocyte ratio was normal. Blood coagulation, electrolytes, serum lipids, antinuclear antibodies, extractable nuclear antigens spectrum, antineutrophil cytoplasmic antibodies, immunoglobulin (κ and λ light chains), hepatitis virus detection, double-stranded DNA, tumor markers, T-cell interferon- release assays and B-type natriuretic peptide were all normal. We performed bilateral thoracentesis and abdominal puncture to detect tuberculosis and tumors. Both pleural and peritoneal effusions were composed of exudates, mainly lymphocytes (60% and 77%, respectively). Chyle test of the pleural fluid was positive. There were no tumor cells in the pleura, pleural as well as peritoneal effusions (Figure 1B), and the tuberculosis nucleic acid test was negative. Lymph node biopsy (right axillary) (Figure 1B) revealed proliferative lesions of the lymphoid tissue, lymphatic sinuses and lymphoid follicles. The toenails were negative for fungi or parasites.
Effusions in these areas, subcutaneous edema, pleural thickening, and enlarged axillary as well as mediastinal lymph nodes were confirmed by computed tomography (CT) (Figure 1C). Positron emission tomography (PET)/CT scans revealed multiple symmetrical distributions of enlarged lymph nodes in the neck, chest, abdomen, pelvis, and left ethmoid sinusitis (Figure 1C). Doppler echocardiography revealed pericardial effusion and a mild decrease of the left ventricular diastolic function. Radionuclide lymphoscintigraphy revealed interrupted lymphatic vessels of the right lower limb, with the imaging agent being accumulated in subcutaneous tissues, inguinal lymph nodes on the right side were not clear, and lymphatic vessels of the left lower limb exhibited collateral circulations (Figure 1D).
YNS.
The patient was treated with vitamin E (0.6 g/d) and clarithromycin (0.5 g/d).
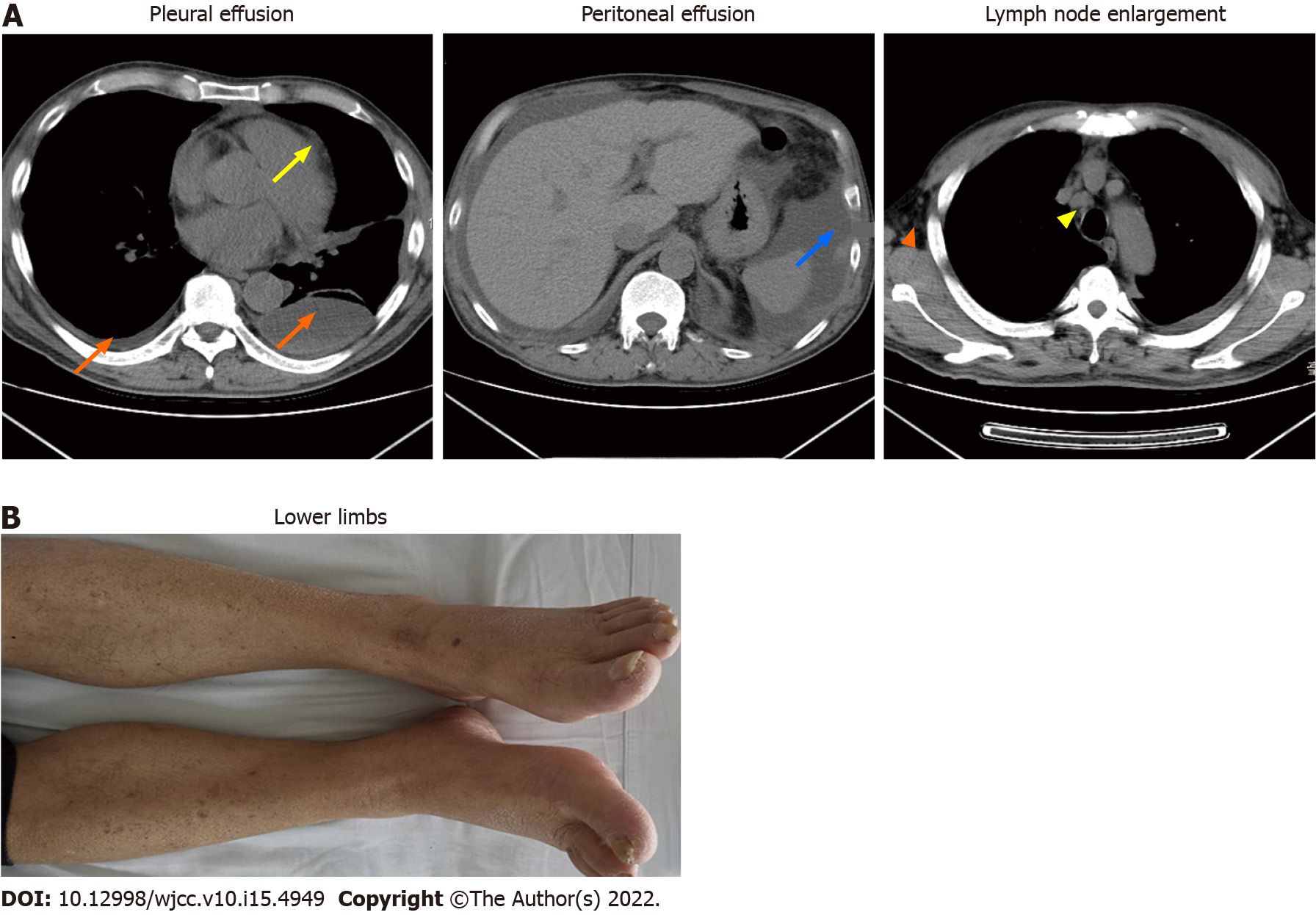
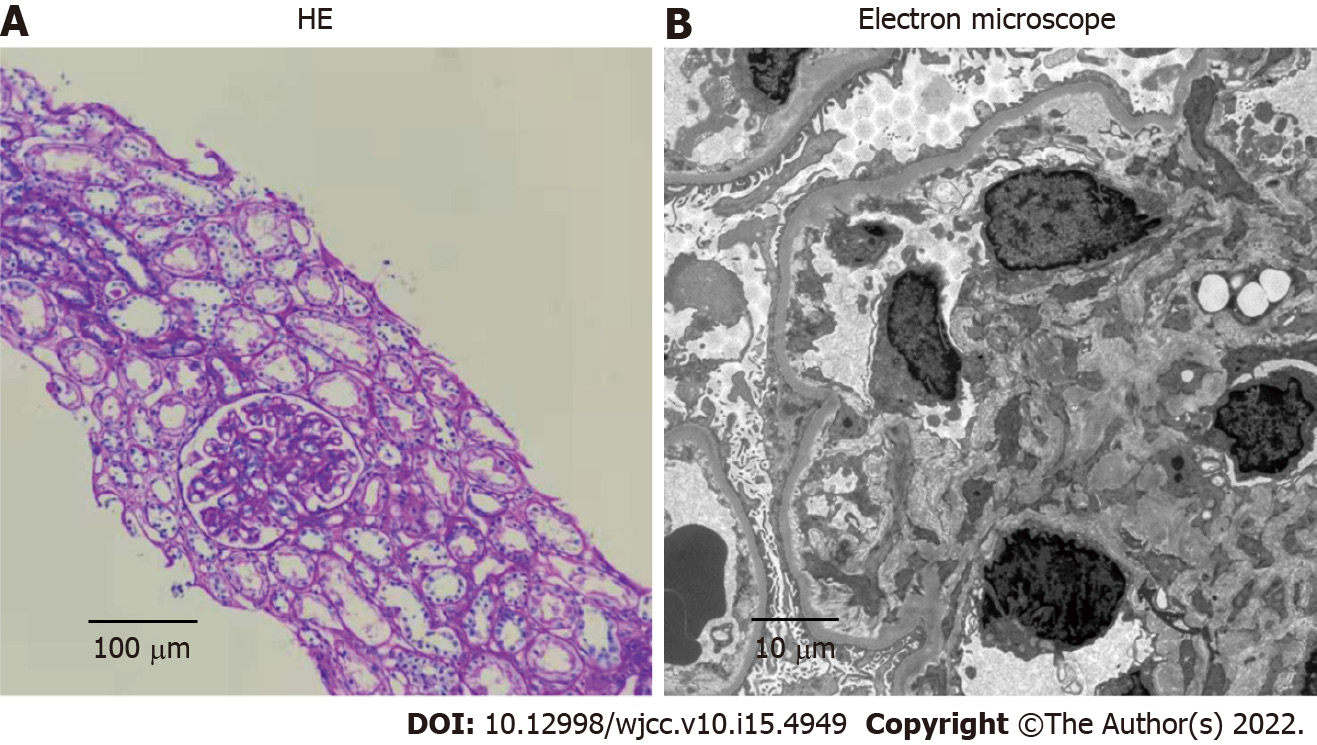
After 1 mo of treatment, dyspnea, pleural effusions, pericardial effusions, ascites and systemic edema were relieved (Figure 2A). However, proteinuria was significantly elevated (4.87 g/24 h). Renal biopsy confirmed minimal-change nephrotic syndrome (Figure 3A and B). The combination of clinical and laboratory findings revealed that the patient had nephrotic syndrome. Therefore, he was treated with oral metoprolol (8 mg/d). Two months later, his proteinuria had been relieved. The clinical course and therapy of the patients are shown in Figure 4.
The first case of YNS was reported by Heller in 1927. The prevalence of YNS has not been fully established; however, based on the number of reported cases, it is estimated that it is less than 1/1000000[5]. Moreover, its pathogenesis has not been conclusively evaluated. At present, it is considered to be as a result of abnormal lymphatic functions, including lymphatic dysplasia, chy
Yellow nail is a characteristic manifestation of YNS. The patients have dark nail discoloration, which varies from pale yellow to more or less dark green, thickening of the nail layer, excess bending, onycholysis and slow growth[5]. These physical signs can appear alone, without lymphedema or respiratory manifestations. Our patient’s toenails were thickened and yellow, with increased curvature and slow growth (0.2 mm/wk), as were his nails (Figure 1A). Microscopic examination of nail slices revealed that normal loose fibrous blood vessels had been replaced with dense collagen fibers[10]. Accumulation of the lipofuscin pigment explains the yellow discoloration. These pathological changes are attributed to poor lymph drainage under the nail[11].
Lung involvement in YNS, which occurs in 56%-71% of the patients, diversely affects some parts of the respiratory tract with a variety of clinical manifestations[5]. Chronic cough, pleural effusion and bronchiectasis are common pulmonary manifestations of YNS[12]. In a meta-analysis, 68.3% of the YNS patients had bilateral pleural effusions and 95% of effusions were described as exudates (median protein level: 4.2 g/dL) with lymphocytic predominance in 96%[13]. In our case, bilateral pleural effusions were the main clinical manifestation (Figure 1C) and presented as chylothoraxexudates. These findings are consistent with those of a previous report[14]. Thoracic duct and lymphatic vessel rupture or obstruction in the thoracic cavity is associated with lymph accumulation in the pleural cavity to produce chylothorax. Besides, the patient developed pericardial and peritoneal effusions (Figure 1C). As far as we know, this is the first report about multiple effusions caused by YNS.
Lymphedema has been reported in 29%-80% of previous cases, and may be the first signal to seek medical care[12]. Lymphedema mainly affects both lower limbs[14]. Lymphoscintigraphy is an effective imaging modality that is widely used for differential diagnosis of lymphedema and for determining the extent of the disease[15]. Lymphoscintigraphy of this case revealed that lymphatic vessels of the right lower limb were interrupted and collateral circulation appeared in the lymphatic vessels below the left knee which resulted in edema of both lower limbs (Figure 1D).
Sinusitis is common among YNS patients. A previous study reported that 44 of 85 YNS patients had chronic rhinosinusitis, which presented with nasal/postnasal drainage (94%), obstruction/congestion (81%), facial pain/pressure (33%) and anosmia (8%)[16]. Sinusitis of this patient was confirmed by imaging studies, with no obvious symptoms (Figure 1C). There are many causes of sinusitis, including microbial infections, allergies, and abnormal anatomical structures. The lymphatic tissue plays an important role in immune responses.
In addition, YNS patients can also present with multiple comorbidities, including nephrotic syndrome, endocrine diseases, infections, and malignant neoplasms. There are three reports on the presence of nephrotic syndrome in YNS[17-19]; two of which were minimal nephritic syndrome change. At the time of reporting, clinical examination of this case was almost complete, and diseases associated with related symptoms such as infections, tumor and immunity excluded. Due to heavy proteinuria, a diagnosis of nephrotic syndrome was made, and subsequent renal biopsy confirmed minimal-change nephrotic syndrome (Figure 3), similar to a previous report[19]. The pathogenic association between YNS and minimal change nephrotic syndrome is unclear. Yáñez et al[18] suggested a causal relationship between the two disorders. Gupta et al[20] found a striking deficiency of naive CD4+ and CD8+ T cells and total B cells, and increased transitional B cells in YNS patients. Furthermore, the dysfunction of T cells and B cells has been confirmed to play a central role in minimal change nephrotic syndrome[21]. We postulate that YNS patients may have extensive lymphatic dysfunctions, and renal lymphatic drainage is blocked, resulting in antibody stasis and deposition in the glomerular capillary network, leading to pathophysiological changes of nephrotic syndromes.
The main therapeutic options for YNS include clinical management of yellow nails, pleural effusion and lymphedema. Vitamin E is also a potential therapeutic option, although its efficacy and putative mechanisms have not been established. Somatostatin analogs, primarily octreotide, have also been used to treat pleural effusions and lymphedema, with varying outcomes[5]. In YNS patients, anti-inflammatory activities of macrolides might improve systemic inflammation, which reduces lymphedema and promotes nail growth[22]. Algain[23] reported the first and only case of a YNS patient who failed to respond to fluconazole and vitamin E, but showed near-complete recovery after administration with terbinafine and topical minoxidil. In this case, the patient was administered with vitamin E, clarithromycin and oral metoprolol. Subsequently, his dyspnea, pleural effusions, pericardial effusions, ascites and systemic edema (Figure 2A and B) were relieved, hypoalbuminemia was also corrected, while proteinuria gradually reduced.
The classic triad is not present in many cases of YNS, especially in comorbid cases, leading to delayed or missed diagnosis. Once YNS is considered, it is recommended to improve relevant examinations of the lymphatic system for further determinations. There is a need to establish a more systematic, comprehensive as well as standardized diagnosis and treatment options for this disease.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Moshref L, Saudi Arabia; Yorioka N, Japan S-Editor: Liu JH L-Editor: Kerr C P-Editor: Liu JH
| 1. | Aslam MZ, De Loughry G, O'Brien B. Anaesthesia and orphan diseases: Yellow nail syndrome. Eur J Anaesthesiol. 2020;37:728-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Hsu TY, Lin CC, Lee MD, Chang BP, Tsai JD. Titanium Dioxide in Toothpaste Causing Yellow Nail Syndrome. Pediatrics. 2017;139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Cecchini M, Doumit J, Kanigsberg N. Atypical presentation of congenital yellow nail syndrome in a 2-year-old female. J Cutan Med Surg. 2013;17:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Razi E. Familial yellow nail syndrome. Dermatol Online J. 2006;12:15. [PubMed] |
| 5. | Vignes S, Baran R. Yellow nail syndrome: a review. Orphanet J Rare Dis. 2017;12:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 6. | Emerson PA. Yellow nails, lymphoedema, and pleural effusions. Thorax. 1966;21:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 138] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Beer DJ, Pereira W Jr, Snider GL. Pleural effusion associated with primary lymphedema: a perspective on the yellow nail syndrome. Am Rev Respir Dis. 1978;117:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Lewis M, Kallenbach J, Zaltzman M, Conlan A, Zwi S, Abramowitz J. Pleurectomy in the management of massive pleural effusion associated with primary lymphoedema: demonstration of abnormal pleural lymphatics. Thorax. 1983;38:637-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Uchida T, Uchida Y, Takahashi M, Masaki K, Sato H, Iemura H, Shinomiya S, Nakamura H, Nagata M. Yellow Nail Syndrome in Which Intranodal Lymphangiography Contributed to the Diagnosis. Intern Med. 2021;60:3599-3603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | DeCoste SD, Imber MJ, Baden HP. Yellow nail syndrome. J Am Acad Dermatol. 1990;22:608-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Norton L. Further observations on the yellow nail syndrome with therapeutic effects of oral alpha-tocopherol. Cutis. 1985;36:457-462. [PubMed] |
| 12. | Piraccini BM, Urciuoli B, Starace M, Tosti A, Balestri R. Yellow nail syndrome: clinical experience in a series of 21 patients. J Dtsch Dermatol Ges. 2014;12:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Valdés L, Huggins JT, Gude F, Ferreiro L, Alvarez-Dobaño JM, Golpe A, Toubes ME, González-Barcala FJ, José ES, Sahn SA. Characteristics of patients with yellow nail syndrome and pleural effusion. Respirology. 2014;19:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Maldonado F, Ryu JH. Yellow nail syndrome. Curr Opin Pulm Med. 2009;15:371-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Kwon HR, Hwang JH, Mun GH, Hyun SH, Moon SH, Lee KH, Choi JY. Predictive role of lymphoscintigraphy undergoing lymphovenous anastomosis in patients with lower extremity lymphedema: a preliminary study. BMC Med Imaging. 2021;21:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Gutierrez CN, Low CM, Stokken JK, Choby G, O'Brien EK. Characterization of Sinus Disease in Patients with Yellow Nail Syndrome. Am J Rhinol Allergy. 2020;34:156-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Cockram CS, Richards P. Yellow nails and nephrotic syndrome. Br J Dermatol. 1979;101:707-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Yáñez S, Val-Bernal JF, Fernández-Llaca H. Yellow nails and minimal change nephrotic syndrome. Nephron. 1999;82:180-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Sakiyama T, Shimizu T, Funakoshi T, Saito M. Case of yellow nail syndrome accompanied by nephrotic syndrome. J Dermatol. 2016;43:585-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Gupta S, Samra D, Yel L, Agrawal S. T and B cell deficiency associated with yellow nail syndrome. Scand J Immunol. 2012;75:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Kim SH, Park SJ, Han KH, Kronbichler A, Saleem MA, Oh J, Lim BJ, Shin JI. Pathogenesis of minimal change nephrotic syndrome: an immunological concept. Korean J Pediatr. 2016;59:205-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Matsubayashi S, Suzuki M, Suzuki T, Shiozawa A, Kobayashi K, Ishii S, Iikura M, Izumi S, Kudo K, Sugiyama H. Effectiveness of clarithromycin in patients with yellow nail syndrome. BMC Pulm Med. 2018;18:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Algain M. Yellow Nail Syndrome Successfully Treated with Oral Terbinafine and Topical Minoxidil. Clin Cosmet Investig Dermatol. 2021;14:249-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |