Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4836
Peer-review started: April 26, 2021
First decision: July 27, 2021
Revised: August 3, 2021
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 26, 2022
Processing time: 392 Days and 19.3 Hours
Postoperative pancreatic fistula (POPF) is the most fearful complication after pancreatic surgery and can lead to severe postoperative complications such as surgical site infections, sepsis and bleeding. A previous study which identified cut-offs of drains amylase levels (DALs) determined on postoperative day (POD) 1 and POD3, was able to significantly predict POPF, abdominal collections and biliary fistulas, when related to specific findings detected at the abdominal computerized tomography (CT) scan routinely performed on POD3.
To validate the cut-offs of DALs in POD1 and POD3, established during the previous study, to assess the risk of clinically relevant POPF and confirm the usefulness of abdominal CT scan on POD3 in patients at increased risk of abdominal collection.
The DALCUT trial is an interventional prospective study. All patients who will undergo pancreatoduodenectomy (PD) for periampullary neoplasms will be considered eligible. All patients will receive clinical staging and, if eligible for surgery, will undergo routine preoperative evaluation. After the PD, daily DALs will be evaluated from POD1. Drains removal and possible requirement of abdominal CT scans in POD3 will be managed on the basis of the outcome of DALs in the first three postoperative days.
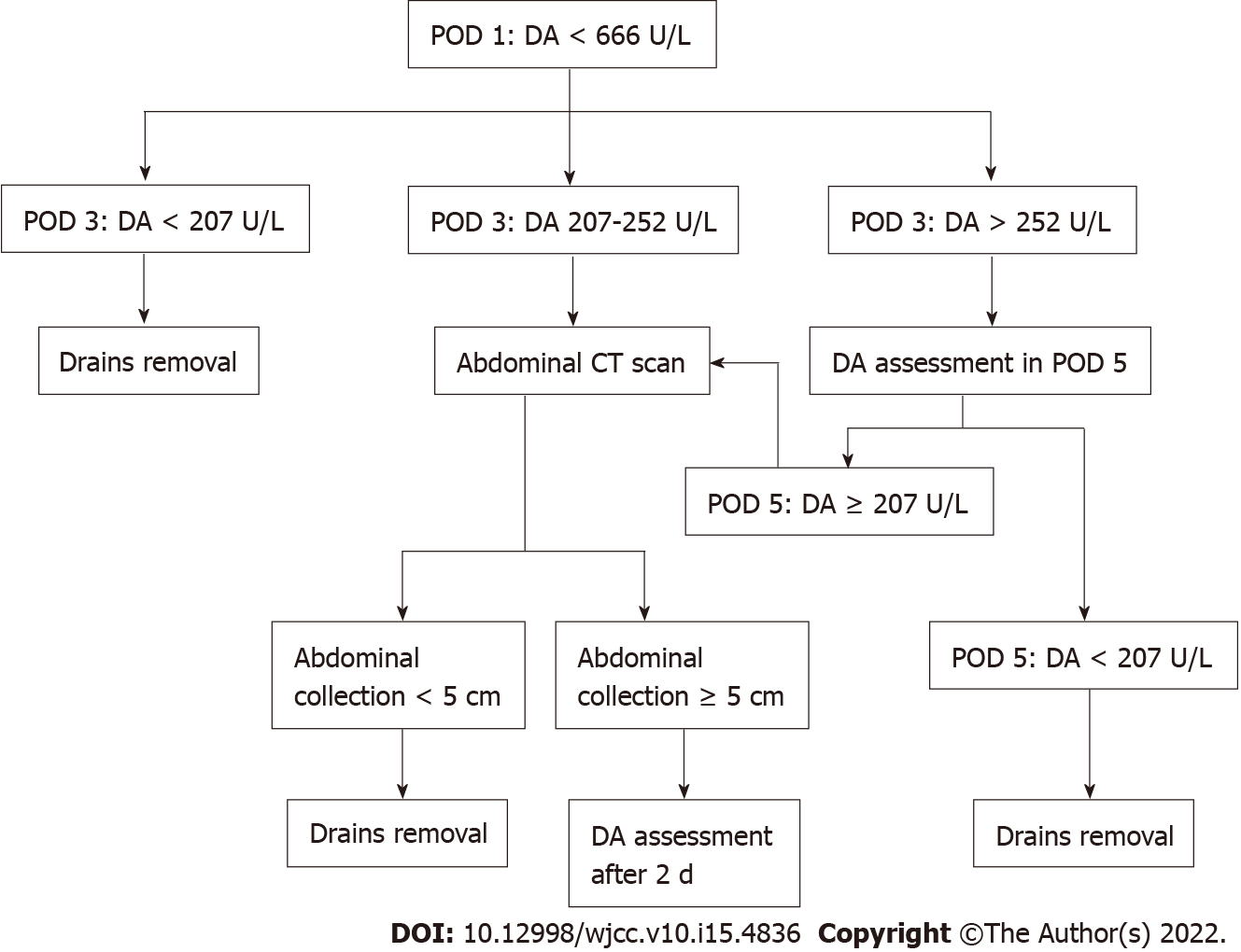
This prospective study could validate the role of DALs in the management of surgical drains and in assessing the risk or relevant complications after PD. Drains could be removed in POD3 in case of POD1 DALs < 666 U/L and POD3 DALs < 207 U/L. In case of POD3 DALs ≥ 252, abdominal CT scan will be performed in POD3 to identify abdominal collections ≥ 5 cm. In this latter category of patients, drains could be maintained beyond POD3.
The results of this trial will contribute to a better knowledge of POPF and management of surgical drains.
Core Tip: Nowadays, postoperative pancreatic fistula (POPF) is the most dreadful complication after pancreatic surgery. POPF can lead to severe postoperative complications such as surgical site infections, sepsis and bleeding. The DALCUT trial is an interventional prospective study with the aim to validate cut-offs of the drains amylase levels in postoperative day (POD) 1 and POD3, found during the previous study, to assess the risk of clinically relevant POPF and confirm the usefulness of abdomen computerized tomography scan on POD3 in patients at increased risk of postoperative abdominal collections.
- Citation: Caputo D, Coppola A, La Vaccara V, Passa R, Carbone L, Ciccozzi M, Angeletti S, Coppola R. Validations of new cut-offs for surgical drains management and use of computerized tomography scan after pancreatoduodenectomy: The DALCUT trial. World J Clin Cases 2022; 10(15): 4836-4842
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4836.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4836
Pancreatoduodenectomy (PD) represents the standard of care for periampullary malignancies[1-2]. Postoperative pancreatic fistula (POPF) remains the main complication following pancreatic surgery, even in hospitals with wide experience[3-6]. POPF can lead to other, even lethal, complications as site infections, sepsis, and hemorrhage. Moreover, the management of POPF could increase hospital costs due to prolonged hospitalization[7-9].
The International Study Group on Pancreatic Fistula described POPF as “the leak from a surgical or percutaneously positioned postoperative drains of any measurable quantity of fluid, starting from the postoperative day (POD) 3, with an amylase content three times higher than the upper normal limit of serum amylases”[10]. Therefore, according to this definition, patients could be affected by POPF even without any signs or symptoms. To overcome this issue, a three grades classification system of POPF has been recently introduced. This classification is based on the clinical impact of POPF. A: A biochemical fistula, also called a biochemical leak; it does not require intervention and does not affect post-operative length of stay (LOS). B: LOS increases, drains are not removed, additional radiological drains placement, bleeding control, antibiotics and artificial feeding are needed. C: A re-surgery is necessary and the patient’s death may occur. Grade B and C amount for the so-called clinically relevant POPF (CR-POPF). Therefore, it is clear that the correct grading of pancreatic fistula can only be assessed afterward.
Nonetheless, taking into account the impact of POPF, it is crucial to assess risk factors and define tools to predict the risk of CR-POPF in order to plan better management before irreversible complications occur. Postoperative drains amylase levels (DALs), at different cut offs and on different postoperative days have been identified and proposed as the main predictive factor of POPF[11-14]. On the other hand, several authors assume that abdominal drains themselves can lead to the development of POPF and of other complications[15-17].
Therefore, it is clear that drains can be useful but they should be removed as soon as possible. On this basis, Molinari et al[18] demonstrated that a POD1 DALs < 5000 IU/L identifies a subgroup of patients, at lower risk of POPF, in which the maintenance of drains is useless. However, in Molinari et al[18]’s work patients with grade A POPF were also considered. Ven Fong et al[19] identified a subgroup of patients at higher risk of POPF after PD in presence of POD1 DALs > 600 IU/L. On this basis, the author proposed the immediate removal of abdominal drains in low-risk patients.
One of the most consistent biases of the study of Ven Fong et al[19] is the use of extracorporeal Wirsung drainage. Recently, Seykora et al[20] demonstrated in PD patients how DALs at different cutoffs in POD1, POD3 and POD5 can predict CR-POPF risk and change surgical drains management. However, in the Seykora et al[20]’s study, the cut-offs have been found according to their negative predictive value and not the positive one.
Recently, at the University Campus Bio-Medico di Roma, Caputo et al[4] confirmed that the drains amylase level represents a significant predictor of POPF and, according to Seykora et al[20], that the management of abdominal drains has to be considered as a dynamic process mainly conditioned by DALs in POD1 and POD3[3].
Furthermore, in the study of Caputo et al[4], DALs > 666 IU/L in POD1 and DALs > 252 IU/L in POD3 predicted more than 80% of the CR-POPF. Nonetheless, it has also been shown that POD3 DALs > 207 IU/L and abdominal collections ≥ 5 cm, detected at the abdominal computerized tomography (CT) scan performed on POD3, was significantly related to the risk of a biliary fistula. According to Koch et al[21], who reported that biliary collections or biliary peritonitis can be defined by the need for radiological drainage or surgery, regardless of the bilirubin concentration in the drains. It is our opinion that if further confirmed, POD3 DALs could also be useful to identify patients at higher risk of biliary fistula. This prospective multicenter study protocol has been designed on the basis of a previously reported experience with the aim to validate the practice of maintaining in place the drains up to POD3 and manage their removal on the basis of specific DALs cut-offs. Whenever DALs are < 666 U/L and < 207 U/L, respectively in POD1 and POD3, drains could be removed in POD3. In presence of the POD3 DALs ≥ 207 U/L and < 252 U/L, due to the risk of biliary fistula in the presence of abdominal collection ≥ 5 cm, an abdominal CT scan on the same day will be performed in order to detect this finding. In these cases, drains could be maintained beyond POD3.
This is a prospective study reviewed and approved by the Ethics Committee of the University Campus Bio-Medico of Rome on February 26th, 2020. This study began in March 2020. The length of the study will be approximately 24 mo. The Protocol was registered at the Clinical Trial.Gov Registry (Registration number NCT04380506).
All patients who will undergo an open or laparoscopic PD for periampullary tumors will be considered eligible for the study if meeting the inclusion criteria reported below. After the clinical staging, patients will undergo routine preoperative evaluation. After the PD, daily DALs will be evaluated from POD1. Drains removal and the need for abdominal CT scans in POD3 will be decided on the basis of the results of DALs in the first three postoperative days as shown in Figure 1.
Each patient will be provided with an information sheet, which summarizes the purpose, targets and methods of the study. Each patient will also sign a standard consent for surgery and a specific consent to be enrolled in the study.
All data will be stored, in compliance with local privacy laws, within the CRF data collection sheet. The CRF will be filled at the time of the enrollment of the patient in the study and will contain the following sections.
Personal data: The patient’s personal data will be collected. The date of the patient’s enrollment will coincide with the date of acquisition of the written informed consent. Every patient will be identified by a unique alphanumeric code consisting of 8 characters. The first two will be two letters obtained by the initial of the name and surname of the patient, respectively (in case of composed first name and/or surname only the first initial will be used [e.g., Mario Rossi (MR), Mario Del Principe (MD)]; the last six characters will identify the patient’s date of birth, the first two digits will be the day of the month, the second two will be the month, and the last two will be the last two digits of the year of birth (in case of day and/or month of the year consisting of a single digit, a zero will be inserted; e.g., for date of birth January 2, 1965 the code will be 020165).
Work up: The patient’s demographic and clinical characteristics (age, sex, body mass index, comorbidity, previous oncological treatments, basic pathologies), laboratory tests, instrumental examination findings resulted from disease staging work-up (e.g., ultrasound, CT scan, magnetic resonance imaging, US-endoscopy, endoscopic retrograde cholangiopancreatography, etc.) and intraoperative data (pancreas texture, diameter of the main pancreatic duct, type of reconstructions, blood loss, etc.) will be reported in this section.
Postoperative course data: For each patient, qualitative and quantitative characteristics of abdominal drains fluid and amylase levels will be registered. Complications that resulted from placement of drains, diagnostic tests and their management will be recorded.
Hospital readmission and mortality data: For each patient, any hospital readmission will be considered within the first 90 postoperative days. In this section, the reason of re-hospitalization and adopted diagnostic and therapeutic measures will be recorded. This section will also record data regarding mortality in the first 90 postoperative days.
Surgical specimen data: For each patient enrolled, the outcome of the pathological staging and the state of the resection margins will be recorded.
Primary endpoint: To validate the cut-offs of DALs established during the previous study[4], and to identify patients with higher risk of CR-POPF and confirm the usefulness of abdominal CT scan on POD3 in patients at higher risk of abdominal collection.
Secondary endpoints: To assess the prevalence of POPF on the basis of early or late removal of abdominal drains according to DALs and radiological findings of CT in POD3, if performed; to identify predictive factors of POPF and calculate a score that can be applied for early diagnosis of POPF.
Sample size calculation: For a power of 80% and an alpha error of 5%, a total of 165 patients have been estimated as needed for structuring this study. The length of the study will be approximately 24 mo (the time required for the enrollment and statistical analysis).
Statistical analysis plan: Data will be analyzed using the Med-Calc 18.11.3 statistical package (MedCalc software, Mariakerke, Belgium). The Shapiro-Wilk test for normal distribution will be used to evaluate if data follows the normal distribution. Descriptive statistics will include mean, standard deviation, minimum, median, maximum, and quartiles for continuous data, as well as, absolute and relative frequencies for categorical data. Possible differences between patients with and without postoperative complications will be calculated using the Wilcoxon rank-sum test. Multivariable binary logistic regression will be used to identify possible risk factors of complications. The significance level will be set at P ≤ 0.05, representing a 95% confidence interval.
Participation in the study is voluntary. The protocol has been approved by the Institutional Review Board of University Campus Bio-Medico di Roma (Prot.: 24/20 PAR ComEt CBM). All subjects will be informed about the aim and procedures of the study and sign an informed consent. Patients’ data will be secured by medical confidentiality. All data will be coded and statistically examined. Third parties will have no access to original patient records. Subjects can quit DALCUT any time. Decision to draw someone from the protocol, because of mentioned exclusion criteria, will be made by the certified board surgeon.
This prospective study could validate the role of DALs in the management of surgical drains and in assessing the risk or relevant complications after PD. Drains could be removed in POD3 in case of POD1 DALs < 666 U/L and POD3 DALs < 207 U/L. In case of POD3 DALs ≥ 252, abdominal CT scan will be performed in POD3 to identify abdominal collections ≥ 5 cm. In this latter category of patients, drains could be maintained beyond POD3.
DALCUT is the first clinical trial designed to validate DALs cutoffs during an earlier study to recognize patients at higher risk of CR-POPF and to confirm the use of abdominal CT scans in POD3. In the DALCUT trial, drains removal would be allowed in POD3 if DALs < 666 U/L and < 207 U/L, respectively in POD1 and POD3 are detected. POD3 DALs ≥ 207 would represent an indication for the use of abdominal CT scans on the same day in order to detect abdominal collections ≥ 5 cm. In the presence of POD3 DALs ≥ 207 U/L and < 252 U/L, due to the risk of biliary fistula in presence of abdominal collection ≥ 5 cm, an abdominal CT scan on the same day will be performed in order to detect this finding. In these cases, drains could be maintained beyond POD3.
This study may also identify predictive factors of POPF with the opportunity of calculating a score that can be applied in the early diagnosis of POPF.
Postoperative pancreatic fistula (POPF) still remains the main complication after pancreatic surgery as it can lead to several and even life-threatening postoperative complications (e.g., surgical site infections, sepsis and bleeding).
A previous study allowed to identify cut-offs of drains amylase levels (DALs) determined on postoperative day (POD) 1 and POD3, able to significantly predict POPF, abdominal collections and biliary leaks, when related to defined findings identified at the abdominal computerized tomography (CT) scan routinely executed on POD3.
The aim of this trial is to validate the cut-offs of DALs in POD1 and POD3, established during the previous study, evaluating the risk of clinically relevant POPF and confirm the usefulness of abdominal CT scan on POD3 in patients at increased risk of abdominal collection.
The DALCUT trial is an interventional prospective study. All patients who will undergo pancreatoduodenectomy (PD) for periampullary neoplasms will be considered eligible. All patients will receive clinical staging and, if eligible for surgery, will undergo routine preoperative evaluation. After the PD, daily DALs will be evaluated from POD1. Drains removal and possible requirement of abdominal CT scans in POD3 will be managed on the basis of the outcome of DALs in the first 3 PODs.
In POD3 drains removal is feasible in presence of levels of drains amylases < 666 U/L in POD1 and < 207 U/L in POD3. In case of POD3 DALs ≥ 252, abdominal CT scan will be performed in POD3 to identify abdominal collections ≥ 5 cm. In this latter category of patients, drains could be maintained beyond POD3.
This prospective study could validate the role of DALs in the management of surgical drains and in assessing the risk or relevant complications after PD.
The results of this trial will contribute to a better knowledge of POPF and management of surgical drains.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li HL, China; Tenreiro N, Portugal S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Wang JJ
| 1. | Grace PA, Pitt HA, Tompkins RK, DenBesten L, Longmire WP Jr. Decreased morbidity and mortality after pancreatoduodenectomy. Am J Surg. 1986;151:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 277] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 2. | Sarmiento JM, Nagomey DM, Sarr MG, Farnell MB. Periampullary cancers: are there differences? Surg Clin North Am. 2001;81:543-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Kamarajah SK, Abu Hilal M, White SA. Does center or surgeon volume influence adoption of minimally invasive versus open pancreatoduodenectomy? Surgery. 2021;169:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Caputo D, Angeletti S, Ciccozzi M, Cartillone M, Cascone C, La Vaccara V, Coppola A, Coppola R. Role of drain amylase levels assay and routinary postoperative day 3 abdominal CT scan in prevention of complications and management of surgical drains after pancreaticoduodenectomy. Updates Surg. 2020;72:727-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | de Wilde RF, Besselink MG, van der Tweel I, de Hingh IH, van Eijck CH, Dejong CH, Porte RJ, Gouma DJ, Busch OR, Molenaar IQ; Dutch Pancreatic Cancer Group. Impact of nationwide centralization of pancreaticoduodenectomy on hospital mortality. Br J Surg. 2012;99:404-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 247] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 6. | Pedrazzoli S. Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): A systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore). 2017;96:e6858. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 161] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 7. | Abbott DE, Tzeng CW, McMillan MT, Callery MP, Kent TS, Christein JD, Behrman SW, Schauer DP, Hanseman DJ, Eckman MH, Vollmer CM. Pancreas fistula risk prediction: implications for hospital costs and payments. HPB (Oxford). 2017;19:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Vollmer CM Jr. The economics of pancreas surgery. Surg Clin North Am. 2013;93:711-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Yuan F, Essaji Y, Belley-Cote EP, Gafni A, Latchupatula L, Ruo L, Serrano PE. Postoperative complications in elderly patients following pancreaticoduodenectomy lead to increased postoperative mortality and costs. A retrospective cohort study. Int J Surg. 2018;60:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2964] [Article Influence: 370.5] [Reference Citation Analysis (35)] |
| 11. | Chen BP, Bennett S, Bertens KA, Balaa FK, Martel G. Use and acceptance of the International Study Group for Pancreatic Fistula (ISGPF) definition and criteria in the surgical literature. HPB (Oxford). 2018;20:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Linnemann RJA, Patijn GA, van Rijssen LB, Besselink MG, Mungroop TH, de Hingh IH, Kazemier G, Festen S, de Jong KP, van Eijck CHJ, Scheepers JJG, van der Kolk M, Dulk MD, Bosscha K, Busch OR, Boerma D, van der Harst E, Nieuwenhuijs VB; Dutch Pancreatic Cancer Group. The role of abdominal drainage in pancreatic resection - A multicenter validation study for early drain removal. Pancreatology. 2019;19:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Caputo D, Coppola A, Cascone C, Angeletti S, Ciccozzi M, La Vaccara V, Coppola R. Preoperative systemic inflammatory biomarkers and postoperative day 1 drain amylase value predict grade C pancreatic fistula after pancreaticoduodenectomy. Ann Med Surg (Lond). 2020;57:56-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Davidson TB, Yaghoobi M, Davidson BR, Gurusamy KS. Amylase in drain fluid for the diagnosis of pancreatic leak in post-pancreatic resection. Cochrane Database Syst Rev. 2017;4:CD012009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, Merchant N, Brennan MF. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 2001;234:487-93; discussion 493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 383] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 16. | Kaminsky PM, Mezhir JJ. Intraperitoneal drainage after pancreatic resection: a review of the evidence. J Surg Res. 2013;184:925-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Mehta VV, Fisher SB, Maithel SK, Sarmiento JM, Staley CA, Kooby DA. Is it time to abandon routine operative drain use? J Am Coll Surg. 2013;216:635-42; discussion 642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 18. | Molinari E, Bassi C, Salvia R, Butturini G, Crippa S, Talamini G, Falconi M, Pederzoli P. Amylase value in drains after pancreatic resection as predictive factor of postoperative pancreatic fistula: results of a prospective study in 137 patients. Ann Surg. 2007;246:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 252] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Ven Fong Z, Correa-Gallego C, Ferrone CR, Veillette GR, Warshaw AL, Lillemoe KD, Fernández-del Castillo C. Early Drain Removal--The Middle Ground Between the Drain Versus No Drain Debate in Patients Undergoing Pancreaticoduodenectomy: A Prospective Validation Study. Ann Surg. 2015;262:378-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Seykora TF, Maggino L, Malleo G, Lee MK 4th, Roses R, Salvia R, Bassi C, Vollmer CM Jr. Evolving the Paradigm of Early Drain Removal Following Pancreatoduodenectomy. J Gastrointest Surg. 2019;23:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, Fan ST, Yokoyama Y, Crawford M, Makuuchi M, Christophi C, Banting S, Brooke-Smith M, Usatoff V, Nagino M, Maddern G, Hugh TJ, Vauthey JN, Greig P, Rees M, Nimura Y, Figueras J, DeMatteo RP, Büchler MW, Weitz J. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1413] [Article Influence: 100.9] [Reference Citation Analysis (0)] |