Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4799
Peer-review started: November 6, 2021
First decision: December 27, 2021
Revised: December 31, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 26, 2022
Processing time: 199 Days and 3.8 Hours
There is no suitable scoring system that can be used to predict mortality in children with acute paraquat intoxication (APP).
To optimize a predictive scoring system for mortality in children with APP.
A total of 113 children with APP from January 1, 2010 to January 1, 2020 were enrolled in this study. These patients were divided into survivors and non-survivors. We compared the clinical characteristics between the two groups and analyzed the independent prognostic risk factors. The survival rates of patients with different values of the pediatric critical illness score (PCIS) were assessed using kaplan-meier survival analysis. The best scoring system was established by using the area under the receiver operating characteristic curve analysis.
The overall mortality rate was 23.4%. All non-survivors died within 20 days; 48.1% (13/27) died within 3 days, and 70.3% (19/27) died within 7 days. Compared to survivors, the non-survivors were older, had higher white blood cell count, alanine aminotransferase (ALT), aspartate aminotransferase, serum creatinine, blood urea nitrogen, glucose, and pediatric early warning score, and had lower platelet count, albumin, Serum sodium (Na+) and PCIS. ALT and PCIS were the independent prognostic risk factors for children with APP. The survival rate of children classified as extremely critical patients (100%) was lower than that of children classified as critical (60%) or noncritical (6.7%) patients. The specificity of ALT was high (96.51%), but the sensitivity was low (59.26%). The sensitivity and specificity of ALT combined with PCIS were high, 92.59% and 87.21%, respectively. The difference in mortality was significantly higher for ALT combined with PCIS (area under the receiver operating characteristic: 0.937; 95%CI: 0.875-0.974; P < 0.05).
In our study, ALT and PCIS were independent prognostic risk factors for children with APP. ALT combined with PCIS is an optimal predictive mortality scoring system for children with APP.
Core Tip: The mortality rate of children with acute paraquat intoxication (APP) was high. Early and accurate prediction of mortality is very important for children with APP in clinical decision-making. However, there is no scoring system that can be used to predict the mortality of children with APP. In our study, we discovered that alanine aminotransferase (ALT) and the pediatric critical illness score (PCIS) were independent prognostic risk factors for children with APP. ALT combined with the PCIS is an optimal predictive mortality scoring system for children with APP.
- Citation: Song Y, Wang H, Tao YH. Risk factors and optimal predictive scoring system of mortality for children with acute paraquat poisoning. World J Clin Cases 2022; 10(15): 4799-4809
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4799.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4799
Paraquat (PQ) is a widely used herbicide worldwide. Since paraquat began to be used in agriculture in 1962, the number of patients with acute paraquat intoxication has gradually increased[1]. With the participation of reduced coenzyme II-cytochrome P450 reductase, xanthine oxidase and other enzymes, PQ produces a single cationic free radical PQ+ in vivo, and PQ+ rapidly reoxidizes into PQ2+. PQ2+ receives electrons from coenzyme II and generates superoxide anions, which then produce peroxynitrite by combining with nitric oxide free radicals. These highly reactive oxygen species and peroxynitrite lead to mitochondrial dysfunction and apoptosis through lipid peroxidation and the activation of nuclear factor-κB, which results in multiple organ damage[2-4]. In the absence of specific antidotes, the mortality rate in children with acute paraquat intoxication (APP) was 14.38%-63.6%[5-7]. Therefore, the early and accurate prediction of mortality is very important in clinical decision-making for children with APP.
At present, several scoring systems have been used to predict the mortality of adult patients with APP, such as the sequential organ failure assessment[8], severity index of paraquat poisoning (SIPP)[9], acute physiology and chronic health evaluation II[10], early warning score (EWS)[11], and modified EWS[12]. However, the predictive powers of the above scoring systems are different, and most importantly, they are unsuitable for children.
There is no scoring system that can be used to predict mortality in children with APP. Because of the simple calculation and available indices, the pediatric critical illness score (PCIS) and pediatric EWS (PEWS) are scoring tools widely used for critically ill children[13-16]. However, there is no report in which the PCIS and PEWS were used to predict the prognosis of children with APP. Our study aimed to investigate the performance of the PCIS, the PEWS, as well as a single clinical index for predicting mortality in children with APP and to provide a theoretical basis for clinical application.
We performed a single-center, retrospective, observational study, which was approved by the ethics committee of the West China Second University Hospital, Sichuan University.
Pediatric patients with APP enrolled in this study were < 18 years old and were admitted to our hospital between January 1, 2010 and January 1, 2020. The inclusion criteria were as follows: (1) A diagnosis of APP[17]; and (2) first visit to the hospital with no history of special treatment, such as gastric lavage and hemodialysis. The diagnostic criteria of APP were as follows: (1) The children or family members could provide the history of paraquat exposure; (2) for those who denied paraquat exposure, evidence was found to the contrary, including black–green residue on the skin, an empty paraquat bottle, vomiting, oral mucosal erosion with unknown causes; and (3) blood or urine was positive for paraquat. The exclusion criteria were as follows: (1) Complicated with chronic diseases; (2) other drug exposure; (3) death within 24 h of admission; and (4) discharge against medical advice.
Age, sex, time to blood purification and consultation and related symptoms (vomiting, abdominal pain, oral ulcer and gastrointestinal bleeding) were collected at admission. Routine laboratory tests, including routine blood tests, blood gas, liver function, renal function and electrolytes, were performed. The PCIS[18] (Table 1) and PEWS[19] (Table 2) were calculated within 24 h after admission. Patients with the score of > 80 were considered noncritical, 71-80 critical, and ≤ 70 extremely critical. All children were followed up for at least 90 days.
| Parameter | Age < 1 yr | Age ≥ 1 yr | Scores |
| Heart rate (beats/min) | < 80 or > 180 | < 60 or > 160 | 4 |
| 80-100 or 160-180 | 60-80 or 140-160 | 6 | |
| Others | Others | 10 | |
| Blood pressure (systolic) [kPa (mmHg)] | < 7.5 (55) or > 17.3 (130) | < 8.7 (65) or > 20 (150) | 4 |
| 7.5-8.7 (55-65) or 13.3-17.3 (100-130) | 8.7-10 (65-75) or 17.3-20 (130-150) | 6 | |
| Others | Others | 10 | |
| Breathing rate (breaths/min) | < 20 or > 70 or irregular breathing | < 15 or > 60 or irregular breathing | 4 |
| 20-25 or 40-70 | 15-20 or 35-60 | 6 | |
| Others | Others | 10 | |
| PaO2 [kPa (mmHg)] (no oxygen inhalation) | < 6.7 (50) | 4 | |
| 6.7-9.3 (50-70) | 6 | ||
| Others | 10 | ||
| pH | < 7.25 or > 7.55 | 4 | |
| 7.25-7.30 or 7.50-7.55 | 6 | ||
| Others | 10 | ||
| Na+ (mmol/L) | < 120 or > 160 | 4 | |
| 120-130 or 150-160 | 6 | ||
| Others | 10 | ||
| K + (mmol/L) | < 3.0 or > 6.5 | 4 | |
| 3.0-3.5 or 5.5-6.5 | 6 | ||
| Others | 10 | ||
| Scr (μmol/L) | > 159 | 4 | |
| 106-159 | 6 | ||
| Others | 10 | ||
| BUN (mmol/L) | > 14.3 | 4 | |
| 7.1-14.3 | 6 | ||
| Others | 10 | ||
| Hb (g/L) | < 60 | 4 | |
| 60-90 | 6 | ||
| Others | 10 | ||
| Digestive system symptoms | Stress ulcer bleeding and intestinal paralysis | 4 | |
| Stress ulcer bleeding | 6 | ||
| Others | 10 | ||
| 0 | 1 | 2 | 3 | |
| Behavior | Playing; alert; appropriate; at baseline | Sleeping; fussy but consolable | Irritable/inconsolable | Lethargic; confused; reduced response to pain |
| Cardiovascular status | Pink cap refill 1-2 s | Gray; cap refill 3 s | Gray; cap refill 4 s; tachycardia of 20 beats/min above the normal rate | Gray; mottled; cap refill 5 s; tachycardia of 30 beats/min above the normal rate; bradycardia |
| Respiratory status | Within normal parameters | Greater than 10 breaths/min above normal parameters; accessory muscle use; 30% FiO2; 3 liters/minute | Greater than 20 breaths/min above normal parameters; retractions; | Below normal parameters with retractions; grunting; 50% FiO2; 8 liters/minute |
Routine blood tests, liver function, renal function, electrolytes, random blood glucose and chest computed tomography tests were performed upon admission. Routine treatments (vomiting induction, oral activated carbon and diuresis) were adapted. Some critically ill children were administered methylprednisolone 15 mg/(kg/d) for 3 d. The patients with infective symptoms were given anti-infective drugs, and those with respiratory failure were given oxygen inhalation or mechanical ventilation. Some critically ill children were treated with hemoperfusion 3-5 times or plasma exchange 3-4 times. Hemodialysis or continuous renal replacement therapy was used for patients with multiple organ dysfunction.
All statistical analyses were conducted using IBM SPSS statistics version 21 (IBM Corp & licensors 1989, 2011). Continuous variables are presented as the mean ± SD or median (interquartile range) [mean (P25, P75)]. Categorical variables were expressed as percentages. The two groups were compared using student’s t-tests, chi-square tests, wilcoxon tests, and mann–whitney U tests. Multivariable logistic regression model was computed to identify whether variables were associated with unfavorable outcomes. The receiver operator characteristic [area under the receiver operating characteristic (AUROC)] curve was used to predict probability of mortality. We analyzed the survival rate of children with different PCIS by kaplan–meier survival analysis. P < 0.05 was considered statistically significant.
In total, 113 patients were included. During the 90-day follow-up, the overall mortality rate was 23.4% (27/113). All non-survivors died within 20 d; 48.1% (13/27) died within 3 d, and 70.3% (19/27) died within 7 d. The causes of poisoning were suicide (22.1%) and accidental ingestion (77.9%).
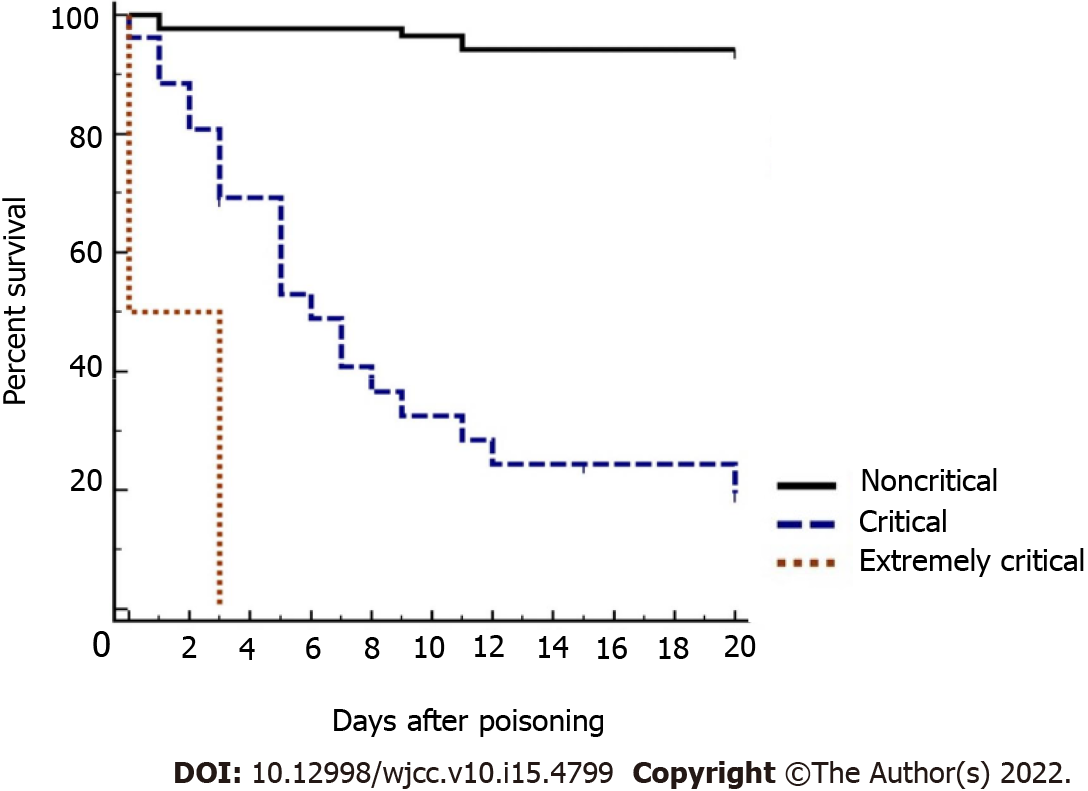
Among the 113 children, 96 (85%), 15 (13.3%) and 2 (1.8%) were categorized as noncritical (PCIS > 80 points), critical (PCIS 71-80 points) and extremely critical (PCIS ≤ 70 points), respectively, and the mortality rates were 16.7% (16/96), 60% (9/15), and 100% (2/2), respectively. As shown in Figure 1, the survival rate of children classified as extremely critical patients (100%) was significantly lower than that of children classified as critical (60%) or noncritical (6.7%) patients (P < 0.05).
Compared to survivors, the non-survivors were older (8.11 ± 4.72 vs. 11.48 ± 2.99 years); had higher white blood cell count, serum creatinine (Scr), blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase, glucose, and PEWS; and had lower platelet count, albumin, Serum sodium (Na+) and PCIS (Table 3) (all P < 0.05).
| Variable | Survivors | Non-survivors | P value |
| Age (yr), n (%) | 0.003 | ||
| ≤ 3 yr | 22 (25.6) | 0 (0.0) | |
| 3-6 yr (not including 3 yr) | 12 (13.4) | 3 (11.1) | |
| 6-12 yr (not including 6 yr) | 27 (31.2) | 10 (37.0) | |
| > 12 yr | 25 (29.1) | 14 (51.9) | |
| Sex, n (%) | 0.039 | ||
| Female | 41 (47.7) | 19 (70.4) | |
| Male | 45 (52.3) | 8 (29.6) | |
| Cause of intoxication, n (%) | 0.585 | ||
| Accidental | 68 (79.1) | 20 (74.1) | |
| Suicide | 18 (20.9) | 7 (25.9) | |
| Time to blood purification, n (%) | 0.185 | ||
| < 6 h | 3 (5.0) | 2 (8.7) | |
| 6-12 h | 12 (20.0) | 7 (30.4) | |
| 12-24 h (not including 12 h) | 13 (21.7) | 5 (21.7) | |
| > 24 h | 32 (53.3) | 9 (39.1) | |
| Time to consultation, n (%) | 0.191 | ||
| < 6 h | 28 (32.6) | 11 (40.7) | |
| 6-24 h | 22 (25.6) | 6 (22.2) | |
| > 24 h | 36 (41.9) | 10 (37.1) | |
| Vomiting, n (%) | 30 (34.9) | 19 (70.4) | 0.001 |
| Abdominal pain, n (%) | 14 (16.3) | 9 (33.3) | 0.055 |
| Oral ulcer, n (%) | 32 (37.2) | 16 (66.7) | 0.043 |
| Gastrointestinal bleeding, n (%) | 3 (3.5) | 5 (18.5) | 0.001 |
| WBC count (× 109/L) | 11.27 ± 4.57 | 15.45 ± 7.15 | 0.007 |
| PLT count (× 109/L) | 267.79 ± 102.52 | 222.48 ± 79.15 | 0.038 |
| ALT [M (P25, P75)] | 32.5 (25, 42) | 99 (41, 494) | < 0.001 |
| AST [M (P25, P75)] | 28 (20, 40) | 108 (38, 295) | < 0.001 |
| Albumin (g/L) | 44.34 ± 5.33 | 40.54 ± 6.23 | 0.002 |
| Glucose (mmol/L) | 6.60 ± 3.43 | 6.75 ± 1.31 | 0.805 |
| Na+ (mmol/L) | 137.04 ± 4.84 | 133.84 ± 6.49 | 0.024 |
| K+ (mmol/L) | 3.72 ± 0.58 | 3.70 ± 0.668 | 0.882 |
| Scr (mmol/L) | 74.17 ± 74.70 | 441.04 ± 267.86 | < 0.001 |
| BUN (mmol/L) | 7.62 ± 6.65 | 23.06 ± 14.99 | < 0.001 |
| PEWS, n (%) | < 0.001 | ||
| 0 | 66 (78.6) | 9 (33.3) | |
| 1 | 12 (14.0) | 9 (33.3) | |
| 2 | 5 (5.8) | 3 (11.1) | |
| 3 | 1 (1.1) | 3 (11.1) | |
| 4 | 1 (1.1) | 0 (0.0) | |
| ≥ 5 | 1 (1.1) | 3 (11.1) | |
| PCIS | 95.57 ± 6.33 | 83.85 ± 7.74 | < 0.001 |
| ≤ 70, n (%) | 0 (0.0) | 2 (7.4) | |
| 71-80, n (%) | 6 (69.8) | 9 (33.3) | |
| > 80, n (%) | 80 (93.0) | 16 (59.3) |
The median time to consultation in survivors and non-survivors was 22.5 (8.75, 48) and 20 (8, 48) hours, respectively. In addition, there was no significant difference between the survivors and non-survivors in the time to consultation < 6 h, 6-24 h, and > 24 h subgroups.
In order to explore the prognostic risk factors for children with APP, we selected variables with P < 0.1 in the univariate analysis to perform a multivariable logistic regression analysis. The indices of Scr, BUN, Na+, Serum potassium (K+), hemoglobin, abdominal pain, vomiting, and gastrointestinal bleeding were included in the PCIS system and were not introduced into the multivariable logistic regression analysis. ALT and PCIS were independent prognostic risk factors for those children with APP (P < 0.05) (Table 4).
| Variables | β Coefficient | SE | Odds ratio (95%CI) | P value |
| ALT | 0.024 | 0.010 | 1.024 (1.003-1.045) | 0.022 |
| PCIS | -0.151 | 0.046 | 0.860 (0.785-0.942) | 0.001 |
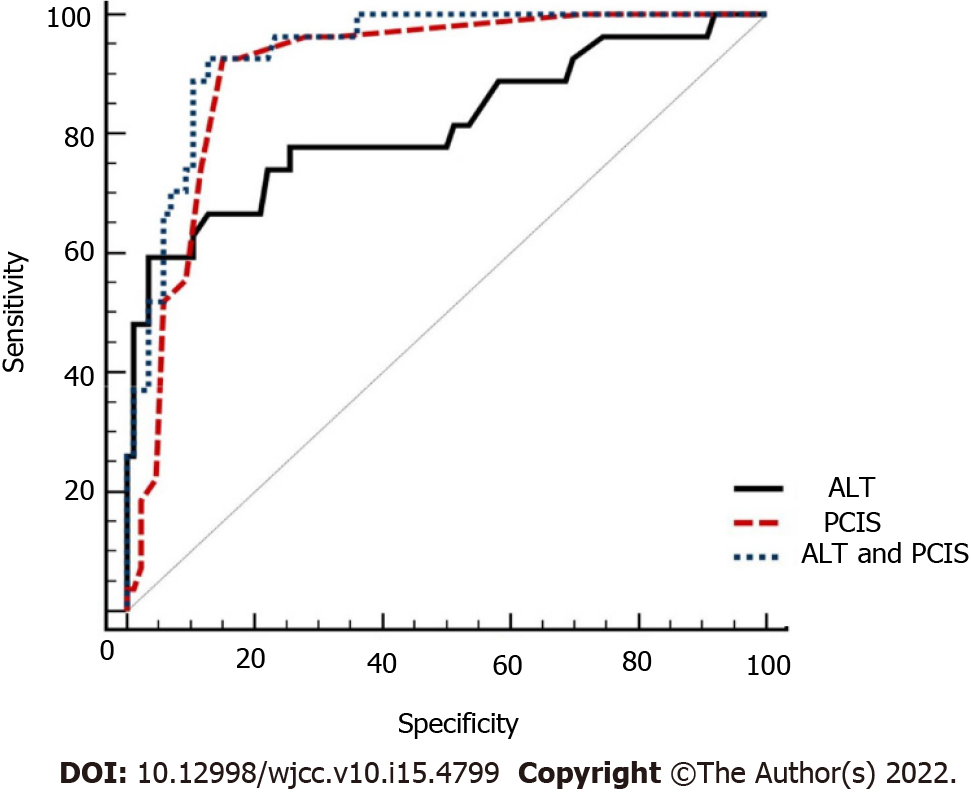
Because the multiple logistic regression analysis revealed that ALT and PCIS were independent prognostic risk factors for children with APP, we further analyzed the predictive performance of ALT, PCIS and ALT combined with PCIS for mortality in children with APP. Table 5 and Figure 2 show the predictive power of ALT, PCIS and ALT combined with PCIS. The specificity of ALT was high (96.51%), but its sensitivity was low (59.26%). The sensitivity and specificity of PCIS and ALT combined with PCIS were high, 92.30% vs. 92.59% and 82.21% vs. 87.21%, respectively. The difference in-outcomes was significantly higher for ALT combined with PCIS (AUROC: 0.937; 95%CI: 0.875-0.974) than for PCIS (AUROC: 0.905; 95%CI: 0.836-0.952) and ALT (AUROC: 0.814; 95%CI: 0.730-0.881) (all P < 0.05). Thus, ALT combined with the PCIS was the optimal scoring system.
| Predictive factors | Sensitivity (%) | Specificity (%) | AUROC (95%CI) | Youden index | P value |
| ALT | 59.26 | 96.51 | 0.814 (0.730-0.881) | 0.557 | < 0.001 |
| PCIS | 92.30 | 82.21 | 0.905 (0.836-0.952) | 0.774 | < 0.001 |
| ALT and PCIS | 92.59 | 87.21 | 0.937 (0.875-0.974) | 0.798 | < 0.001 |
The early prediction of mortality is important in clinical decision-making for patients with APP. Previous studies have indicated that plasma paraquat concentration can effectively predict the mortality of patients with APP[20,21]. However, Gil et al[21] found that some patients with low paraquat concentration in plasma still had poor outcomes. The reasons are as follows. The plasma paraquat concentration reached a peak value within 0.5-2 h after ingestion[22], and the half-life is 5 h[23]. The concentration of paraquat in plasma decreased obviously in the early stage, and the survival expectations would decrease from 70% to 30% for a delay of 1 h[23]. The time of detecting paraquat concentration in plasma had an impact on the accuracy of paraquat measurements. In addition, most children with APP accidentally ingested paraquat; therefore, clinicians cannot estimate the dose of paraquat they ingested. Paraquat measurement was unavailable in almost all primary hospitals. Therefore, the paraquat concentration in plasma was not included in the prognostic risk factor analysis of children with APP. In addition, clinical indices such as serum lactic acid[24], K[25], blood amylase[26] and peripheral blood monocyte count[27] were considered to be effective in predicting the prognosis of patients with APP. Paraquat binds to plasma protein after entering the bloodstream and is quickly distributed to many organs, resulting in multiple organ damage[28]. Therefore, using a single index to predict the mortality of APP patients is inaccurate, while the combination of multiple indicators is more comprehensive and reasonable[29].
The poisoning severity score[30,31] and pediatric logistic organ dysfunction (PELOD) score[32] can be used to predict the prognosis of children with APP. Nevertheless, complex calculations and the availability of indices (such as PO2/FiO2) in the general ward limit their application in clinical work. In addition, the SIPP[9] and clinical classification of APP[33] have good predictive ability for the prognosis of children with APP. Due to the unavailability of ingestion doses and paraquat concentrations, the clinical classification of APP and SIPP was not adopted in this study.
The EWS is widely used in adult patients. However, the vital signs and physiological indices of children of different ages vary widely, and there is a long compensation period before rapid deterioration due to disease. Therefore, the PEWS was established according to the physiological and pathological characteristics of children. The PEWS includes three parts: Consciousness, respiratory status and cardiovascular status. The PEWS is easy to administer and calculate. Nevertheless, multivariate logistic analysis suggested that the PEWS was not an independent risk factor for mortality in children with APP; therefore, the PEWS cannot be used to predict mortality in children with APP. The reasons are as follows: (1) The PEWS comprises physical signs and a few parameters but no objective laboratory indices; and (2) repeated evaluations by doctors and nurses and the anxiety and panic of parents may affect the vital signs of the children, resulting in a decrease in the predictive efficacy of the PEWS[34].
PCIS comprises heart rate, blood pressure, breathing rate, PaO2, pondus hydrogenii, Na+, K+, BUN/Scr, hemoglobin, and digestive system symptoms, and the PCIS system can evaluate the condition of children with APP more comprehensively and objectively than the PEWS system. PCIS is widely used to evaluate the severity and prognosis of critically ill children[13,15]. The PCIS system was first used to predict the prognosis of children with APP, as shown in our study. Our study showed that the survival rate of children classified as extremely critical patients (PCIS ≤ 70 points) was significantly higher than that of children classified as critical (PCIS 71-80 points) and noncritical (PCIS > 80 points) patients. In addition, the AUROC of PCIS was 0.905 (95%CI: 0.836-0.952), with a high sensitivity of 92.3% and a high specificity of 82.21%. A recent study showed that there was no significant difference among the AUROC curves for PCIS, PRISM IV and PELOD-2 in predicting the prognosis of children in pediatric intensive care units[35]. Thus, PCIS can be used to predict the prognosis of children with APP.
The liver is the main source of endogenous antioxidants and plays an important role in metabolism and detoxification. The liver is considered to be the main target of exogenous organism-mediated oxidative damage[36]. Oxidative damage to the liver was observed in rats after a single oral administration of 150 mg/kg paraquat for 20 h[37]. Yang et al[38] found that 46.52% of paraquat patients suffered from hepatic complications. Metabolic disorders or insufficient antioxidants induced by liver injury are associated with poor outcome after paraquat intoxication. In our study, the multiple regression analysis revealed that ALT was an independent risk factor for mortality in children with APP. The AUROC of ALT was 0.814 (95%CI: 0.730-0.881), the sensitivity was 96.51%, and the specificity was 51.26%. In addition, Zhang et al[39] found that ALT and BUN reflect organ injuries and paraquat excretion capability of patients, and the two parameters are negatively correlated with the urine-to-plasma paraquat ratio. Therefore, we combined ALT with the original PCIS system and evaluated its predictive ability for mortality in children with APP. Our study showed that ALT combined with PCIS had optimal predictive ability, with the AUROC of 0.921 (95%CI: 0.854-0.963) and a high sensitivity of 87.21% and specificity of 92.59%. It is suggested that ALT combined with PCIS has a good predictive ability for mortality in children with APP.
The early and accurate prediction of mortality can help clinicians with clinical decision-making and treatment of children with acute paraquat intoxication. However, there are few articles about acute paraquat poisoning in children, and there is no suitable scoring system that can be used to predict the mortality of these patients. In this study, we studied the risk factors and optimal predictive scoring system of mortality for children with acute paraquat poisoning. To date, this is the first article on the predictive score of acute paraquat poisoning in children. Of course, a limited number of children with APP were included in our study. The results of this study need to be further verified by large-sample and multicenter research.
The mortality rate in children with APP was high. ALT and PCIS were independent prognostic risk factors for children with APP. ALT combined with PCIS is an optimal predictive scoring system for mortality in children with APP.
The mortality rate in children with acute paraquat intoxication (APP) was 14.38%-63.6%, the early and accurate prediction of mortality is very important in clinical decision-making for children with APP.
The mortality rate in children with APP was high. The early prediction of mortality is important in clinical decision-making for patients with APP. Therefore, our aim is to optimize a predictive scoring system for mortality in children with APP.
Our aim is to optimize a predictive scoring system for mortality in children with APP, and help doctors to make clinical decisions.
We compared the clinical characteristics between the two groups and analyzed the independent prognostic risk factors. The survival rates were assessed using kaplan-meier survival analysis. The best scoring system was established by using the area under the receiver operating characteristic curve analysis.
Alanine aminotransferase (ALT) and pediatric critical illness score (PCIS) were independent prognostic risk factors for children with APP. The survival rate of children classified as extremely critical patients was significantly lower than that of children classified as critical or noncritical patients. The sensitivity and specificity of ALT combined with PCIS were high.
ALT and PCIS were independent prognostic risk factors for children with APP. ALT combined with PCIS is an optimal predictive mortality scoring system for children with APP.
The results of this study need to be further verified by large-sample and multicenter research.
We thank the doctors at the Department of pediatrics of West China Second University Hospital for their help with data collection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Pediatrics
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bianchi F, Spain; Wierzbicka A, Poland S-Editor: Guo XR L-Editor: A P-Editor: Guo XR
| 1. | Lee WJ, Cha ES, Park ES, Kong KA, Yi JH, Son M. Deaths from pesticide poisoning in South Korea: trends over 10 years. Int Arch Occup Environ Health. 2009;82:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | Gil HW, Hong JR, Jang SH, Hong SY. Diagnostic and therapeutic approach for acute paraquat intoxication. J Korean Med Sci. 2014;29:1441-1449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Xu L, Xu J, Wang Z. Molecular mechanisms of paraquat-induced acute lung injury: a current review. Drug Chem Toxicol. 2014;37:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 4. | Sun B, Chen YG. Advances in the mechanism of paraquat-induced pulmonary injury. Eur Rev Med Pharmacol Sci. 2016;20:1597-1602. [PubMed] |
| 5. | Lee EY, Hwang KY, Yang JO, Hong SY. Predictors of survival after acute paraquat poisoning. Toxicol Ind Health. 2002;18:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Hsieh YW, Lin JL, Lee SY, Weng CH, Yang HY, Liu SH, Wang IK, Liang CC, Chang CT, Yen TH. Paraquat poisoning in pediatric patients. Pediatr Emerg Care. 2013;29:487-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Elenga N, Merlin C, Le Guern R, Kom-Tchameni R, Ducrot YM, Pradier M, Ntab B, Dinh-Van KA, Sobesky M, Mathieu D, Dueymes JM, Egmann G, Kallel H, Mathieu-Nolf M. Clinical features and prognosis of paraquat poisoning in French Guiana: A review of 62 cases. Medicine (Baltimore). 2018;97:e9621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Sawada Y, Yamamoto I, Hirokane T, Nagai Y, Satoh Y, Ueyama M. Severity index of paraquat poisoning. Lancet. 1988;1:1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Xu S, Hu H, Jiang Z, Tang S, Zhou Y, Sheng J, Chen J, Cao Y. APACHE score, Severity Index of Paraquat Poisoning, and serum lactic acid concentration in the prognosis of paraquat poisoning of Chinese Patients. Pediatr Emerg Care. 2015;31:117-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Huang NC, Hung YM, Lin SL, Wann SR, Hsu CW, Ger LP, Hung SY, Chung HM, Yeh JH. Further evidence of the usefulness of Acute Physiology and Chronic Health Evaluation II scoring system in acute paraquat poisoning. Clin Toxicol (Phila). 2006;44:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Siddiqui S, Chua M, Kumaresh V, Choo R. A comparison of pre ICU admission SIRS, EWS and q SOFA scores for predicting mortality and length of stay in ICU. J Crit Care. 2017;41:191-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Mitsunaga T, Hasegawa I, Uzura M, Okuno K, Otani K, Ohtaki Y, Sekine A, Takeda S. Comparison of the National Early Warning Score (NEWS) and the Modified Early Warning Score (MEWS) for predicting admission and in-hospital mortality in elderly patients in the pre-hospital setting and in the emergency department. PeerJ. 2019;7:e6947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Yang YW, Wu CH, Ko WJ, Wu VC, Chen JS, Chou NK, Lai HS. Prevalence of acute kidney injury and prognostic significance in patients with acute myocarditis. PLoS One. 2012;7:e48055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Piano S, Rosi S, Maresio G, Fasolato S, Cavallin M, Romano A, Morando F, Gola E, Frigo AC, Gatta A, Angeli P. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 15. | Sampaio MC, Máximo CA, Montenegro CM, Mota DM, Fernandes TR, Bianco AC, Amodeo C, Cordeiro AC. Comparison of diagnostic criteria for acute kidney injury in cardiac surgery. Arq Bras Cardiol. 2013;101:18-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Benediktsson S, Frigyesi A, Kander T. Routine coagulation tests on ICU admission are associated with mortality in sepsis: an observational study. Acta Anaesthesiol Scand. 2017;61:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Fengjun J, Wen Z, Taoning W, Yaying Y, Kai K, Liu M. Analysis of risk factors for prognosis of patients with acute paraquat intoxication. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2015;27:906-910. [PubMed] |
| 18. | An K, Wang Y, Li B, Luo C, Wang J, Chen J. Prognostic factors and outcome of patients undergoing hematopoietic stem cell transplantation who are admitted to pediatric intensive care unit. BMC Pediatr. 2016;16:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Monaghan A. Detecting and managing deterioration in children. Paediatr Nurs. 2005;17:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 81] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Proudfoot AT, Stewart MS, Levitt T, Widdop B. Paraquat poisoning: significance of plasma-paraquat concentrations. Lancet. 1979;2:330-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 230] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Gil HW, Kang MS, Yang JO, Lee EY, Hong SY. Association between plasma paraquat level and outcome of paraquat poisoning in 375 paraquat poisoning patients. Clin Toxicol (Phila). 2008;46:515-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Pond SM, Rivory LP, Hampson EC, Roberts MS. Kinetics of toxic doses of paraquat and the effects of hemoperfusion in the dog. J Toxicol Clin Toxicol. 1993;31:229-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Novaes RD, Gonçalves RV, Marques DC, Cupertino Mdo C, Peluzio Mdo C, Leite JP, Maldonado IR. Effect of bark extract of Bathysa cuspidata on hepatic oxidative damage and blood glucose kinetics in rats exposed to paraquat. Toxicol Pathol. 2012;40:62-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Liu XW, Ma T, Li LL, Qu B, Liu Z. Predictive values of urine paraquat concentration, dose of poison, arterial blood lactate and APACHE II score in the prognosis of patients with acute paraquat poisoning. Exp Ther Med. 2017;14:79-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Liu ZQ, Wang HS, Gu Y. Hypokalemia is a biochemical signal of poor prognosis for acute paraquat poisoning within 4 hours. Intern Emerg Med. 2017;12:837-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Huang C, Bai L, Xue X, Peng L, Jiang J, Zhang X. Hyperamylasemia as an early predictor of mortality in patients with acute paraquat poisoning. J Int Med Res. 2020;48:300060520910037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Zhao Y, Song YQ, Gao J, Feng SY, Li Y. Monocytes as an Early Predictor for Patients with Acute Paraquat Poisoning: A Retrospective Analysis. Biomed Res Int. 2019;2019:6360459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Minakata K, Suzuki O, Horio F, Saito S, Harada N. Increase in production of ascorbate radical in tissues of rat treated with paraquat. Free Radic Res. 2000;33:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Hu L, Li H, Cai Z, Lin F, Hong G, Chen H, Lu Z. A new machine-learning method to prognosticate paraquat poisoned patients by combining coagulation, liver, and kidney indices. PLoS One. 2017;12:e0186427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Chartier C, Penouil F, Blanc-Brisset I, Pion C, Descatha A, Deguigne M. Pediatric cannabis poisonings in France: more and more frequent and severe. Clin Toxicol (Phila). 2021;59:326-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | von Fabeck K, Boulamery A, Glaizal M, de Haro L, Simon N. Buprenorphine poisoning in children: a 10-year-experience of Marseille Poison Center. Fundam Clin Pharmacol. 2020;34:265-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Duan Y, Wang Z. To explore the characteristics of fatality in children poisoned by paraquat--with analysis of 146 cases. Int J Artif Organs. 2016;39:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 586] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 34. | Brown SR, Martinez Garcia D, Agulnik A. Scoping Review of Pediatric Early Warning Systems (PEWS) in Resource-Limited and Humanitarian Settings. Front Pediatr. 2018;6:410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Zhang Z, Huang X, Wang Y, Li Y, Miao H, Zhang C, Pan G, Zhang Y, Zhu X, Chen W, Li J, Su D, Bi Y, Chen Z, Jin B, Kong X, Cheng Y, Chen Y, Yan G, Yan W, Lu G. Performance of Three Mortality Prediction Scores and Evaluation of Important Determinants in Eight Pediatric Intensive Care Units in China. Front Pediatr. 2020;8:522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Zeinvand-Lorestani H, Nili-Ahmadabadi A, Balak F, Hasanzadeh G, Sabzevari O. Protective role of thymoquinone against paraquat-induced hepatotoxicity in mice. Pestic Biochem Physiol. 2018;148:16-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Konstantinova SG, Russanov EM. Studies on paraquat-induced oxidative stress in rat liver. Acta Physiol Pharmacol Bulg. 1999;24:107-111. [PubMed] |
| 38. | Yang CJ, Lin JL, Lin-Tan DT, Weng CH, Hsu CW, Lee SY, Lee SH, Chang CM, Lin WR, Yen TH. Spectrum of toxic hepatitis following intentional paraquat ingestion: analysis of 187 cases. Liver Int. 2012;32:1400-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 39. | Zhang S, Song S, Luo X, Liu J, Liu M, Li W, Cao T, Li N, Zeng C, Zhang B, Cai H. Prognostic value of liver and kidney function parameters and their correlation with the ratio of urine-to-plasma paraquat in patients with paraquat poisoning. Basic Clin Pharmacol Toxicol. 2021;128:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |