Published online May 26, 2022. doi: 10.12998/wjcc.v10.i15.4717
Peer-review started: November 1, 2021
First decision: December 26, 2021
Revised: January 10, 2022
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 26, 2022
Processing time: 203 Days and 23.2 Hours
Patients with chronic liver diseases (CLDs) develop acute liver injury and/or acute decompensation under the attack of various precipitants and present with significantly elevated alanine aminotransferase and/or total bilirubin levels, liver failure, or acute decompensation of liver cirrhosis, which is called acute-on-CLD (AoCLD). AoCLD accounts for the majority of patients hospitalized in the Department of Hepatology or Infectious Diseases. AoCLD is complicated by various clinical types, the severity of the disease, and may pose a high risk of death. To date, the definition of AoCLD is still vague, and a consensus concept of the clinical classification is lacking. This review aimed to define the concept and clinical types of AoCLD based on related studies and the literature.
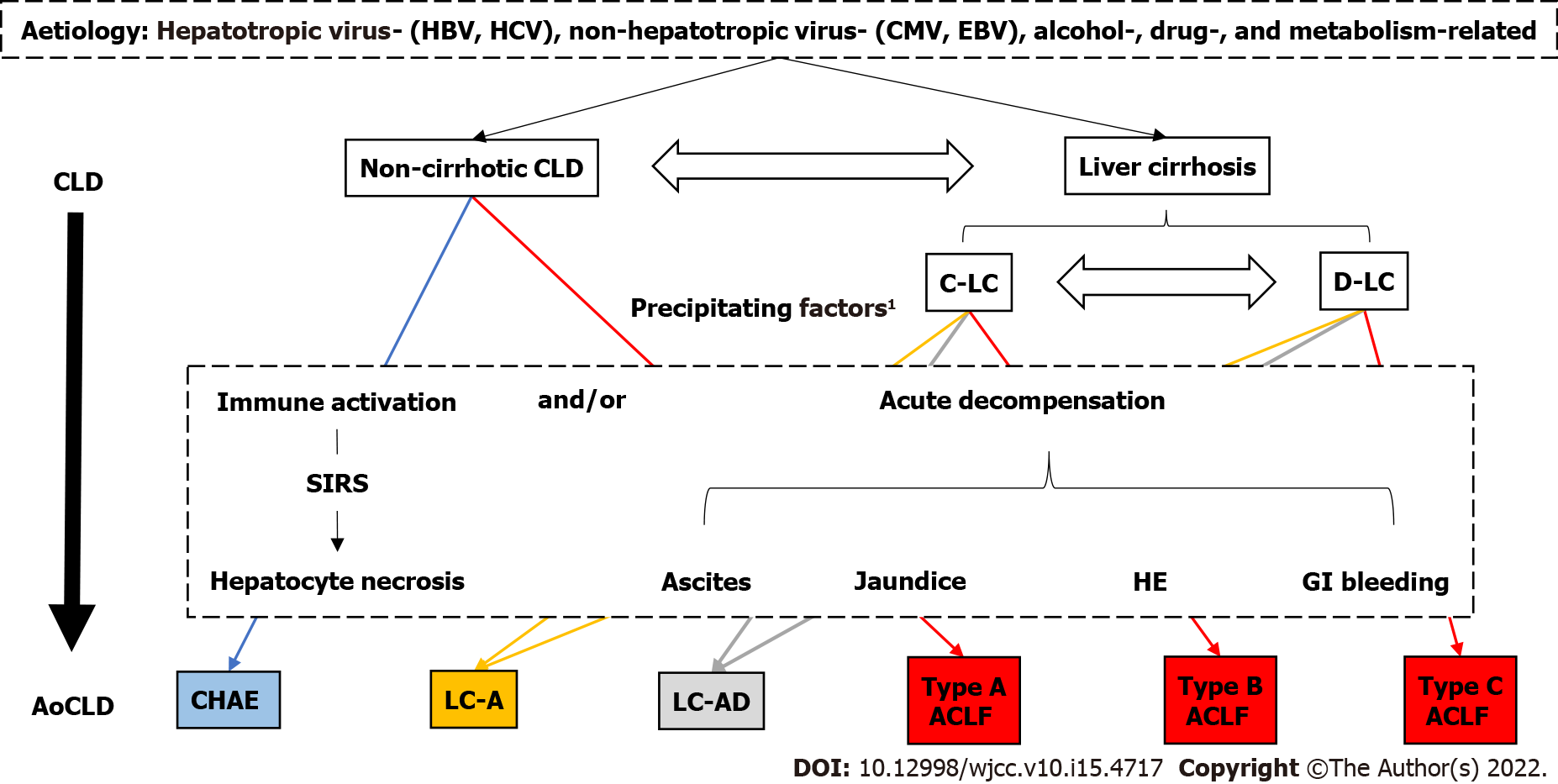
Core Tip: Acute–on-chronic liver disease (AoCLD) can be defined as a group of diseases that experience acute liver injury (ALI) or acute decompensation in patients with pre-existing chronic liver disease (CLD). AoCLD can be divided into acute-on-chronic liver failure (ACLF) and non-ACLF according to the degree of ALI and the presence or absence of organ failure. According to the basic state of CLD, ACLF can be classified as type A (on the basis of chronic hepatitis), type B (on the basis of compensatory cirrhosis), and type C (on the basis of decompensated cirrhosis), and non-ACLF can be further classified as chronic hepatitis with acute exacerbation, the active phase of liver cirrhosis, and liver cirrhosis-acute decompensation.
- Citation: Zhang YY, Meng ZJ. Definition and classification of acute-on-chronic liver diseases. World J Clin Cases 2022; 10(15): 4717-4725
- URL: https://www.wjgnet.com/2307-8960/full/v10/i15/4717.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i15.4717
It is estimated that at least 1.5 billion people worldwide suffer from chronic liver diseases (CLDs), and an average of 2 million people die of CLDs each year[1,2]. The latest data from research investigating the global burden of disease released by The Lancet in 2020 show that the disability-adjusted life years caused by CLD in 2019 have increased by 33.0% over the past 30 years, accounting for 1.8% of the global burden. These data indicate that CLD imposes an increasing burden on public health[3], likely because most CLD patients are in a stable state for a long time without obvious symptoms or signs in the early stage. In most cases, CLD patients are often unaware of their disease and are exposed to various liver injury factors until the onset of symptoms, such as nausea, vomiting, abdominal distension, jaundice, etc., and require hospitalization. At this point, the disease progressed to severe hepatitis, decompensated cirrhosis, and even acute-on-chronic liver failure (ACLF) characterized by high short-term mortality[4,5], placing a serious economic burden on the family and society. These patients are collectively referred to as acute-on-CLD (AoCLD) patients[6]. Given such a large group of patients, it is of great importance for clinicians to quickly identify patients at a high risk of death and make corresponding clinical decisions that can improve the prognosis of patients and save medical resources. To date, the definition of AoCLD is still vague, and a consensus concept of the clinical classification is lacking. Therefore, a definitive definition and classification of AoCLD is urgently needed.
In the early 1990s, Kohn et al[7], for the first time, proposed the concept of AoCLD and mentioned that “AoCLD may lead to hepatic encephalopathy”. At the end of the 1990s, AoCLD was preliminarily defined as a type of disease with hepatic encephalopathy based on CLD[8,9]. In 2009, the definition of AoCLD was expanded to “acute decompensation occurring on CLD”[10]. With increasing attention to ACLF, some scholars[11] described AoCLD as acute liver injury (ALI) superimposed on CLD and further classified AoCLD into ACLF and non-ACLF. However, other scholars defined AoCLD as ALI on the basis of CLD and patients who did not meet the ACLF criteria[12]. In 2019, Caracuel et al[13] proposed another interpretation of the concept of AoCLD, arguing that AoCLD is a clinical syndrome characterized by decompensated cirrhosis, portal hypertension, and visceral hyperdynamic circulation. Recently, in the Chinese ACLF multicentre prospective cohort study launched by the Chinese ACLF Consortium, AoCLD was redefined as an acute exacerbation of liver cirrhosis and non-cirrhotic CLD, including ACLF and non-ACLF (other unstable CLD)[6,14-16]. The evolution of the definition of AoCLD is listed in Table 1.
| Ref. | Definition of AoCLD | Chronic liver disease status | Clinical features |
| Kohn et al[7], 1993 | A type of disease that may develop into hepatic encephalopathy | Liver cirrhosis | Hepatic encephalopathy |
| Clemmesen et al[8,9], 1999 | A class of diseases of hepatic encephalopathy based on CLD | Liver cirrhosis or non-cirrhosis | Hepatic encephalopathy |
| Agarwal et al[10], 2009 | Acute decompensation occurs on CLD | Liver cirrhosis | Acute decompensation events |
| Jagadisan et al[11], 2012 | Acute liver injury superimposed on the basis of CLD including ACLF and non-ACLF | Liver cirrhosis or non-cirrhosis | Acute liver injury and/or acute decompensation events |
| Tasneem and Luck[12], 2017 | Acute liver injury on the basis of CLD that does not meet the criteria of ACLF | Liver cirrhosis or non-cirrhosis | Acute liver injury |
| Caracuel et al[13], 2019 | A clinical syndrome characterized by decompensated cirrhosis, portal hypertension, and visceral hyperdynamic circulation | Liver cirrhosis | Acute decompensation events |
| Qiao et al[6], 2021 | Acute exacerbations of various CLD (including cirrhosis and non-cirrhosis), including ACLF and non-ACLF | Liver cirrhosis or non-cirrhosis | Acute liver injury and/or acute decompensation events |
Considering the evolution of the definition of AoCLD (Table 1), there are two necessary conditions as follows: An underlying disease of CLD and acute exacerbation of the disease in a short period. CLD refers to a cluster of diseases with varying degrees of intrahepatic inflammatory necrosis and/or fibrosis caused by different aetiologies with a history of at least 6 mo. CLDs usually include cirrhosis and non-cirrhotic chronic liver diseases[16], including different forms of chronic hepatitis [chronic hepatitis B (CHB) and chronic hepatitis C], alcohol-associated liver disease, metabolic associated fatty liver disease, autoimmune liver disease, genetic metabolic liver disease and chronic drug-induced liver injury. Acute exacerbation is manifested as the new occurrence of acute inflammatory necrosis in the liver under the attack of different inducements (such as hepatitis virus mutation, overlap virus infection, bacterial infection, excessive alcohol intake, drugs or immune damage), causing further aggravation of the original inflammation and/or fibrosis and leading to liver dysfunction, decompensation, or even liver failure[17-19]. The period of the onset of acute aggravation varies in different basic states of CLD. Upon ALI, acute exacerbation usually presents in patients with chronic hepatitis within 1 wk[20,21], and acute decompensation of liver cirrhosis (LC-AD) usually occurs within 1 mo[6]. Since the definition of ACLF has not been unified in Eastern and Western countries, the time window of the acute exacerbation of ACLF is unclear; however, most studies suggest that ACLF patients display increased mortality at 28 d and that the most adverse outcomes (death or liver transplantation) occur within 3 mo[22-24]. Notably, if ALI occurs in CLD patients without underlying intrahepatic inflammation or fibrosis, AoCLD should not be diagnosed[25].
Thus, AoCLD can be defined as a cluster of diseases in which ALI or acute decompensation occurs in patients with pre-existing CLD, triggered by different precipitants. AoCLD may histologically present with intrahepatic mild to severe inflammatory necrosis and/or advanced fibrosis and clinically manifest as significantly increased alanine aminotransferase (ALT)/aspartate aminotransferase (AST) and total bilirubin (TBil) levels within 1 wk, acute decompensation of liver cirrhosis, or liver failure within 1 mo.
Inflammation and/or fibrosis in patients with chronic hepatitis or compensatory cirrhosis can be alleviated or even reversed (for compensatory cirrhosis) if a proper treatment regimen is applied, such as continuous nucleos(t)ide analogue (NUC) treatment for CHB[26,27]. Once patients with cirrhosis develop acute decompensation, the prognosis is poor, and the median survival time is approximately 5 years[28]. A mild ALI imposed on CLD may not lead to liver dysfunction, and liver injury can recover upon active treatment with minimal effects on the quality of life and longevity. However, once massive or submassive hepatic necrosis occurs, ACLF is triggered with a short-term (28-d) mortality rate of more than 15%[19,29]. The prognosis of AoCLD highly differs depending on both the various types of CLD and the degree of ALI. Therefore, AoCLD can be divided into ACLF and non-ACLF according to the degree of ALI; furthermore, according to the basic state of CLD (non-cirrhotic chronic liver diseases, compensatory cirrhosis, or decompensated cirrhosis), non-ACLF can be further divided into chronic hepatitis with acute exacerbation (CHAE), the active phase of liver cirrhosis (LC-A), and liver cirrhosis-acute decompensation (LC-AD) (Figure 1). A brief definition and the diagnostic criteria for each clinical type of AoCLD are shown in Table 2.
| Clinical classification | Brief definition | Diagnostic criteria |
| CLD | It refers to a cluster of diseases with varying degrees of intrahepatic inflammatory necrosis and/or fibrosis caused by different aetiologies with a history of liver dysfunction for over 6 mo[16] | No |
| Liver cirrhosis | Liver cirrhosis is a consequence of chronic liver inflammation that is followed by diffuse hepatic fibrosis, where in the normal hepatic architecture is replaced by regenerative hepatic nodules[57]. (1) C-LC, patients with cirrhosis without any cirrhosis-related symptoms or complication; and (2) D-LC, patients with cirrhosis with cirrhosis-related complications such as ascites, variceal bleeding, hepatic encephalopathy, or non-obstructive jaundice | Diagnosis of cirrhosis is based on one of the following criteria[56]: (1) Histologically cirrhosis; (2) gastroesophageal varices or digestive tract ectopic varices on the basis of excluding non-cirrhotic portal hypertension; (3) imaging reveals cirrhosis or portal hypertension; and (4) meeting two or more of the four criteria: PLT < 100 × 109/L without any other reasons; ALB < 35g/L, excluding malnutrition or kidney diseases; INR > 1.3 or PT prolonged; APRI > 2 |
| AoCLD | Acute liver injury, acute decompensation or acute liver failure occurs on the basis of CLD in a short period[16] | (1) Increased ALT/AST and TBil levels on the basis of CLD within 1 wk[16]; and (2) acute decompensation of liver cirrhosis, or liver failure on the basis of CLD within 1 mo[16] |
| ACLF | Acute liver failure or decompensation occurs on the basis of CLD in a short period: (1) Type-A, ACLF occurs on the basis of chronic hepatitis; (2) Type-B, ACLF occurs on the basis of compensated cirrhosis; and (3) Type-C, ACLF occurs on the basis of decompensated cirrhosis | (1) Acute or subacute deterioration of pre-existing chronic liver disease[34]; (2) extreme fatigue with severe digestive symptoms; and (3) TBil ≥ 10 mg/dL or daily rise ≥ 1 mg/dL, and INR ≥ 1.5 (or) PTA ≤ 40%[34] |
| Non-ACLF | ||
| CHAE | Chronic hepatitis acute aggravation in a short period | Intermittent transaminase elevation that exceeds 5 times the ULN or 2 times the baseline level in a short period (usually 1 wk)[44] |
| LC-A | Cirrhosis changes from the quiescent to the active stage without acute decompensation | (1) Liver fibrosis and liver inflammation simultaneously coexist; (2) a rapid increase in the liver stiffness value and serum liver fibrosis markers in a short period (usually 1 wk)[52,53]; and (3) increase in ALT and TBil and decrease in the albumin level to varying degrees[54] |
| LC-AD | Occurrence of acute decompensation in cirrhotic patients with/without previous decompensation in a short period (within 1 mo) under the action of acute incentives | Acute decompensated events, including ascites, hepatic encephalopathy, jaundice and gastrointestinal bleeding that occur in cirrhotic patients within 1 mo[14,58,60] |
ACLF refers to a syndrome that occurs in CLD patients under the action of various ALI factors and is characterized by acute jaundice, coagulatory dysfunction, and rapid disease progression with high mortality[12,30]. The differences in the aetiology of CLD and the induction of acute injury between Eastern and Western countries have led to nonuniform diagnostic criteria for ACLF. The definition of ACLF in Asia focuses on liver failure caused by ALI, while the definition in Europe and America pays more attention to systemic multiorgan failure, considering liver failure an unnecessary condition[31]. In 2015, the World Gastrointestinal Organization proposed consensus definitions of ACLF integrated from the East and the West and proposed the following three clinical types of ACLF based on the different CLDs: Type A (based on chronic hepatitis), type B (based on compensatory cirrhosis), and type C (based on decompensated cirrhosis)[22,32,33]. The Chinese Medical Association summarized the definition of ACLF in the Guidelines for the Diagnosis and Treatment of Liver Failure as follows: Acute liver failure (ALF) occurs on the basis of CLD (with or without cirrhosis), mainly manifesting as jaundice (serum TBil attaining a level over 10 times the upper limit of normal (ULN) value or a daily increase ≥ 17.1 μmol/L) and a bleeding tendency (prothrombin activity ≤ 40% or international normalized ratio ≥ 1.5), accompanied by failure of one or more extrahepatic organs with significantly increased mortality within 28 d and 3 mo after onset[34]. According to the basic status of CLD, ACLF was also classified into three clinical types consistent with those of the World Gastrointestinal Organization.
In the diagnosis of ACLF, attention should be given to discriminating ACLF from ALF or subacute LF (SALF) in which liver failure develops within 2 wk or 26 wk, respectively, in patients without pre-existing chronic liver injury[35]. Therefore, the difference between ACLF and ALF/SALF mainly lies in the presence or absence of underlying chronic liver injury. Hepatitis B virus (HBV) infection is the main cause of ACLF[36]. The diagnosis of HBV-associated ACLF is sometimes difficult due to the complexity of the natural history of HBV infection. According to the European Association for the Study of the Liver, the natural history of chronic HBV infection is divided into five stages, namely, hepatitis B e antigen (HBeAg)-positive chronic HBV infection, HBeAg-positive CHB, HBeAg-negative chronic HBV infection, HBeAg-negative CHB, and hepatitis B surface antigen (HBsAg)-negative stage[37]. The nomenclature is based on the description of the following two main characteristics of the history of HBV infection: infection and hepatitis. In a state of chronic HBV infection, there is limited or no chronic inflammation or fibrosis in the liver[25]. Studies have shown that the survival rate of chronic HBV-infected patients is comparable to that of non-HBV-infected patients[38]. Therefore, ALF/SALF rather than ACLF should be diagnosed once liver failure occurs in patients with chronic HBV infection or CHB whose intrahepatic inflammation and fibrosis have completely disappeared for more than half a year upon NUC treatment[39,40]. In contrast, ACLF should be diagnosed once liver failure occurs in CHB patients with active intrahepatic inflammation and/or fibrosis. However, since most patients with chronic HBV infection lack liver histological evidence, the status of liver inflammatory activity and fibrosis can be judged only indirectly by referring to the levels of serum ALT/AST and liver stiffness (detected by transient elastography)[41,42]. Thus, for patients with chronic HBV infection who do not show obvious signs and symptoms of active hepatitis but may have different degrees of inflammatory activity and fibrosis histologically, the dynamic monitoring of the ALT/AST levels and liver stiffness could facilitate the assessment of liver inflammatory activity and fibrosis, respectively, which is helpful for distinguishing ALF/SALF from ACLF[41,43].
In patients with chronic hepatitis, the presence of various precipitants, such as HBV reactivation, leads to acute exacerbation of liver inflammation and/or focal necrosis of hepatocytes, which is manifested as repeated or continuous increases in the serum ALT and/or AST levels, a decrease in albumin or the albumin/globulin ratio, an increase in the TBil level, and even the presence of abnormal coagulation function[17]. The 2015 edition of the Asia-Pacific Liver Association clinical practice guidelines for hepatitis B define CHAE as an intermittent transaminase elevation that exceeds 5 times the ULN or 2 times the baseline level[44]. CHAE clinically manifests as the activation of chronic hepatitis, which can be classified as mild, moderate, and severe according to the degree of inflammation and fibrosis of liver tissue[45].
Notably, due to the particularity of the natural history of HBV infection, HBV-related ALI occurring in a state of chronic HBV infection can be divided into the following two situations: one situation involves a mild transient liver injury that does not activate HBV or require anti-HBV treatment, and the patient is still in a state of “chronic HBV infection” after recovery from ALI[46,47], while in the other situation, the precipitants persist, leading to severe liver damage and even HBV activation, and anti-HBV treatment is needed to control the disease progression. In this case, “chronic HBV infection” transitions into “chronic hepatitis B”[48]. Therefore, ALI occurring under a chronic HBV infection status should not be diagnosed as CHAE.
According to the status of inflammatory activity in liver tissue, liver cirrhosis can be divided into the active and quiescent stages[49,50]. In patients with quiescent cirrhosis, the liver is histologically characterized by pseudolobules and does not show hepatocyte necrosis, lymphocyte infiltration or new fibrogenesis. The presence of acute aggravating factors leads to intrahepatic inflammatory cell infiltration, hepatocyte necrosis and new fibrogenesis, indicating that liver cirrhosis transitioned to the active phase[51]. LC-A is histologically defined as a state of active intrahepatic inflammation and fibrogenesis in patients with cirrhosis and clinically manifests as a sharp increase in serum liver fibrosis markers (laminin, hyaluronic acid, pro-peptide of type III procollagen and collagen IV) or liver stiffness within a short period (usually 1 wk)[52,53] and is accompanied by elevated ALT and TBil levels and decreased albumin[54]. LC-A can occur in both compensatory LC (C-LC) and decompensated LC (D-LC). Due to obvious portal hypertension in patients with D-LC, haemodynamic disorders are very likely to occur, causing hepatic tissue ischaemia and hypoxia, and immunodeficiency renders D-LC patients vulnerable to secondary infections, leading to sepsis and further liver injury; thus, D-LC is rarely in a quiescent state[55]. Therefore, LC-A occurs in patients with C-LC more commonly than in those with D-LC.
In patients with C-LC, the remaining liver cells can maintain liver functions, such as synthesis and catabolism, even in the presence of portal hypertension[56,57]. D-LC is defined as the occurrence of ascites, hepatic encephalopathy, jaundice, or oesophageal-gastric varices bleeding in patients with C-LC[56,58,59]. Both C-LC and D-LC patients may experience acute decompensation in a short period (within 1 mo) under the action of acute inducement, which is called LC-AD[58].
LC-AD is mainly manifested by the following two types of pathophysiological changes: portal hypertension and liver dysfunction. The complications of LC-AD may interact with each other, forming a vicious cycle and promoting the progression of LC-AD[59]. Studies have shown that the prognosis of LC-AD patients with previous decompensation is worse than that of LC-AD patients without previous decompensation[28,60], likely because D-LC patients are more prone to intractable ascites and endotoxaemia than C-LC patients. Under the triple attack of immune injury, ischaemia and hypoxia, and endotoxaemia, patients with liver cirrhosis experience massive/submassive necrosis of the liver tissue, resulting in rapid deterioration, and easily develop ACLF[18,61,62].
The present review preliminarily summarized the definition, aetiology and inducement, and clinical types of AoCLD (Figure 1). However, the aetiology and precipitants of AoCLD are complex, and the clinical classification and definition of AoCLD are divergent; in particular, the diagnostic criteria for ACLF are controversial. For individual clinical types of AoCLD, the relevant factors, including the assessment of the degree of the disease and the prognosis, need to be characterized, and the mechanism driving the progression of AoCLD needs to be further clarified. Therefore, a multicentre, prospective cohort study is needed to systematically analyse the clinical characteristics and prognostic factors of the individual clinical types of AoCLD, which could provide an evidence-based definition and characterization of the diseases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Fallatah H, Saudi Arabia; Popovic DD, Serbia S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Li M, Wang ZQ, Zhang L, Zheng H, Liu DW, Zhou MG. Burden of Cirrhosis and Other Chronic Liver Diseases Caused by Specific Etiologies in China, 1990-2016: Findings from the Global Burden of Disease Study 2016. Biomed Environ Sci. 2020;33:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 2. | Moon AM, Singal AG, Tapper EB. Contemporary Epidemiology of Chronic Liver Disease and Cirrhosis. Clin Gastroenterol Hepatol. 2020;18:2650-2666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 805] [Cited by in RCA: 720] [Article Influence: 144.0] [Reference Citation Analysis (0)] |
| 3. | GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11327] [Cited by in RCA: 9637] [Article Influence: 1927.4] [Reference Citation Analysis (35)] |
| 4. | Kuo CC, Huang CH, Chang C, Chen PC, Chen BH, Chen WT, Ho YP. Comparing CLIF-C ACLF, CLIF-C ACLFlactate, and CLIF-C ACLF-D Prognostic Scores in Acute-on-Chronic Liver Failure Patients by a Single-Center ICU Experience. J Pers Med. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Lin X, Huang X, Wang L, Feng S, Chen X, Cai W, Huang Z. Prognostic Value of Acute-On-Chronic Liver Failure (ACLF) Score in Critically Ill Patients with Cirrhosis and ACLF. Med Sci Monit. 2020;26:e926574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Qiao L, Wang X, Deng G, Huang Y, Chen J, Meng Z, Zheng X, Shi Y, Qian Z, Liu F, Gao Y, Lu X, Liu J, Gu W, Zhang Y, Wang T, Wu D, Dong F, Sun X, Li H. Cohort profile: a multicentre prospective validation cohort of the Chinese Acute-on-Chronic Liver Failure (CATCH-LIFE) study. BMJ Open. 2021;11:e037793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Kohn CL, Brozenec S, Foster PF. Nutritional support for the patient with pancreatobiliary disease. Crit Care Nurs Clin North Am. 1993;5:37-45. [PubMed] |
| 8. | Clemmesen JO, Larsen FS, Kondrup J, Hansen BA, Ott P. Cerebral herniation in patients with acute liver failure is correlated with arterial ammonia concentration. Hepatology. 1999;29:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 451] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Clemmesen JO, Gerbes AL, Gülberg V, Hansen BA, Larsen FS, Skak C, Tygstrup N, Ott P. Hepatic blood flow and splanchnic oxygen consumption in patients with liver failure. Effect of high-volume plasmapheresis. Hepatology. 1999;29:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Agarwal B, Shaw S, Shankar Hari M, Burroughs AK, Davenport A. Continuous renal replacement therapy (CRRT) in patients with liver disease: is circuit life different? J Hepatol. 2009;51:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Jagadisan B, Srivastava A, Yachha SK, Poddar U. Acute on chronic liver disease in children from the developing world: recognition and prognosis. J Pediatr Gastroenterol Nutr. 2012;54:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Tasneem AA, Luck NH. Acute-On-Chronic Liver Failure: Causes, Clinical Characteristics and Predictors of Mortality. J Coll Physicians Surg Pak. 2017;27:8-12. [PubMed] |
| 13. | Caracuel L, Sastre E, Llévenes P, Prieto I, Funes T, Aller MÁ, Arias J, Balfagón G, Blanco-Rivero J. Acute-on-chronic liver disease enhances phenylephrine-induced endothelial nitric oxide release in rat mesenteric resistance arteries through enhanced PKA, PI3K/AKT and cGMP signalling pathways. Sci Rep. 2019;9:6993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Zhang Y, Xu BY, Wang XB, Zheng X, Huang Y, Chen J, Meng ZJ, Gao YH, Qian ZP, Liu F, Lu XB, Shi Y, Shang J, Li H, Wang SY, Yin S, Sun SN, Hou YX, Xiong Y, Li BL, Lei Q, Gao N, Ji LJ, Li J, Jie FR, Zhao RH, Liu JP, Lin TF, Chen LY, Tan WT, Zhang Q, Zou CC, Huang ZB, Jiang XH, Luo S, Liu CY, Zhang YY, Li T, Ren HT, Wang SJ, Deng GH, Xiong SE, Liu XX, Wang C, Yuan W, Gu WY, Qiao L, Wang TY, Wu DD, Dong FC, Hua J. Prevalence and Clinical Significance of Portal Vein Thrombosis in Patients With Cirrhosis and Acute Decompensation. Clin Gastroenterol Hepatol. 2020;18:2564-2572.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 15. | Long L, Li H, Deng G, Wang X, Lu S, Li B, Meng Z, Gao Y, Qian Z, Liu F, Lu X, Ren H, Shang J, Wang S, Zheng Y, Yan H, Yin S, Tan W, Zhang Q, Zheng X, Chen J, Luo S, Zhao J, Yuan W, Li T, Zheng R, Liu J, Liu X, Gu W, Li S, Mei X, Chen R, Huang Y. Impact of Hepatic Encephalopathy on Clinical Characteristics and Adverse Outcomes in Prospective and Multicenter Cohorts of Patients With Acute-on-Chronic Liver Diseases. Front Med (Lausanne). 2021;8:709884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Ouyang R, Li H, Xia J, Wang X, Zheng X, Huang Y, Meng Z, Gao Y, Qian Z, Liu F, Lu X, Shi Y, Shang J, Liu J, Deng G, Zheng Y, Yan H, Zhang W, Qiao L, Jiang X, Wang H, Zhong G, Li B, Chen J. Lower platelet counts were associated with 90-day adverse outcomes in acute-on-chronic liver disease patients. Ann Palliat Med. 2021;10:9342-9353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Crismale JF, Friedman SL. Acute Liver Injury and Decompensated Cirrhosis. Med Clin North Am. 2020;104:647-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Dienes HP, Drebber U. Pathology of immune-mediated liver injury. Dig Dis. 2010;28:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Thawley V. Acute Liver Injury and Failure. Vet Clin North Am Small Anim Pract. 2017;47:617-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Green TJ, Sivilotti ML, Langmann C, Yarema M, Juurlink D, Burns MJ, Johnson DW. When do the aminotransferases rise after acute acetaminophen overdose? Clin Toxicol (Phila). 2010;48:787-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Wu HL, Kao JH, Chen TC, Wu WH, Liu CH, Su TH, Yang HC, Chen DS, Chen PJ, Liu CJ. Serum cytokine/chemokine profiles in acute exacerbation of chronic hepatitis B: clinical and mechanistic implications. J Gastroenterol Hepatol. 2014;29:1629-1636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Jalan R, Yurdaydin C, Bajaj JS, Acharya SK, Arroyo V, Lin HC, Gines P, Kim WR, Kamath PS; World Gastroenterology Organization Working Party. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 23. | Chen T, Yang Z, Choudhury AK, Al Mahtab M, Li J, Chen Y, Tan SS, Han T, Hu J, Hamid SS, Huei LG, Ghazinian H, Nan Y, Chawla YK, Yuen MF, Devarbhavi H, Shukla A, Abbas Z, Sahu M, Dokmeci AK, Lesmana LA, Lesmana CRA, Xin S, Duan Z, Guo W, Ma K, Zhang Z, Cheng Q, Jia J, Sharma BC, Sarin SK, Ning Q. Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: a multi-national study from the Asia-Pacific region. Hepatol Int. 2019;13:695-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Hernaez R, Liu Y, Kramer JR, Rana A, El-Serag HB, Kanwal F. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure. J Hepatol. 2020;73:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 25. | Sugawara K, Nakayama N, Mochida S. Acute liver failure in Japan: definition, classification, and prediction of the outcome. J Gastroenterol. 2012;47:849-861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 26. | Bedossa P. Reversibility of hepatitis B virus cirrhosis after therapy: who and why? Liver Int. 2015;35 Suppl 1:78-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Saffioti F, Pinzani M. Development and Regression of Cirrhosis. Dig Dis. 2016;34:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Nilsson E, Anderson H, Sargenti K, Lindgren S, Prytz H. Patients with liver cirrhosis show worse survival if decompensation occurs later during course of disease than at diagnosis. Scand J Gastroenterol. 2018;53:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Cao Z, Liu Y, Cai M, Xu Y, Xiang X, Zhao G, Cai W, Wang H, Wang W, Xie Q. The Use of NACSELD and EASL-CLIF Classification Systems of ACLF in the Prediction of Prognosis in Hospitalized Patients With Cirrhosis. Am J Gastroenterol. 2020;115:2026-2035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 30. | Sarin SK, Choudhury A, Sharma MK, Maiwall R, Al Mahtab M, Rahman S, Saigal S, Saraf N, Soin AS, Devarbhavi H, Kim DJ, Dhiman RK, Duseja A, Taneja S, Eapen CE, Goel A, Ning Q, Chen T, Ma K, Duan Z, Yu C, Treeprasertsuk S, Hamid SS, Butt AS, Jafri W, Shukla A, Saraswat V, Tan SS, Sood A, Midha V, Goyal O, Ghazinyan H, Arora A, Hu J, Sahu M, Rao PN, Lee GH, Lim SG, Lesmana LA, Lesmana CR, Shah S, Prasad VGM, Payawal DA, Abbas Z, Dokmeci AK, Sollano JD, Carpio G, Shresta A, Lau GK, Fazal Karim M, Shiha G, Gani R, Kalista KF, Yuen MF, Alam S, Khanna R, Sood V, Lal BB, Pamecha V, Jindal A, Rajan V, Arora V, Yokosuka O, Niriella MA, Li H, Qi X, Tanaka A, Mochida S, Chaudhuri DR, Gane E, Win KM, Chen WT, Rela M, Kapoor D, Rastogi A, Kale P, Sharma CB, Bajpai M, Singh V, Premkumar M, Maharashi S, Olithselvan A, Philips CA, Srivastava A, Yachha SK, Wani ZA, Thapa BR, Saraya A, Shalimar, Kumar A, Wadhawan M, Gupta S, Madan K, Sakhuja P, Vij V, Sharma BC, Garg H, Garg V, Kalal C, Anand L, Vyas T, Mathur RP, Kumar G, Jain P, Pasupuleti SSR, Chawla YK, Chowdhury A, Song DS, Yang JM, Yoon EL; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party. Correction to: Acute-on-chronic liver failure: consensus recommendations of the Asian Pacific association for the study of the liver (APASL): an update. Hepatol Int. 2019;13:826-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 31. | Leão GS, Lunardi FL, Picon RV, Tovo CV, de Mattos AA, de Mattos ÂZ. Acute-on-chronic liver failure: A comparison of three different diagnostic criteria. Ann Hepatol. 2019;18:373-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Tang X, Qi T, Li B, Li H, Huang Z, Zhu Z, Tu M, Gao J, Zhu C, Jiang X, Yu X, Lu G, Xiong M, He Q, Zhou F, Wen W, Chen J, Hou J. Tri-typing of hepatitis B-related acute-on-chronic liver failure defined by the World Gastroenterology Organization. J Gastroenterol Hepatol. 2021;36:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 33. | Mu X, Tong J, Xu X, Chen J, Su H, Liu X, Pang F, Zhai X, Wang L, Wang Y, Guan C, Wang F, Hu J. World Gastroenterology Organisation classification and a new type-based prognostic model for hepatitis B virus-related acute-on-chronic liver failure. Clin Res Hepatol Gastroenterol. 2021;45:101548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Zhang Q, Li Y, Han T, Nie C, Cai J, Liu H, Liu Y. Comparison of current diagnostic criteria for acute-on-chronic liver failure. PLoS One. 2015;10:e0122158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Guo X, Ma Z, Wang B, Lu H, Qi X. Characteristics and in-hospital outcomes of COVID-19 patients with acute or subacute liver failure. Dig Liver Dis. 2021;53:1069-1070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Gu WY, Xu BY, Zheng X, Chen J, Wang XB, Huang Y, Gao YH, Meng ZJ, Qian ZP, Liu F, Lu XB, Shang J, Li H, Wang SY, Sun X. Acute-on-Chronic Liver Failure in China: Rationale for Developing a Patient Registry and Baseline Characteristics. Am J Epidemiol. 2018;187:1829-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Hadziyannis SJ. Unrevealing the natural course of the so-called "inactive HBsAg or HBV carrier state". Hepatol Int. 2007;1:281-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Villa E, Fattovich G, Mauro A, Pasino M. Natural history of chronic HBV infection: special emphasis on the prognostic implications of the inactive carrier state vs chronic hepatitis. Dig Liver Dis. 2011;43 Suppl 1:S8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Oketani M, Uto H, Ido A, Tsubouchi H. Management of hepatitis B virus-related acute liver failure. Clin J Gastroenterol. 2014;7:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Mochida S, Nakayama N, Ido A, Takikawa Y, Yokosuka O, Sakaida I, Moriwaki H, Genda T, Takikawa H. Revised criteria for classification of the etiologies of acute liver failure and late-onset hepatic failure in Japan: A report by the Intractable Hepato-biliary Diseases Study Group of Japan in 2015. Hepatol Res. 2016;46:369-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Sonneveld MJ, Brouwer WP, Hansen BE, Chan HL, Piratvisuth T, Jia JD, Zeuzem S, Chien RN, Choi H, de Knegt RJ, Wat C, Pavlovic V, Gaggar A, Xie Q, Buti M, de Man RA, Janssen HLA; SONIC-B Study Group. Very low probability of significant liver inflammation in chronic hepatitis B patients with low ALT levels in the absence of liver fibrosis. Aliment Pharmacol Ther. 2020;52:1399-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 42. | He T, Li J, Ouyang Y, Lv G, Ceng X, Zhang Z, Ding J. FibroScan Detection of Fatty Liver/Liver Fibrosis in 2266 Cases of Chronic Hepatitis B. J Clin Transl Hepatol. 2020;8:113-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | Rahayu ES, Mariyatun M, Putri Manurung NE, Hasan PN, Therdtatha P, Mishima R, Komalasari H, Mahfuzah NA, Pamungkaningtyas FH, Yoga WK, Nurfiana DA, Liwan SY, Juffrie M, Nugroho AE, Utami T. Effect of probiotic Lactobacillus plantarum Dad-13 powder consumption on the gut microbiota and intestinal health of overweight adults. World J Gastroenterol. 2021;27:107-128. [PubMed] [DOI] [Full Text] |
| 44. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 1960] [Article Influence: 217.8] [Reference Citation Analysis (0)] |
| 45. | Zhou XJ, Huang WB. [Evaluation of different grading and staging systems of chronic hepatitis and problems in application]. Zhonghua Bing Li Xue Za Zhi. 2008;37:636-640. [PubMed] |
| 46. | Salpini R, Battisti A, Colagrossi L, Di Carlo D, Fabeni L, Piermatteo L, Cerva C, Lichtner M, Mastroianni C, Marignani M, Maylin S, Delaugerre C, Morisco F, Coppola N, Marrone A, Angelico M, Sarmati L, Andreoni M, Perno CF, Ceccherini-Silberstein F, Svicher V. A snapshot of virological presentation and outcome of immunosuppression-driven HBV reactivation from real clinical practice: Evidence of a relevant risk of death and evolution from silent to chronic infection. J Viral Hepat. 2019;26:846-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Zhang ZQ, Shi BS, Lu W, Huang D, Wang YB, Feng YL. Quantitative serum HBV markers in predicting phases of natural history of chronic HBV infection. J Virol Methods. 2021;296:114226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Huang SC, Yang HC, Kao JH. Hepatitis B reactivation: diagnosis and management. Expert Rev Gastroenterol Hepatol. 2020;14:565-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | de Oliveira da Silva B, Ramos LF, Moraes KCM. Molecular interplays in hepatic stellate cells: apoptosis, senescence, and phenotype reversion as cellular connections that modulate liver fibrosis. Cell Biol Int. 2017;41:946-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Liu X, Xu J, Rosenthal S, Zhang LJ, McCubbin R, Meshgin N, Shang L, Koyama Y, Ma HY, Sharma S, Heinz S, Glass CK, Benner C, Brenner DA, Kisseleva T. Identification of Lineage-Specific Transcription Factors That Prevent Activation of Hepatic Stellate Cells and Promote Fibrosis Resolution. Gastroenterology. 2020;158:1728-1744.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 51. | Fung J, Lai CL, Chan SC, But D, Seto WK, Cheng C, Wong DK, Lo CM, Fan ST, Yuen MF. Correlation of liver stiffness and histological features in healthy persons and in patients with occult hepatitis B, chronic active hepatitis B, or hepatitis B cirrhosis. Am J Gastroenterol. 2010;105:1116-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Koch A, Horn A, Dückers H, Yagmur E, Sanson E, Bruensing J, Buendgens L, Voigt S, Trautwein C, Tacke F. Increased liver stiffness denotes hepatic dysfunction and mortality risk in critically ill non-cirrhotic patients at a medical ICU. Crit Care. 2011;15:R266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Zhu C, Qi X, Li H, Peng Y, Dai J, Chen J, Xia C, Hou Y, Zhang W, Guo X. Correlation of serum liver fibrosis markers with severity of liver dysfunction in liver cirrhosis: a retrospective cross-sectional study. Int J Clin Exp Med. 2015;8:5989-5998. [PubMed] |
| 54. | Fujita K, Masaki T. Serum Biomarkers of Liver Fibrosis Staging in the Era of the Concept "Compensated Advanced Chronic Liver Disease". J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 55. | Bothou C, Rüschenbaum S, Kubesch A, Quenstedt L, Schwarzkopf K, Welsch C, Zeuzem S, Welzel TM, Lange CM. Anemia and Systemic Inflammation Rather than Arterial Circulatory Dysfunction Predict Decompensation of Liver Cirrhosis. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Xu XY, Ding HG, Li WG, Xu JH, Han Y, Jia JD, Wei L, Duan ZP, Ling-Hu EQ, Zhuang H. Chinese guidelines on the management of liver cirrhosis (abbreviated version). World J Gastroenterol. 2020;26:7088-7103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 57. | Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398:1359-1376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 860] [Article Influence: 215.0] [Reference Citation Analysis (1)] |
| 58. | Pfortmueller CA, Wiemann C, Funk GC, Leichtle AB, Fiedler GM, Exadaktylos AK, Lindner G. Hypoglycemia is associated with increased mortality in patients with acute decompensated liver cirrhosis. J Crit Care. 2014;29:316.e7-316.12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 59. | Bernardi M, Caraceni P. Novel perspectives in the management of decompensated cirrhosis. Nat Rev Gastroenterol Hepatol. 2018;15:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, Valsecchi MG. New concepts on the clinical course and stratification of compensated and decompensated cirrhosis. Hepatol Int. 2018;12:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 61. | Yue S, Zhou H, Wang X, Busuttil RW, Kupiec-Weglinski JW, Zhai Y. Prolonged Ischemia Triggers Necrotic Depletion of Tissue-Resident Macrophages To Facilitate Inflammatory Immune Activation in Liver Ischemia Reperfusion Injury. J Immunol. 2017;198:3588-3595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 62. | Van den Broecke A, Van Coile L, Decruyenaere A, Colpaert K, Benoit D, Van Vlierberghe H, Decruyenaere J. Epidemiology, causes, evolution and outcome in a single-center cohort of 1116 critically ill patients with hypoxic hepatitis. Ann Intensive Care. 2018;8:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |