Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4669
Peer-review started: January 9, 2022
First decision: February 21, 2022
Revised: March 3, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: May 16, 2022
Processing time: 123 Days and 20.4 Hours
PD-1 inhibitors in combination with fruquintinib have not previously been reported as neoadjuvant therapy for patients with colorectal cancer. In this case report, the combination of a PD-1 inhibitor and fruquintinib demonstrated good efficacy in patients with MSI-H colorectal cancer.
The patient was a young man in his 30s who had MSI-H type colon cancer. The patient underwent four cycles of neoadjuvant therapy with a PD-1 inhibitor combined with fruquintinib before surgery, resulting in regression of the mass and a successful surgery.
Some patients with colorectal cancer have the MSI-H type, and the first-line chemotherapy regimen is not effective. However, PD-1 monoclonal antibody immunotherapy has a good therapeutic effect, which can be improved by combination therapy with fruquintinib. We recommend that patients with a history of colon or rectal cancer receive universal MSI testing; then, neoadjuvant therapy should be used.
Core Tip: A 30-year-old man was diagnosed with microsatellite unstable colon cancer. Prior to his surgical treatment, he was treated with a neoadjuvant regimen of PD1 inhibitors in combination with fruquintinib. This neoadjuvant regimen is rarely used; however, this patient had a good outcome. Subsequently, we performed a laparoscopic radical resection of his right colon cancer, which revealed no microscopic tumour cells. The patient was in good condition after the surgical treatment.
- Citation: Zhang HQ, Huang CZ, Wu JY, Wang ZL, Shao Y, Fu Z. PD-1 inhibitor in combination with fruquintinib therapy for initial unresectable colorectal cancer: A case report. World J Clin Cases 2022; 10(14): 4669-4675
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4669.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4669
Colorectal cancer is the third most common malignancy worldwide, and MSI-H is present in approximately 15% of colorectal cancers[1]. Microsatellites are short tandem repeats throughout the human genome, with single nucleotide, double nucleotide or high nucleotide repeats that are comprised of 10-50 repeats. Changes in the microsatellite length that result from the insertion or deletion of repeating units in tumour cells (rather than normal cells) are called microsatellite instability. A large number of studies have shown that MSI is caused by defects in mismatch repair (MMR) genes, which are closely related to the occurrence of tumours. MSI has been clinically used as a molecular marker for the prognosis and adjuvant treatment of colorectal cancer and other solid tumours[2].
Most patients with microsatellite instability colorectal cancer develop resistance to first-line chemotherapy when having a longer course of treatment. After its successful application in the treatment of melanomas, immunotherapy has been actively explored in different types of solid cancers, including colorectal cancer. PD-1 inhibitors have been shown to be effective in the MSI-H subtypes that are associated with mismatch repair defects in patients with metastatic colorectal cancer (CRC). Immunotherapy can allow for long-term remission in a subset of patients with metastatic CRC[3]. The latest guidelines also recommend pembrolizumab or nivolumab as first-line treatment options for patients with MSI-H CRC[2].
For patients with CRC who have failed to receive standard first-line and second-line treatments, the third-line treatment consists of regorafenib, fruquintinib, and TAS-102[4]. Clinical studies have shown that antiangiogenic drugs combined with an immune checkpoint blockade can significantly improve the effectiveness of the malignant tumour treatments[5-8]. In addition, patients with MSI-H colorectal cancer are characterized by high VEGF expression, which can be inhibited by fruquintinib. In this case, the combination of fruquintinib resulted in a better efficacy of the neoadjuvant therapy.
A 30-year-old man had an increased stool frequency and unformed stool over a year.
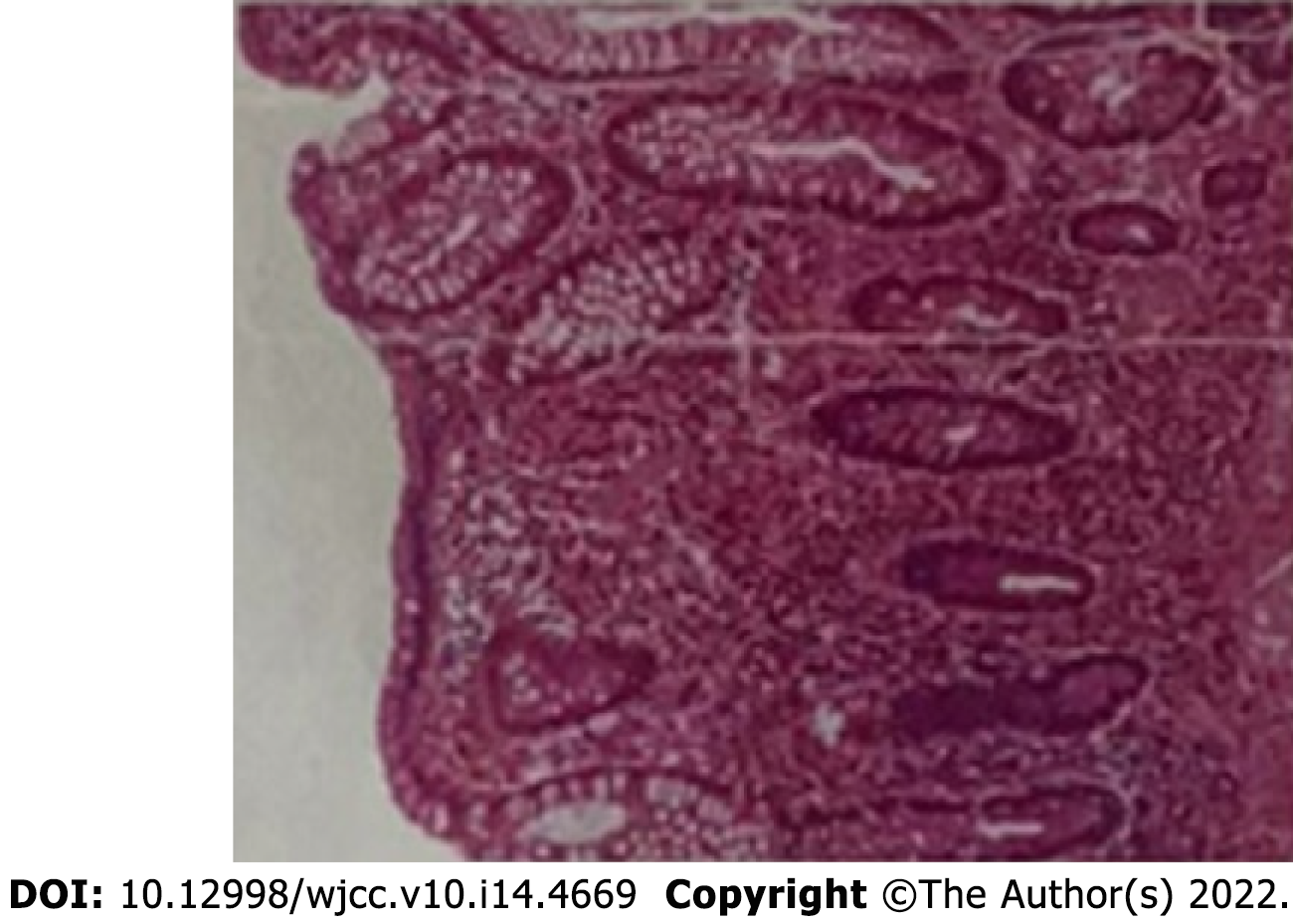
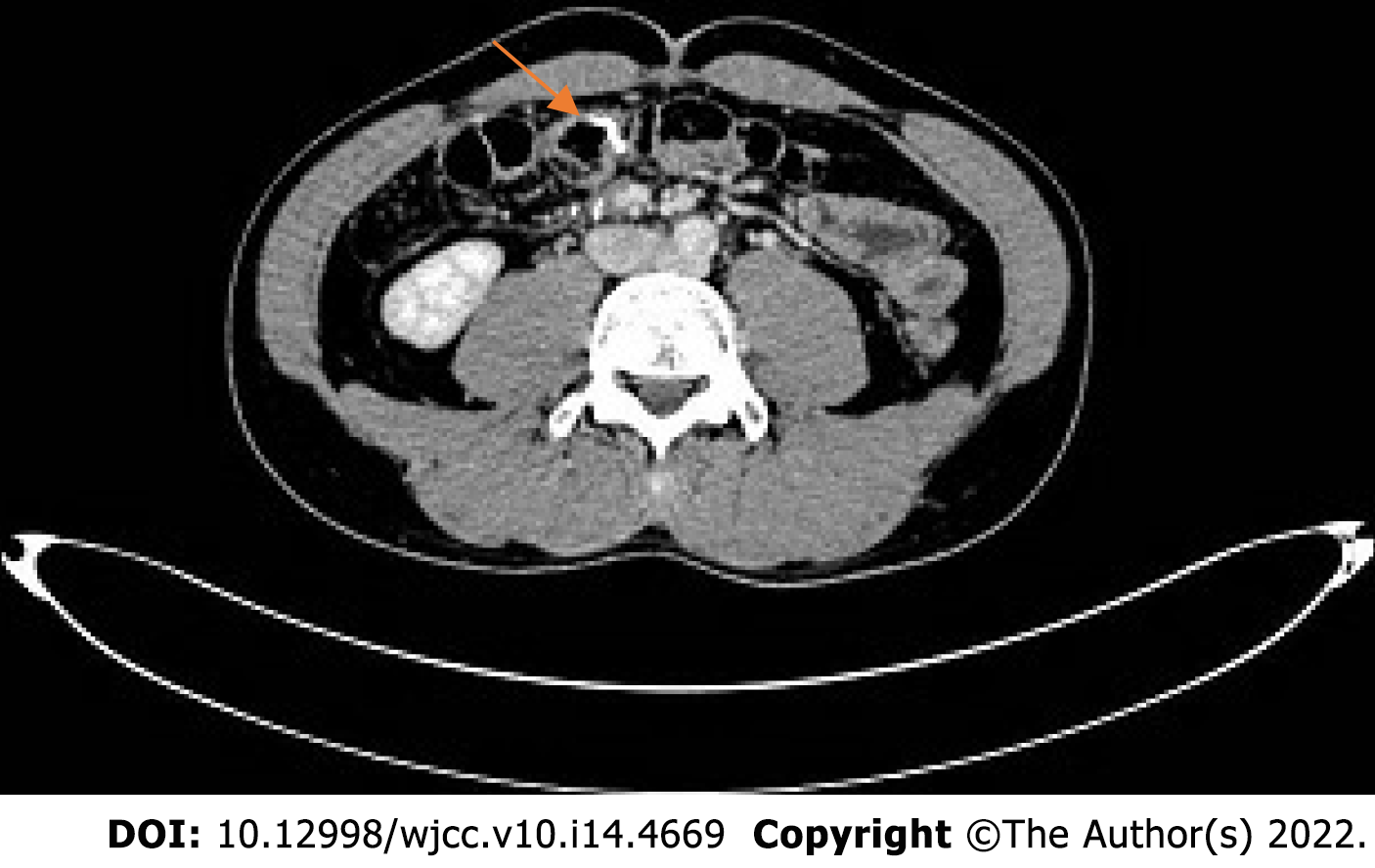
One year ago, the patient had an increased stool frequency without any obvious inciting cause, and his stool properties had changed. He had abdominal distension for 2 mo prior to presentation, and his stools were loose and unformed 3 to 4 times a day. On May 26, 2021, the patient underwent a colonoscopy. The colonoscopy found that the patient had a mass that was approximately 60 cm away from the anus. Pathology showed severe atypical hyperplasia and adenocarcinoma in parts of the glandular epithelium (Figure 1 and Table 1).
| Date | [T]/[S]/[E] |
| May, 2021 | [E] Colonoscopy, pathological examination, Abdominal CT examination |
| June, 2021 | [E] The evaluation of three tumour markers in the serum (AFP, CEA, CA199), Faecal occult blood test; [T] Four cycles of immunotherapy (paprizumab+ fruquintinib) |
| July, 2021 | [E] Abdominal CT examination |
| September, 2021 | [E] Abdominal CT examination |
| October, 2021 | [T] Radical resection of the right colon cancer; [E] Pathologic evaluation of the posttreatment tumour regression after neoadjuvant therapy; [S] The patient’s general appearance had improved; the patient was discharged smoothly |
| November, 2021 | [E] An abdominal CT was re-evaluated after surgery |
The patient’s previous medical history was free of disease.
The patient’s personal and family history was free of disease.
The patient’s vital signs were as follows: his temperature was 36.5 °C, his heart rate was 78 bpm, his respiratory rate was 17 breaths per minute, and his blood pressure was 121/84 mmHg. His abdomen was flat and soft, there were no gastrointestinal peristaltic waves, and he had no abdominal varicose and no tenderness or rebound pain in any part of his abdomen. He did not have any abdominal muscle tension, or any pain in his liver, spleen or ribs, no obvious masses were detected, and Murphy’s sign was negative. The test for shifting dullness was negative, no percussion pain was present in the area of the liver or in the areas of either of his kidneys, and his bowel sounds were heard at a frequency of 4 times/min. A digital rectal examination showed no obvious abnormalities.
CEA 3.23 ng/mL, faecal occult blood test (+).
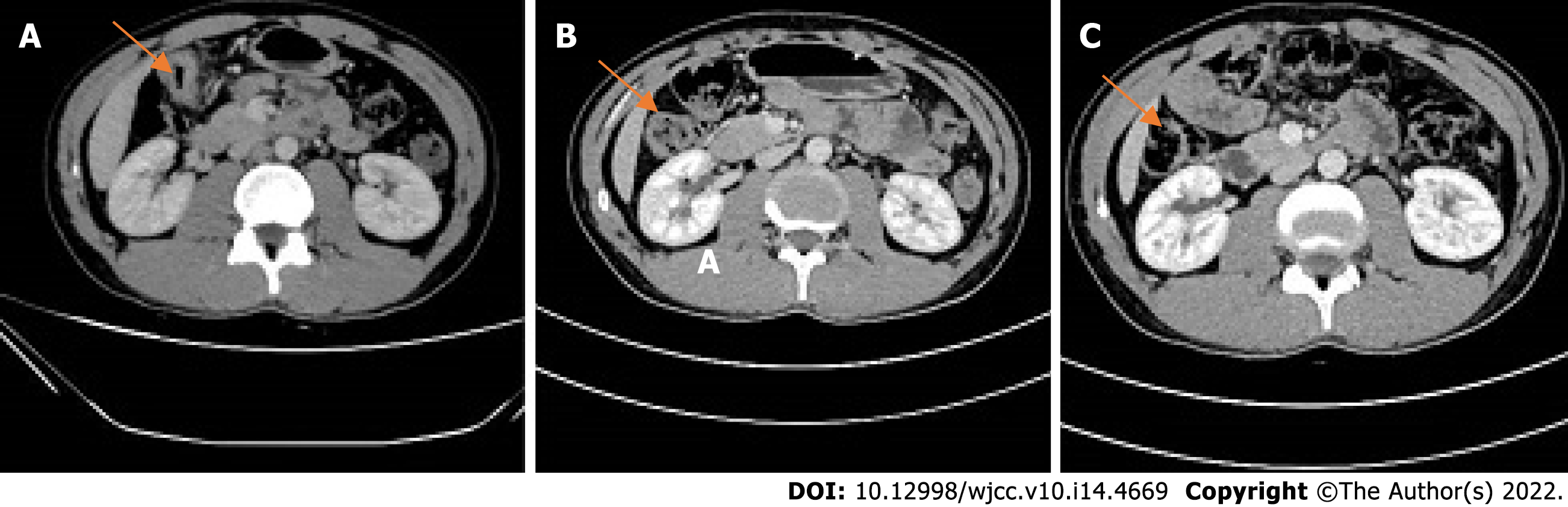
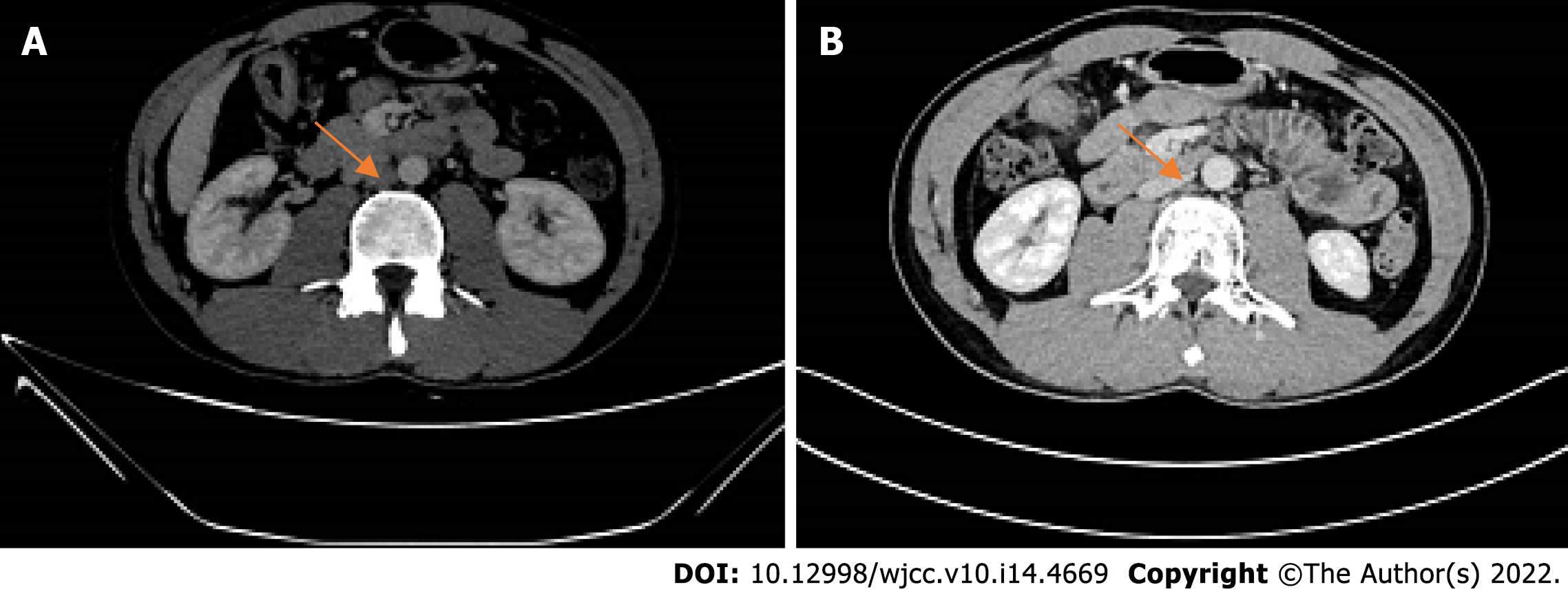
2021-05 Whole abdominal computed tomography (CT): The intestinal wall at the hepatic curvature of the colon was significantly thickened with enhancement, the surrounding fat space was blurred, and the adjacent lymph nodes were enlarged (Figure 2A). Enlarged lymph nodes were found beside the abdominal aorta and were approximately 1 cm in size (Figure 3A). It was recommended that an abnormal density of the left liver lobe be further evaluated.
The final diagnosis was a hepatic flexure tumour of the colon.
Considering the results of the relevant examinations, the patient's tumour had a high degree of malignancy. We decided to treat him with neoadjuvant therapy to decrease the size of the mass, reduce the stage of the tumour, and improve the complete resection rate, instead of going straight to surgery. After the exclusion of contraindications, the patient received the first cycle of the "PD-1 inhibitor (Paprizumab) 200 mg DL + Fruquintinib 1# Po QD DL -- 21" from 2021-06, and the process went smoothly without any complaints of discomfort from the patient.
Another abdominal CT was obtained on 2021-07. The range of the colonic lesions and the surrounding lymph nodes were reduced (Figure 2B). The para-aortic lymph nodes were smaller than before, with a reduction in size from 1 cm to 0.5 cm (Figure 3B). Abdominal CT (2021-09) showed further shrinkage of the colonic lesion (Figure 2C).
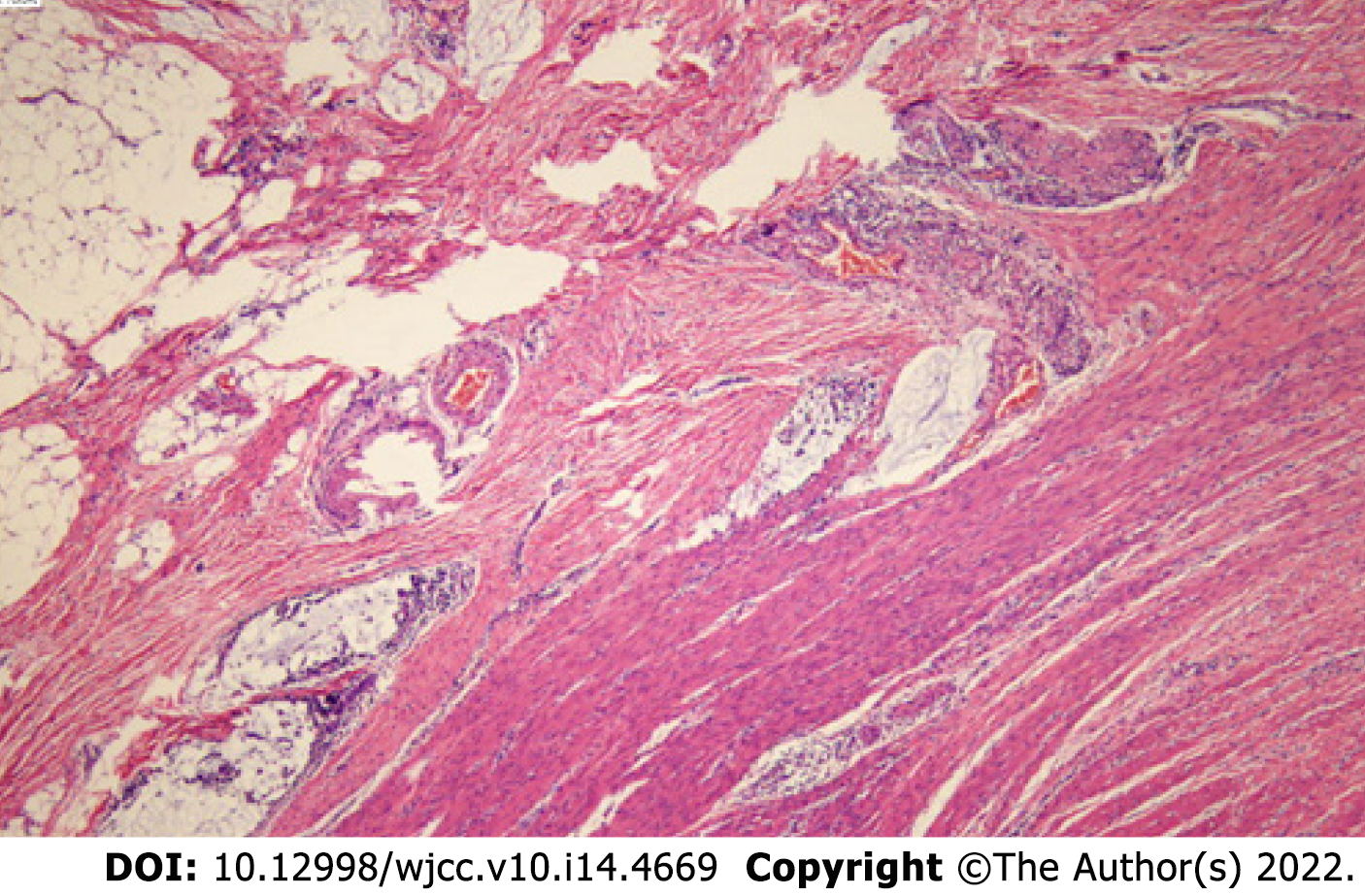
After 4 cycles of PD1+ fruquintinib treatment, the patient was admitted to the Department of Colorectal Surgery on 2021-10 for a radical colon cancer resection.Radical resection of a right colon cancer occurred on 2021-10. Laparoscopic examination showed that after the patient received neoadjuvant chemotherapy for the colon cancer, the tumour was located in the hepatic curvature of the transverse colon and did not invade the serous membrane, so a laparoscopic CME was planned. The operation went smoothly, the bleeding was minimal, the patient's vital signs were stable, and the postoperative specimens were sent out for examination. The pathologic evaluation of the posttreatment tumour regression (TRG) after neoadjuvant therapy showed a complete regression microscopically; no tumour cells were visible; and there were only a few mucous lakes that were infiltrating below the serosal membrane (Figure 4).
Postoperative immunomodulatory, anti-inflammatory, anti-venous thrombosis, nutritional and other treatments were provided to the patient. The patient's symptoms improved, and no special complaints of discomfort or general conditions were observed. The patient was successfully discharged 7 d after surgery. A postoperative abdominal CT (2021-11) showed that a metal suture shadow was present in the operative area, and no obvious mass shadow or abnormal enhancement was observed in the anastomosis (Figure 5).
In this case report, the neoadjuvant therapy for colon cancer included a combination of a PD-1 inhibitor and fruquintinib, which is a third-line treatment for colorectal cancer. PD1 inhibitors have fewer side effects than the existing neoadjuvant therapies, such as FOLFIRI, FOLFOX or CAPOX. The chemotherapeutic drugs of the previous regimen, such as capecitabine and oxaliplatin, can cause gastrointestinal reactions and abnormalities in the blood cell counts. Patients are also less likely to develop resistance to PD1 inhibitors. The patient was very responsive to immunotherapy and had excellent results.
The main treatment mode of microsatellite instability in colorectal cancer is immunomonotherapy. However, the current response rate of immunomonotherapy is only 60%. Immunomonotherapy combined with antiangiogenic drugs is an important method to improve the treatment efficacy. A study showed[9] that, compared with monotherapy, combination therapy could significantly inhibit tumour growth and could prolong the survival time of mice. In addition, fruquintinib combined with anti-PD-1 therapy reduced angiogenesis, enhanced the normalization of the vascular structure, and alleviated the tumour hypoxia. In addition, combination therapy reprogrammed the immune microenvironment by enhancing the release of chemokines, increasing the infiltration and activation of CD8+ T cells, decreasing the proportion of regulatory T cells, and increasing the proportion of M1/M2 macrophages. Therefore, a combination therapy that is comprised of PD1 inhibitors and antiangiogenic therapy is needed.
Regular CT examination also played an important role in observing the efficacy of the neoadjuvant therapy in this case. CT showed that there was a progressive regression of the intestinal masses after the neoadjuvant therapy. This also suggests that the examination of intestinal masses is convenient and intuitive, and people with a family history of this condition should undergo early screening with abdominal imaging examinations or colonoscopies, as well as regular screening.
It is also worth noting that the patient was in his 30 s, and this young of a patient has been very rare in previous studies. Is age a factor affecting the efficacy of immunotherapy? Are young people more resistant to PD1 inhibitors and drug combinations, such as fruquintinib? Further research is needed.
According to the NCCN guidelines for colon cancer (https://www.nccn.org/), it is recommended that all patients with a personal history of colon or rectal cancer undergo universal MMR or MSI testing[10]. After the test is completed, an appropriate treatment plan can be developed according to the results. For patients with colorectal cancer who have the opportunity to undergo surgery, preoperative neoadjuvant therapy is helpful for mass retraction and symptom relief and is conducive to the implementation of later surgery. Surgical treatment of colorectal tumours is still the most important treatment method, and neoadjuvant therapy is of great benefit prior to surgery. For example, in patients with rectal cancer who originally did not have anal preservation after neoadjuvant therapy, their masses had retreated, the distances between the lower edges of the mass and the anus had increased, and there was a chance to retain the anuses in these patients[11].
To our knowledge, PD-1 inhibitors in combination with fruquintinib have not previously been reported as a neoadjuvant therapy option in patients with colorectal cancer. This case report suggests that immunotherapy has a significant effect on microsatellite unstable colorectal cancer patients and that combining it with antiangiogenic drugs can improve the treatment efficacy. It is of great importance to improve relevant examinations, specify appropriate neoadjuvant therapy and combine medical treatment with surgical treatment in these patients.
We thank Sun J, MD (Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu Province, China).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Bhusal U, Nepal; Jeong KY, South Korea; Tchilikidi KY, Russia; Vyshka G, Albania S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, Vilar E, Tie J, Broaddus R, Kopetz S, Desai J, Overman MJ. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol. 2014;25:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, Garrido-Laguna I, Grem JL, Gunn A, Hecht JR, Hoffe S, Hubbard J, Hunt S, Johung KL, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Gregory KM, Gurski LA. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 947] [Article Influence: 236.8] [Reference Citation Analysis (16)] |
| 3. | Kishore C, Bhadra P. Current advancements and future perspectives of immunotherapy in colorectal cancer research. Eur J Pharmacol. 2021;893:173819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Dhillon S. Regorafenib: A Review in Metastatic Colorectal Cancer. Drugs. 2018;78:1133-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 5. | Hodi FS, Lawrence D, Lezcano C, Wu X, Zhou J, Sasada T, Zeng W, Giobbie-Hurder A, Atkins MB, Ibrahim N, Friedlander P, Flaherty KT, Murphy GF, Rodig S, Velazquez EF, Mihm MC Jr, Russell S, DiPiro PJ, Yap JT, Ramaiya N, Van den Abbeele AD, Gargano M, McDermott D. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res. 2014;2:632-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 463] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 6. | Konecny GE. Inhibition of PD-1 and VEGF in microsatellite-stable endometrial cancer. Lancet Oncol. 2019;20:612-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Shigeta K, Datta M, Hato T, Kitahara S, Chen IX, Matsui A, Kikuchi H, Mamessier E, Aoki S, Ramjiawan RR, Ochiai H, Bardeesy N, Huang P, Cobbold M, Zhu AX, Jain RK, Duda DG. Dual Programmed Death Receptor-1 and Vascular Endothelial Growth Factor Receptor-2 Blockade Promotes Vascular Normalization and Enhances Antitumor Immune Responses in Hepatocellular Carcinoma. Hepatology. 2020;71:1247-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 299] [Article Influence: 59.8] [Reference Citation Analysis (0)] |
| 8. | Zhao S, Ren S, Jiang T, Zhu B, Li X, Zhao C, Jia Y, Shi J, Zhang L, Liu X, Qiao M, Chen X, Su C, Yu H, Zhou C, Zhang J, Camidge DR, Hirsch FR. Low-Dose Apatinib Optimizes Tumor Microenvironment and Potentiates Antitumor Effect of PD-1/PD-L1 Blockade in Lung Cancer. Cancer Immunol Res. 2019;7:630-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 9. | Wang Y, Wei B, Gao J, Cai X, Xu L, Zhong H, Wang B, Sun Y, Guo W, Xu Q, Gu Y. Combination of Fruquintinib and Anti-PD-1 for the Treatment of Colorectal Cancer. J Immunol. 2020;205:2905-2915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 10. | Cui S, Zhang X, Zou R, Ye F, Wang Y, Sun J. MLH1 Exon 12 Gene Deletion Leading to Lynch Syndrome: A Case Report. Oncol Res Treat. 2021;44:414-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Hata T, Takahashi H, Sakai D, Haraguchi N, Nishimura J, Kudo T, Chu M, Takemasa I, Taroh S, Mizushima T, Doki Y, Mori M. Neoadjuvant CapeOx therapy followed by sphincter-preserving surgery for lower rectal cancer. Surg Today. 2017;47:1372-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |