Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4661
Peer-review started: December 1, 2021
First decision: January 12, 2022
Revised: January 18, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 16, 2022
Processing time: 163 Days and 6.3 Hours
Papillary thyroid carcinoma (PTC) is the most common endocrine malignancy. Papillary thyroid microcarcinoma (PTMC) accounts for the majority of PTC cases. However, concurrent pulmonary and hepatic metastases of PTMC are rarely seen. Here, we present a patient with coexisting liver and lung metastases from PTMC.
We describe a 26-year-old woman with PTMC with multiple concurrent metastases. After 3 d of unexplained fever, she was admitted to our hospital. Her thyroid functional tests were abnormal. Her positron emission tomography (PET)/magnetic resonance imaging (MRI) examination showed increased fluorodeoxyglucose (FDG) metabolism and space-occupying lesions in the left lobe of the thyroid. Additionally, PET/MRI images revealed multiple nodules in the lung and liver with increased FDG metabolism. Chest computer tomography (CT) showed multiple pulmonary metastases. Abdominal ultrasound and liver MRI showed multiple space-occupying lesions in the liver. The patient underwent total thyroidectomy and central lymph node dissection. Postoperative patho
Since patients with thyroid cancer concurrent with hepatopulmonary metastases have rarely been reported, our case will highlight the clinical and pathological profiles of these patients.
Core Tip: Concurrent pulmonary and hepatic metastases of papillary thyroid microcarcinoma are not often seen due to their rarity and nonspecific presentations. Herein, we provided a successful example of the diagnosis and treatment of pulmonary and hepatic metastases of papillary thyroid microcarcinoma in a young female patient. Our case emphasizes that distant metastases of papillary thyroid carcinoma can occur in young patients.
- Citation: Yang CY, Chen XW, Tang D, Yang WJ, Mi XX, Shi JP, Du WD. Hepatopulmonary metastases from papillary thyroid microcarcinoma: A case report. World J Clin Cases 2022; 10(14): 4661-4668
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4661.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4661
Thyroid cancer is the most common endocrine tumor with a strong female preponderance (3:1)[1]. Papillary thyroid carcinoma (PTC) is a well-differentiated endocrine malignancy. Papillary thyroid microcarcinoma (PTMC) is defined by the World Health Organization as PTC with a maximum diameter ≤ 10 mm. Papillary thyroid microcarcinoma increases the incidence of thyroid cancer by 50%[2]. The main manifestations of PTCs are neck masses and thyroid nodules. However, distant metastasis of PTMC is rare, affecting bone, lung and chest lymph nodes, although local regional metastases in neck lymph nodes are commonly seen[3-5]. PTMC simultaneously metastasized to the liver and lung is very rare. Here, we report a case of PTMC concurrent with liver and pulmonary metastases.
A 26-year-old woman who presented with unexplained fever was admitted to our hospital for further examination.
The patient showed a clear mind and no significant weight loss in the past three months.
She had no smoking or drinking history and no family history of tumors. She had no cough or expectoration. Ethical approval for publishing this case was obtained from the First Medical College of Zhejiang Chinese Medical University Research Ethics Committee.
She denied a family history of hereditary disease.
Her physical examination showed nothing abnormal.
Her thyroid function tests showed elevated levels of thyroid-stimulating hormone and antithyroglobulin antibody, with a decreased level of thyroglobulin. Her biochemical tests showed elevated levels of triglycerides and cholesterol. Routine blood tests showed neutrophilia and lymphocytosis. Humoral tumor screening presented an elevated level of CA50. Her blood coagulation function was normal.
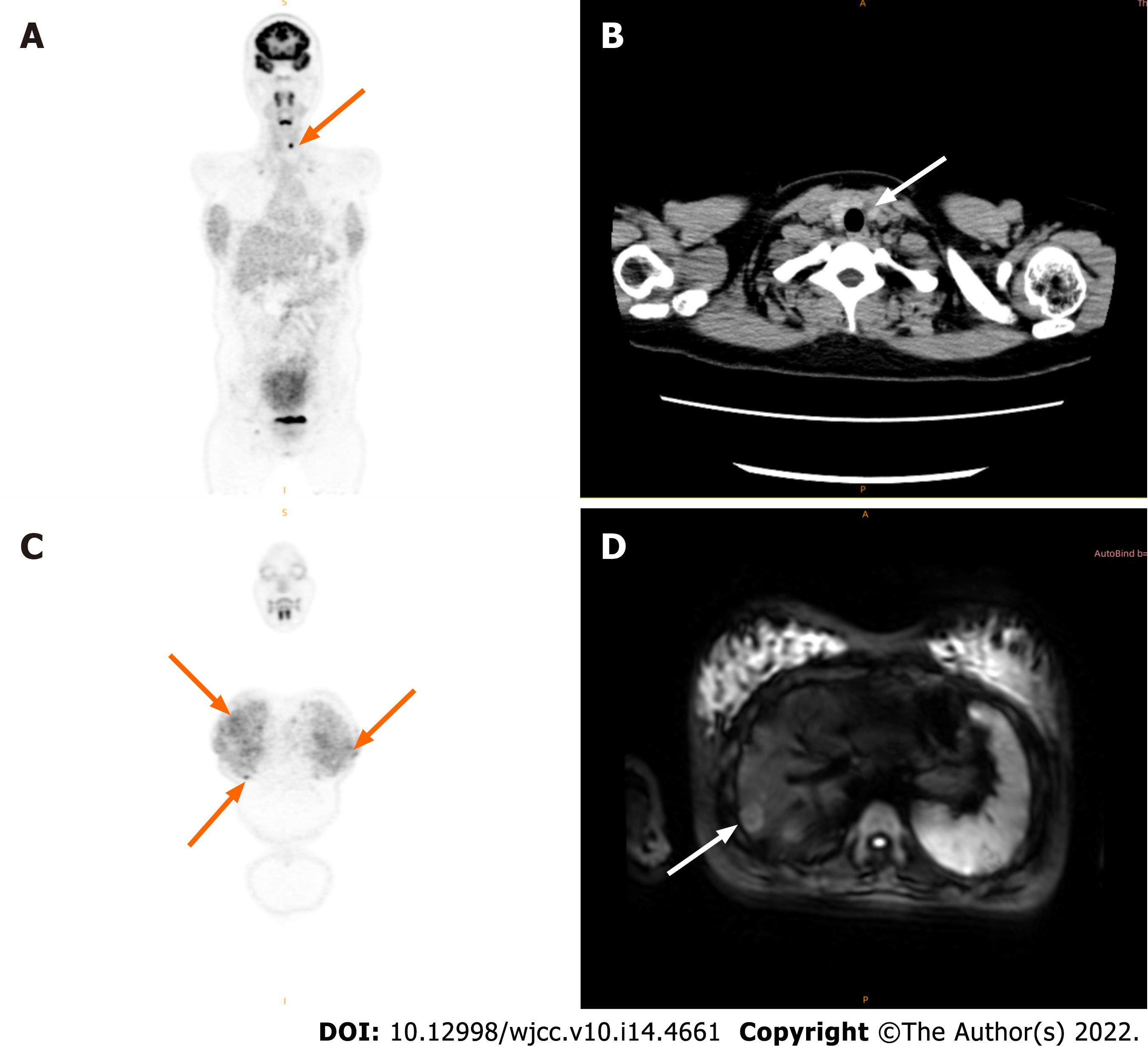
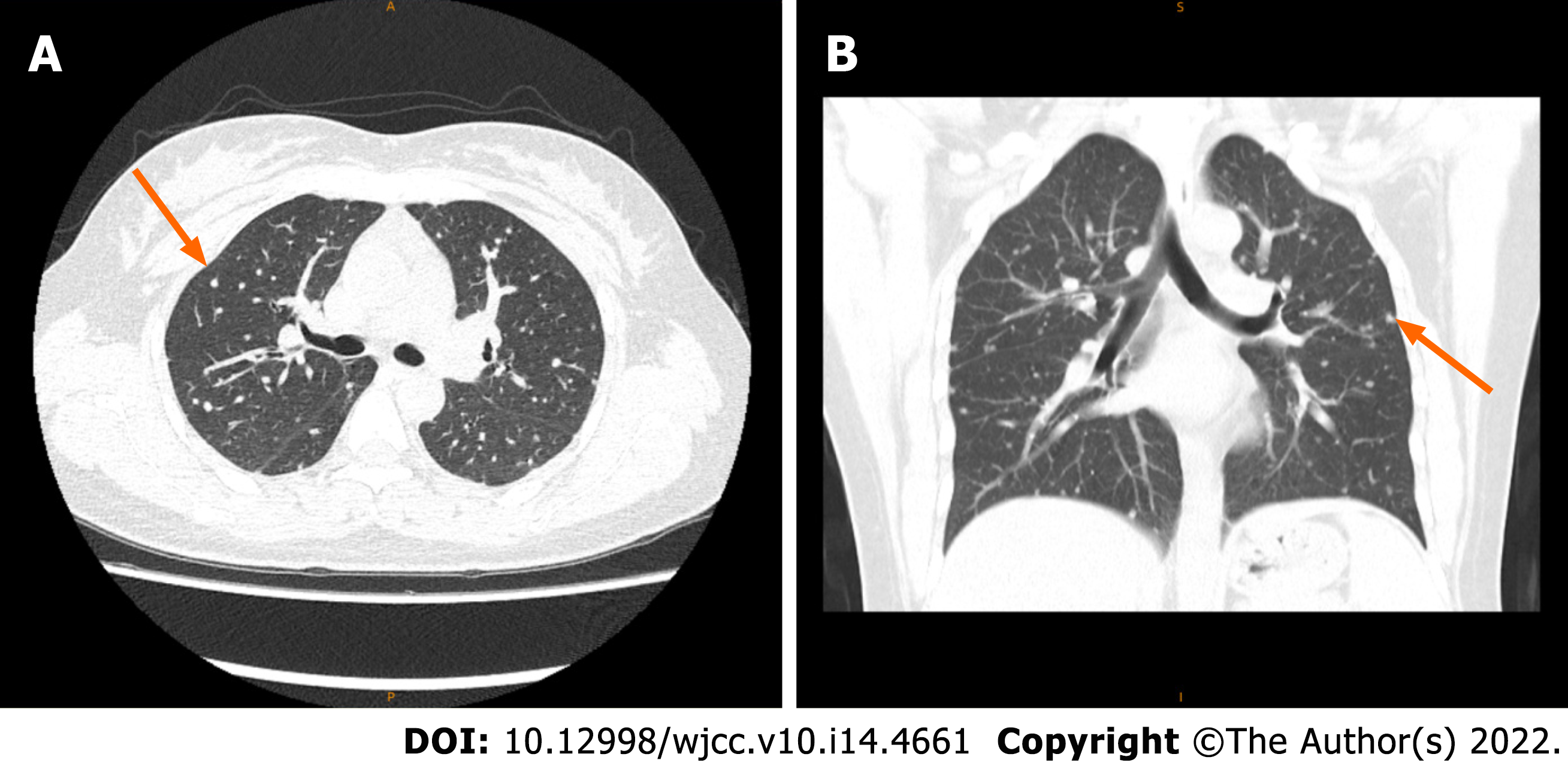
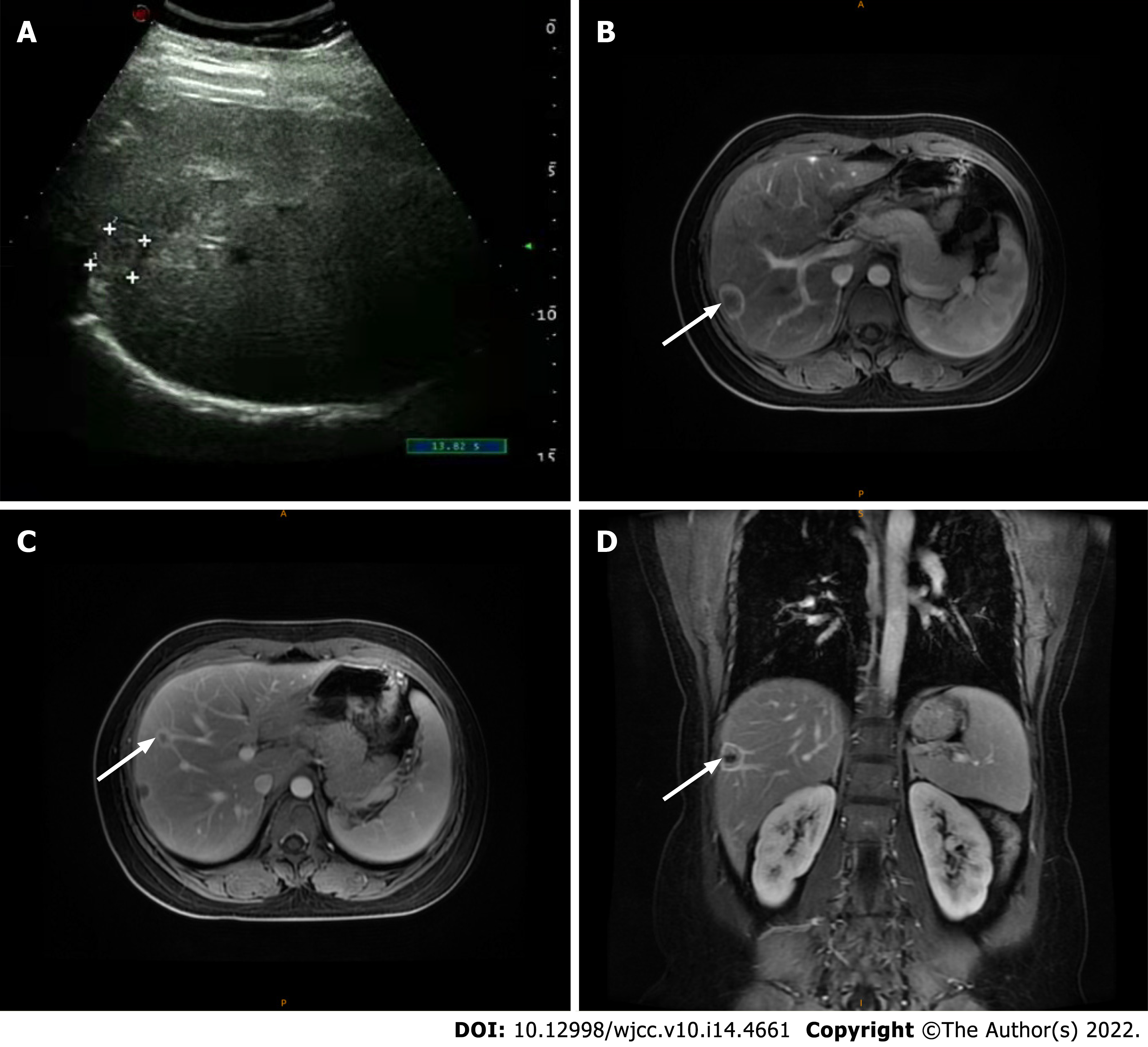
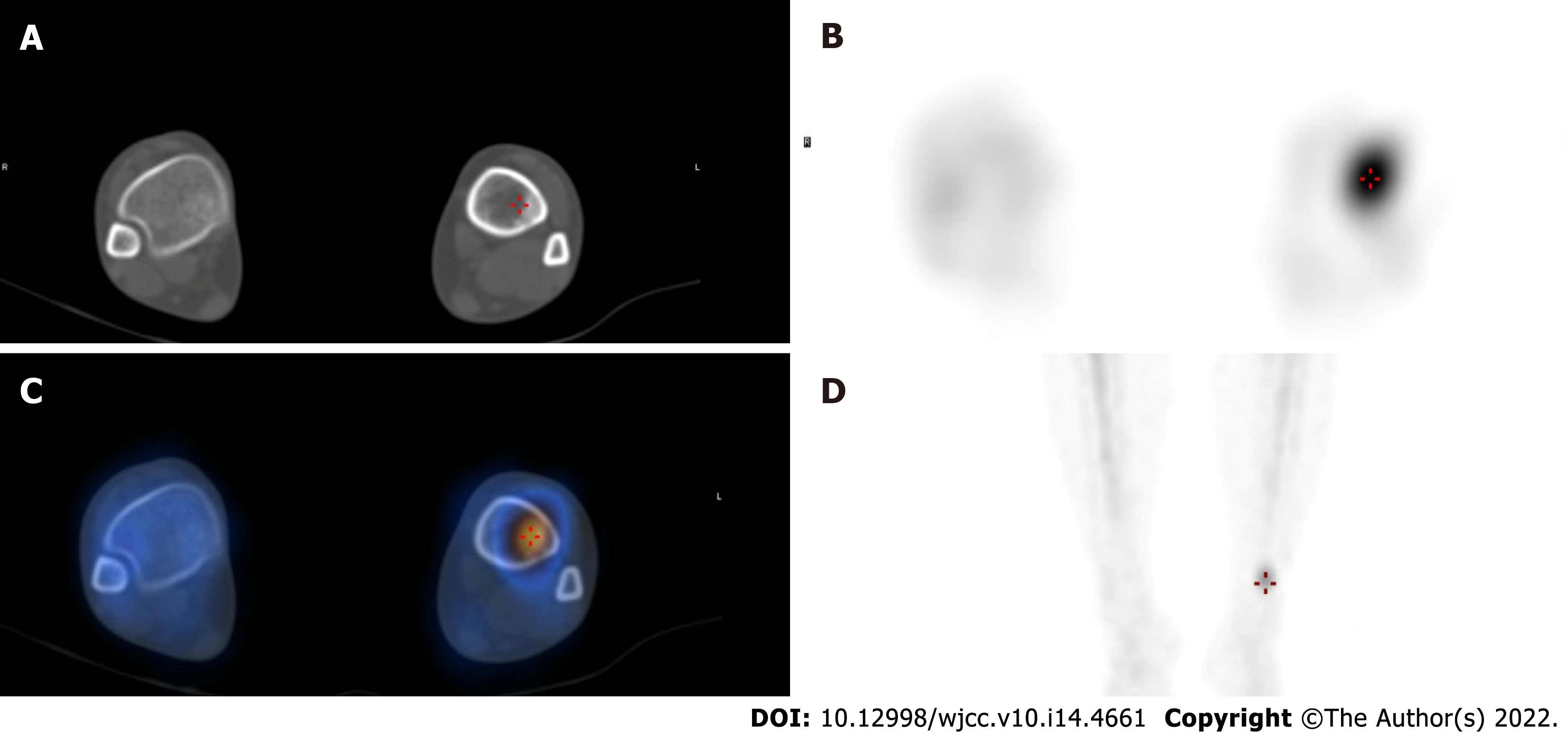
She underwent a positron emission tomography/magnetic resonance imaging (PET/MRI) examination in our hospital. The PET/MRI images showed a space-occupying lesion in the left thyroid with increased fluorodeoxyglucose (FDG) metabolism (Figure 1A), and a Computer tomography (CT) scan revealed that the lesions in the left lobe of the thyroid showed low-density nodular changes involving the thyroid capsule (Figure 1B). The PET/MRI images also showed multiple diffuse nodules (maximum 0.8 cm) in the lung with increased FDG metabolism and multiple nodules (maximum 2.0 cm) in the liver with increased FDG metabolism (Figure 1). Chest CT showed multiple metastases in both lungs, multiple low-density shadows in the liver, and small calcifications in the left breast (Figure 2). Abdominal ultrasound showed a fatty liver and multiple liver nodules (Figure 3A). MRI showed multiple space-occupying lesions in the liver (Figure 3B-D). Whole-body bone imaging and organ tomography showed a metabolically active left tibia and unevenly increased local bone density (Figure 4).
Collectively, based on the medical imaging results and pathological features, a diagnosis of hepatopulmonary metastasis from papillary thyroid microcarcinoma was made.
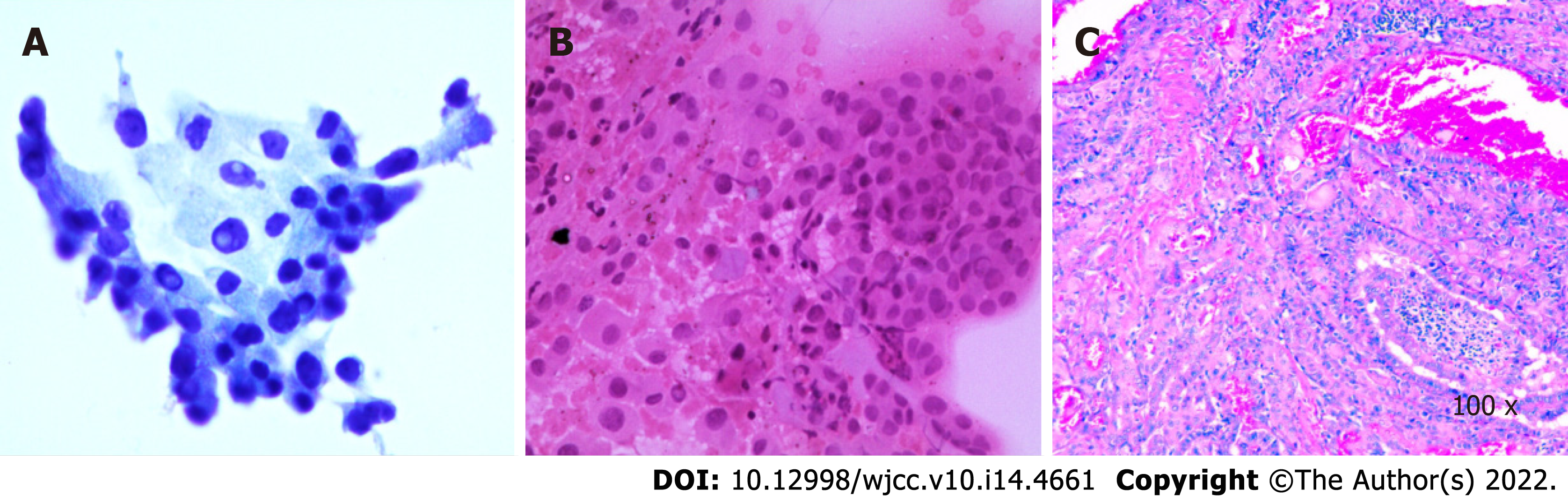
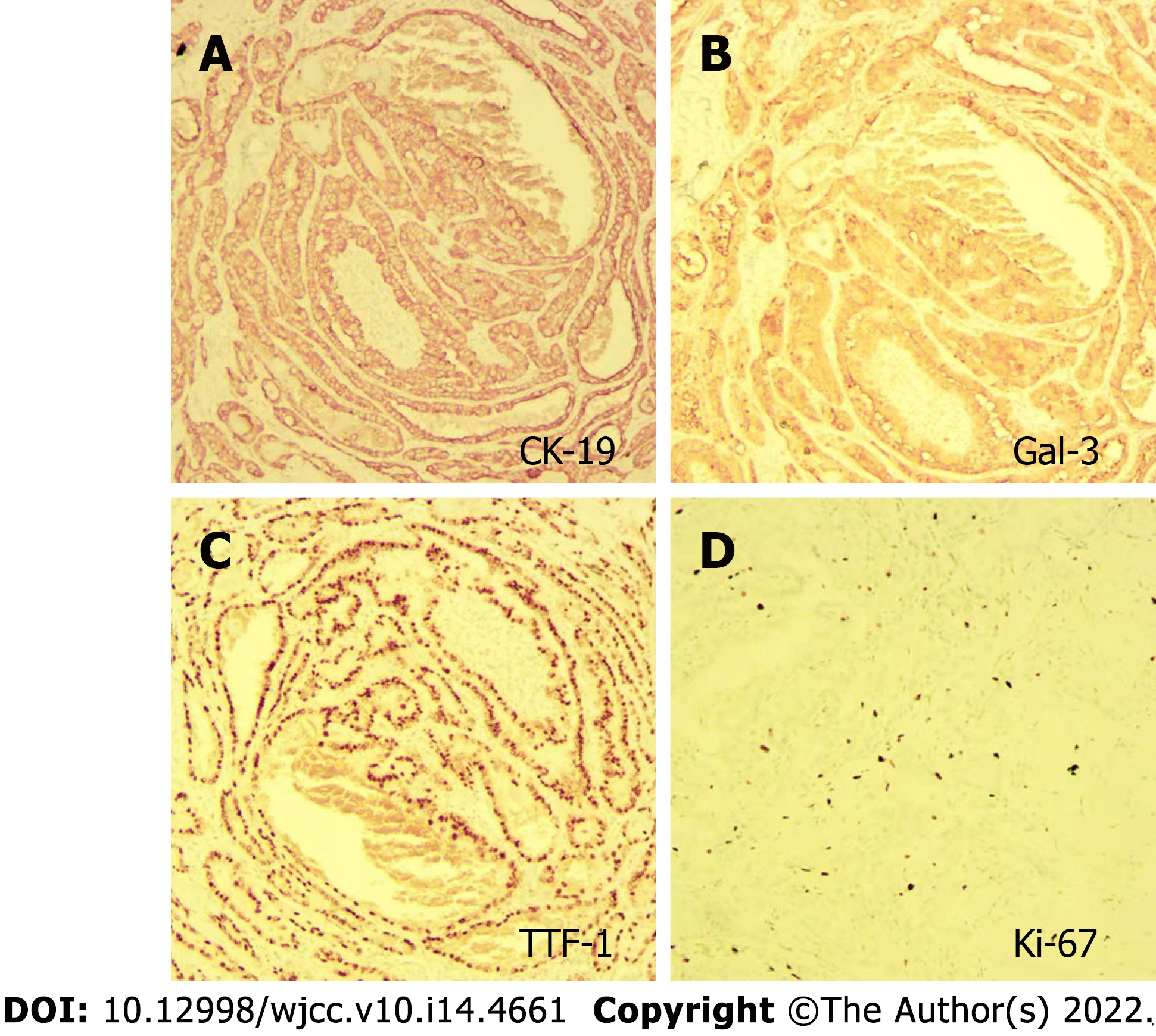
The patient underwent thyroidectomy and central node dissection. Postoperative pathology revealed multiple papillary microcarcinomas in the left thyroid and one foci with the follicular subtype (Figure 5). The carcinomas had invaded the capsule and presented no lymph node metastasis. The immunohistochemical results showed positive signals for CK-19, Gal-3, TTF, and Ki-67 (3%) (Figure 6).
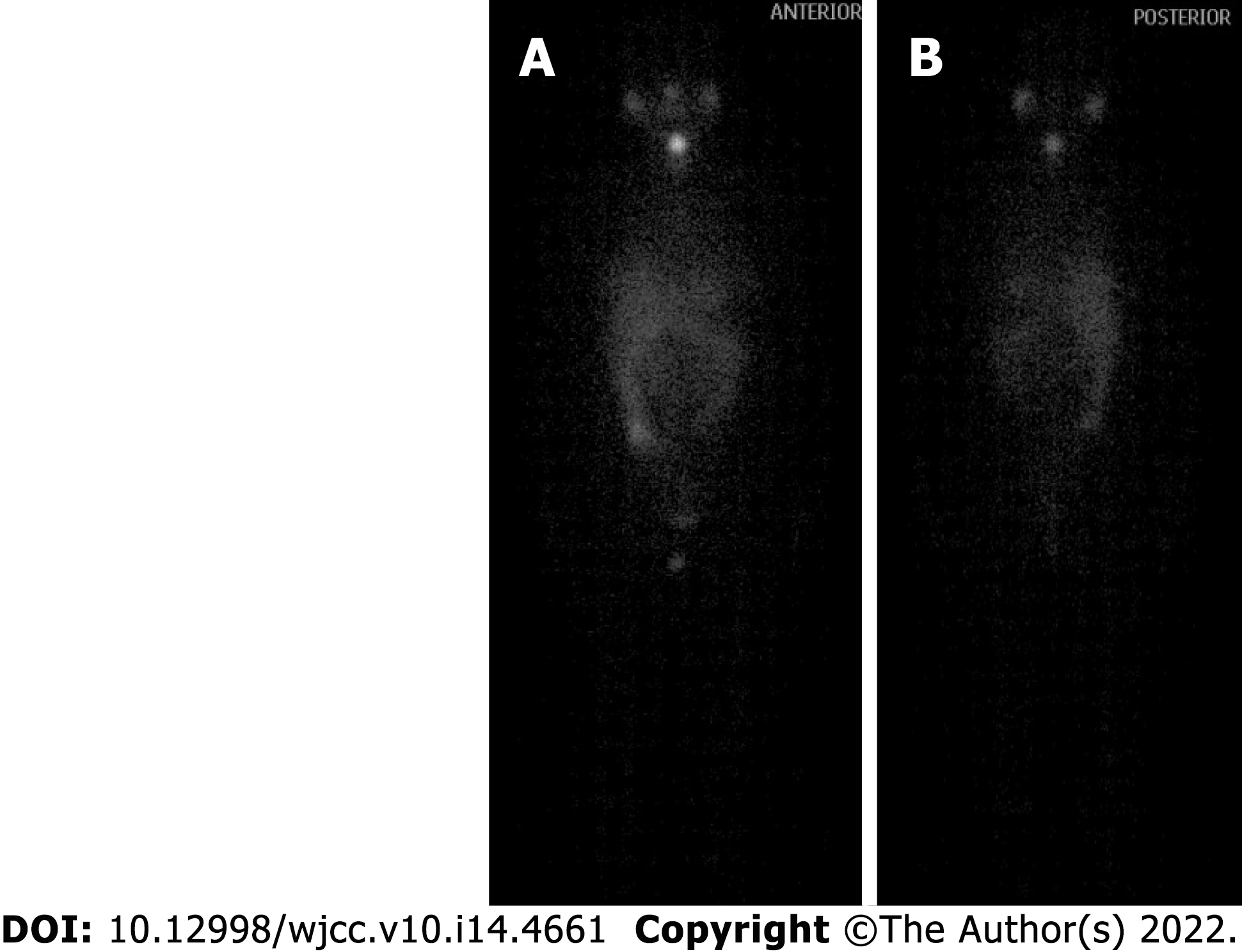
One year after thyroid cancer surgery, the patient was given iodine-131 treatment in our hospital. As shown in Figure 7, iodine imaging in the thyroid area was considered to be residual thyroid tissue. Multiple small nodules were found in both lungs, with no significant iodine uptake. A small amount of pleural effusion was observed on both sides of the lung. Multiple lymph nodes were present in the bilateral neck and supraclavicular areas without iodine intake. Physiological iodine intake was observed in the nasopharynx, oral cavity, salivary glands, gastrointestinal tract, and bladder (Figure 7). The patient had recovered well after the operation, and the incision had healed well. The patient was treated after the iodine therapy with daily oral levothyroxine sodium administration.
PTC accounts for approximately 85% of all follicular-derived well-differentiated thyroid cancers[6]. The 10-year survival rate is more than 80%, and these tumors are considered to be indolent[7,8]. PTMC is a subtype of PTC with a foci diameter ≤ 10 mm. PTMC accounts for the majority of PTC cases. Distant metastasis of PTMC is rare. The most common metastatic sites include the bone and lung, while brain, eye, breast, liver, kidney, muscle, and skin metastases are not commonly seen and only appear in patients with advanced tumor diseases. Here, we present a patient with PTMC who had simultaneous metastases to the lung and liver. Coexisting lung and liver metastases in PTC patients are not commonly seen.
Predictive factors for PTC metastasis included age, sex, thyroid function, Hashimoto's thyroiditis, multifocal tumor, tumor size, capsular invasion, and extrathyroidal extensions. The histopathological characteristics of tumors, such as their bilaterality, multifocality, extrathyroidal extension, capsular invasion, and lymph node metastasis, are important indicators of their invasiveness and they affect the prognosis[9,10].
Liver metastasis from PTMC is a rare event with a reported frequency of only 0.5%[11]. Liver masses can be detected by various imaging modalities, such as ultrasonography, computed tomography, and magnetic resonance imaging. Liver masses are usually 131I-negative in PTMC patients with liver metastasis[12], which is consistent with the observations in our patient. PTC liver metastasis has a poor prognosis. Surgical resection of liver lesions has been reported to offer the best chance for prolonged survival[13]. An increased age in cases of thyroid cancer with lung metastasis increases the mortality risk. In a study performed by Huang, the mortality rates of thyroid cancer with lung metastasis were 32.78% (118/360), 46.71% (156/334), 53.93% (199/369), 58.96% (158/268) and 82.76% (72/87) in patients aged ≤ 55 years, > 55 ≤ 65 years, > 65 ≤ 75 years, > 75 ≤ 85 years and > 85 years[14]. Since our patient was a young mother of a young child, we suggested routine follow-up liver function tests to monitor the pathophysiology of the liver.
PTMC has no early typical symptoms due to its anatomic location. Therefore, a delay in clinical diagnosis is inevitable, which leads to its diagnosis in the advanced stage. Thus, a primary tumor in the thyroid is usually not diagnosed until the symptoms of a secondary metastatic tumor appear[15]. In this case, the patient did not exhibit clinical manifestations of the disease at the initial stage, and no corresponding clinical symptoms were found even after metastasis occurred. Multiple metastases of thyroid cancer were incidentally found during the patient’s examination for unexplained fever, indicating that the disease is easily missed. Therefore, a better clinical index or screening method is needed.
Male patients with distant metastases from PTMC have a high risk of death[8,15]. However, PTC patients with distant metastases have lower levels of dedifferentiation than differentiated thyroid carcinoma patients with distant metastases. Therefore, PTC exhibits more indolent behaviors than differentiated thyroid carcinoma, even with distant metastases. PTC nevertheless has a generally favorable prognosis for long-term survival, even with distant metastases. The clinical manifestations vary from the early to late stages of the disease[6]. The maximal PTMC foci is 1 cm or less[16]. In our patient, postoperative pathology revealed one PCT foci with a diameter of 0.8 cm. Total thyroidectomy with central node dissection is an effective treatment for PTC patients[17]. Long-term follow-up of PTMC patients is needed after surgical treatment[18,19]. Periodic thyroglobulin and thyroglobulin autoantibody measurements are recommended for PTMC patients[20]. An active surveillance approach is recommended by the American Thyroid Association guidelines as an alternative option for patients with low-risk PTMC[18]. Our patient was a 26-year-old woman with PTMC with multiple metastases. She was recommended to take levothyroxine sodium tablets and consume a low iodine diet after her surgery. We also suggested routine thyroid function test and liver imaging during follow-up. She is still alive and actively engaging in daily life.
Our patient's diagnosis of hepatopulmonary metastasis from papillary thyroid carcinoma was based on imaging findings and pathological results. PET/MRI, ultrasonography, CT, and MRI revealed multiple nodules in the liver and lung. Our case will provide a valuable reference for the diagnosis and treatment of papillary thyroid microcarcinoma patients in the future.
In conclusion, we present the case of a young woman with PTMC metastasis to her liver and lung. Since patients with thyroid cancer concurrent with hepatopulmonary metastases have rarely been reported, our case highlights the clinical and pathological profiles of these patients.
The authors would like to thank colleagues from the Institute of Translational Medicine of the Affiliated Hospital of Hangzhou Normal University and colleagues from the Department of Hepatobiliary Surgery of the First Clinical Medical College of Zhejiang Chinese Medical University for their support and collaboration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cainap C, Romania; Pop TL, Romania S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Stewart LA, Kuo JH. Advancements in the treatment of differentiated thyroid cancer. Ther Adv Endocrinol Metab. 2021;12:20420188211000251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12:646-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 709] [Article Influence: 78.8] [Reference Citation Analysis (0)] |
| 3. | Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1879] [Cited by in RCA: 1726] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 4. | Sampson E, Brierley JD, Le LW, Rotstein L, Tsang RW. Clinical management and outcome of papillary and follicular (differentiated) thyroid cancer presenting with distant metastasis at diagnosis. Cancer. 2007;110:1451-1456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 191] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 5. | Carmel Neiderman NN, Duek I, Ravia A, Yaka R, Warshavsky A, Ringel B, Muhanna N, Horowitz G, Ziv Baran T, Fliss DM. The incidence of postoperative re-stratification for recurrence in well-differentiated thyroid cancer-a retrospective cohort study. Gland Surg. 2021;10:2354-2367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA. 2017;317:1338-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1537] [Article Influence: 192.1] [Reference Citation Analysis (0)] |
| 7. | Yu XM, Wan Y, Sippel RS, Chen H. Should all papillary thyroid microcarcinomas be aggressively treated? Ann Surg. 2011;254:653-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 224] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 8. | Weng HY, Yan T, Qiu WW, Xi C, Hou LY, Yang ZL, Qiu ZL. Long-term outcomes and prognostic factors in papillary thyroid microcarcinoma patients with distant metastases. Endocrine. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Zhao L, Sun X, Luo Y, Wang F, Lyu Z. Clinical and pathologic predictors of lymph node metastasis in papillary thyroid microcarcinomas. Ann Diagn Pathol. 2020;49:151647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Jeon MJ, Kim WG, Choi YM, Kwon H, Lee YM, Sung TY, Yoon JH, Chung KW, Hong SJ, Kim TY, Shong YK, Song DE, Kim WB. Features Predictive of Distant Metastasis in Papillary Thyroid Microcarcinomas. Thyroid. 2016;26:161-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Salvatori M, Perotti G, Rufini V, Maussier ML, Summaria V, Fadda G, Troncone L. Solitary liver metastasis from Hürthle cell thyroid cancer: a case report and review of the literature. J Endocrinol Invest. 2004;27:52-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Song HJ, Xue YL, Xu YH, Qiu ZL, Luo QY. Rare metastases of differentiated thyroid carcinoma: pictorial review. Endocr Relat Cancer. 2011;18:R165-R174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Kuo CS, Tang KT, Lin JD, Yang AH, Lee CH, Lin HD. Diffuse sclerosing variant of papillary thyroid carcinoma with multiple metastases and elevated serum carcinoembryonic antigen level. Thyroid. 2012;22:1187-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Huang X, Xia Q, Huang Y, Peng A, Yang J. Age increased the cancer-specific mortality risk of thyroid cancer with lung metastasis. Clin Endocrinol (Oxf). 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Nunes KS, Matos LL, Cavalheiro BG, Magnabosco FF, Tavares MR, Kulcsar MA, Hoff AO, Kowalski LP, Leite AK. Risk factors associated with disease-specific mortality in papillary thyroid cancer patients with distant metastases. Endocrine. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Pu W, Shi X, Yu P, Zhang M, Liu Z, Tan L, Han P, Wang Y, Ji D, Gan H, Wei W, Lu Z, Qu N, Hu J, Hu X, Luo Z, Li H, Ji Q, Wang J, Zhang X, Wang YL. Single-cell transcriptomic analysis of the tumor ecosystems underlying initiation and progression of papillary thyroid carcinoma. Nat Commun. 2021;12:6058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 17. | Pastorčić Grgić M, Stubljar B, Perše P, Zekan Vučetić M, Šitić S. Total Thyroidectomy with Central Node Dissection is a Valuable Option in Papillary Thyroid Cancer Treatment. Acta Clin Croat. 2020;59:102-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9686] [Article Influence: 1076.2] [Reference Citation Analysis (1)] |
| 19. | Gao M, Ge M, Ji Q, Cheng R, Lu H, Guan H. 2016 Chinese expert consensus and guidelines for the diagnosis and treatment of papillary thyroid microcarcinoma. Cancer Biol Med. 2017;14:203-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 20. | Knappe L, Giovanella L. Life after thyroid cancer: the role of thyroglobulin and thyroglobulin antibodies for postoperative follow-up. Expert Rev Endocrinol Metab. 2021;16:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |