Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4648
Peer-review started: December 8, 2021
First decision: January 25, 2022
Revised: January 29, 2022
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: May 16, 2022
Processing time: 156 Days and 0.1 Hours
Pleomorphic adenoma (PA) is the most common type of salivary gland tumor, and its common sites are parotid gland, sinus, nasal septum and cleft palate. PA is an uncommon benign type of tumor occurring in the breast, and there are few reports of cases in Asia.
An 84-year-old woman found a mass in the upper outer quadrant of the right breast > 1 year ago. The patient underwent a right breast lumpectomy and sentinel lymph node biopsy. The pathological diagnosis was PA in the upper outer quadrant of the right breast, and the malignant component was malignant adenomyoepithelioma. The postoperative course was uneventful, and no chemotherapy was administered. At 18 mo of follow-up, the patient is alive and well, with no evidence of recurrent disease.
Patients with breast PA should first undergo extended excision of breast masses followed by pathological examination. If malignancy is confirmed or the surgical margin is positive, modified radical mastectomy should be performed.
Core Tip: Pleomorphic adenoma (PA) is an uncommon benign type of tumor occurring in the breast, and there are few reports of cases in Asia. We here present an extremely rare case of malignant adenomyoepithelioma in PA. We performed a detailed pathological examination, immunohistochemistry and fluorescence in situ hybridization (including detection of PLAG1 and HMGA2 gene rearrangement). We also summarize the pathological differential diagnosis of PA.
- Citation: Zhang WT, Wang YB, Ang Y, Wang HZ, Li YX. Diagnosis of an extremely rare case of malignant adenomyoepithelioma in pleomorphic adenoma: A case report. World J Clin Cases 2022; 10(14): 4648-4653
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4648.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4648
Pleomorphic adenoma (PA) of the breast is a rare tumor in postmenopausal women[1]. It is a mixed tumor with diverse morphology and ability to differentiate in different forms[2]. Because of its rarity, accurate diagnosis via histopathological analysis is important to determine the treatment plan. We here present an extremely rare case of malignant adenomyoepithelioma in PA and performed a detailed pathological examination, immunohistochemistry and fluorescence in situ hybridization (FISH). We also summarize the pathological differential diagnosis of PA.
An 84-year-old woman found a mass in the upper outer quadrant of the right breast > 1 year ago.
On April 8, 2020, she went to the clinic complaining of pain and discomfort intermittently in the lump, which had not subsided for 12 mo.
Her personal and family history was unremarkable.
There was nothing special to mention in her personal and family history.
In the upper outer quadrant of the right breast, a mass of about 3.0 cm × 2.0 cm × 2.0 cm was palpable, with a hard texture, clear boundary, and no tenderness. No abnormalities were seen in the nipple and skin. There was no enlarged lymph node in the right axilla.
Laboratory examinations were normal.
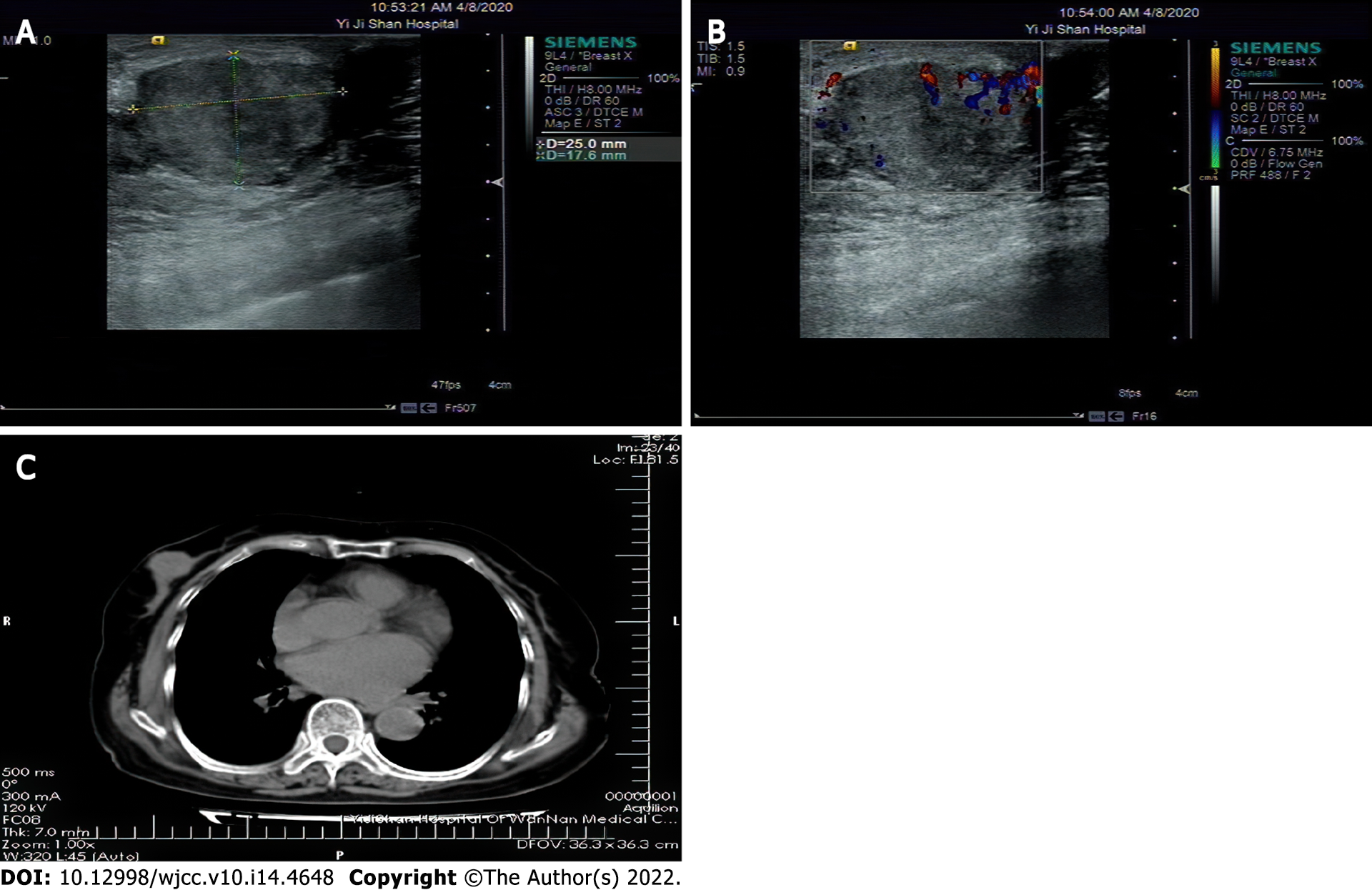
Breast ultrasound examination detected a mixed mass was detected in the right breast (Figure 1A). The boundary between the mass and its surroundings was clear, the echo was uneven, and the blood flow signal was disordered in some areas. Breast Imaging Reporting and Data System score: 4C (Figure 1B). There were no enlarged lymph nodes in the right axilla. Chest computed tomography (CT) examination (Figure 1C): There is nodular soft tissue shadow in the right breast area, about 2.0 cm in diameter.
One piece of gray-yellow tissue, measured 4.0 cm × 2.5 cm × 2.5 cm (including surrounding normal tissue); a gray-white, gray-yellow nodular mass was seen on the cut surface, the boundary was clear, and the length was 2.2 cm.
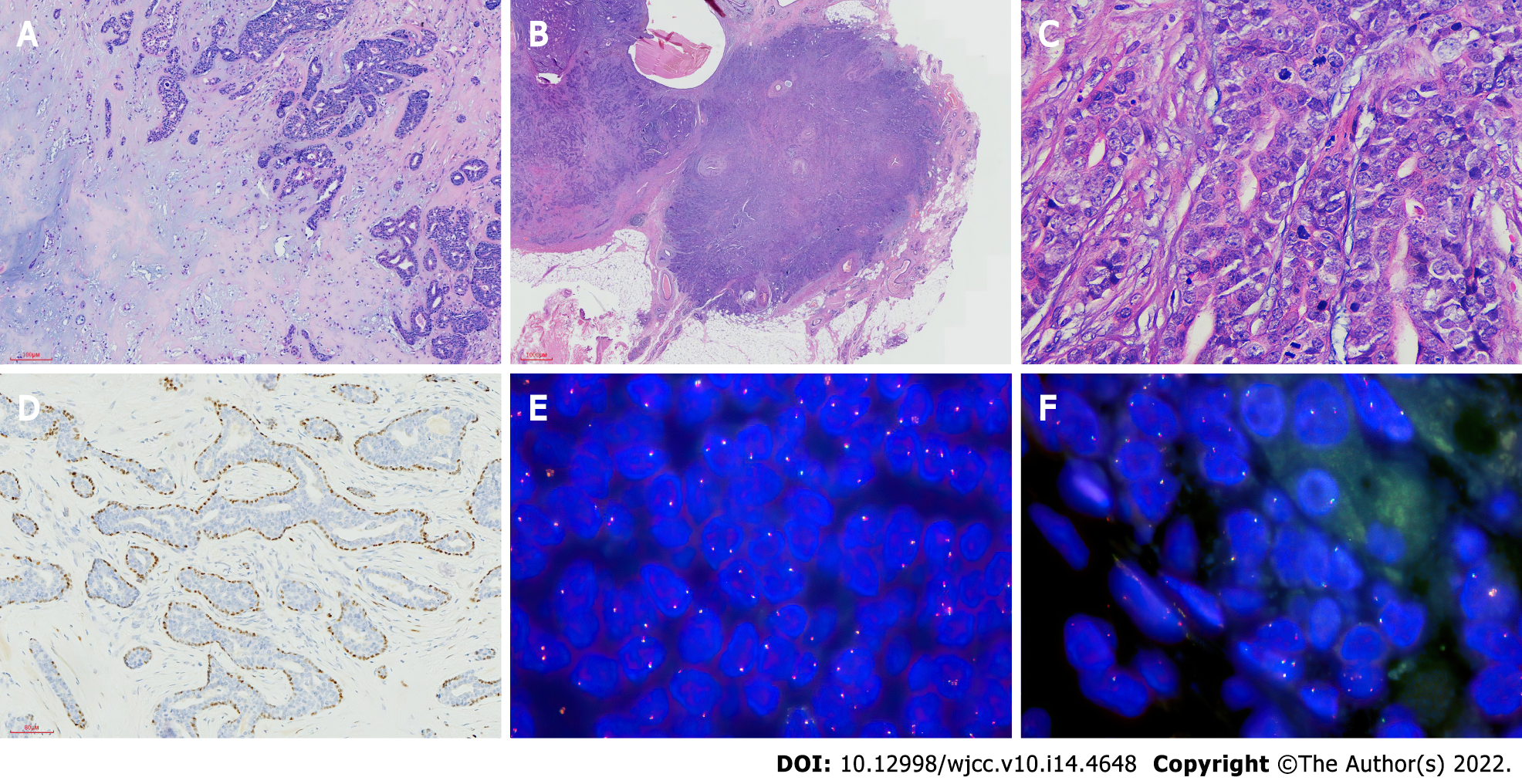
Microscopic observation showed that most areas of the mass and the surrounding boundary are clear; glandular tubular structures were seen in the lesions, which contain pale eosin secretions (Figure 2A). Double-layered cells can be seen in some glandular ducts, and apocrine metaplasia were seen locally. A large area of cartilage mucus-like matrix can be seen, and oval-shaped myoepithelial cells are trapped in the matrix. Myoepithelial cells are polygonal, oval and star-shaped. Cell atypia was not obvious, and there was no mitotic phenomenon or necrosis. In some areas, the tumor and the surrounding area are unclear, showing invasive growth (Figure 2B and C), infiltrating into the fatty tissue and close to the thick-walled blood vessels (Figure 2D), and there is a phenomenon of arranging around the catheter. The abnormality of cells in this area is obvious, and mitotic figures are easy to see.
Immunohistochemistry test results were as follows. The glandular tubular structure expressed EMA, CK7, CD117, dim surface E-cadherin and p120, and partial glandular epithelium was also positive for CK5/6 and GCDFP-15. They were negative for ER, PR and C-erbB-2/HER-2. The myoepithelial cells were positive for spinal muscular atrophy and p63, and negative for S-100 protein. Immunohistochemical staining demonstrated the dual epithelial and myoepithelial components. Ki-67 proliferation index was low (10%).
FISH showed no rearrangement of PLAG1 and HMGA2 genes (Figure 2E and F).
A PA in the upper outer quadrant of the right breast, and the malignant component was malignant adenomyoepithelioma.
A right breast lumpectomy and sentinel lymph node biopsy were performed on April 10, 2020, pending rapid pathological findings, to decide whether to perform modified radical mastectomy on the right breast. During the intraoperative exploration, a 2 cm × 2.5 cm mass on the right breast was found, which was solid, bounded, and smooth in surface. The surrounding 1-cm normal gland was cut off and sent for intraoperative rapid pathology examination. Intraoperative pathology showed epithelial hyperplasia of the right mammary duct, with a small amount of cartilage and the margin was negative. There were two sentinel lymph nodes, showing reactive hyperplasia. After the routine diagnosis was confirmed after the operation, the patient refused to undergo modified radical resection and chemotherapy, and was discharged on the third day after surgery.
We followed up the patient via telephone. From April 2020 to October 2021, the patient underwent breast color ultrasound in a local hospital every 3 mo, chest CT and abdominal color ultrasound every 6 mo, and bone static imaging every year, and no abnormalities were found.
PA is the most common tumor of the salivary glands, accounting for about 60% of salivary gland tumors. PA of the breast was first described in 1906. The morphological characteristics of nine cases of breast tumors containing cartilage and bone tissue have been reported[2]. It is believed that PA of the breast is an intraductal papilloma with interstitial bone and cartilage metaplasia, and a rare specific interstitial component is the stimulated proliferation of papilloma myoepithelial cells. It was not until 1978 that Sheth et al[1] reported a case using the name PA of the breast. The World Health Organization (WHO) Classification of Tumors of the Breast, 5th edition classified PA of the breast under epithelial–myoepithelial tumors, and assigned a new ICD-11 coding: 2F30.Y and XH2KC1 other special benign breasts tumors and PAs. In the 3rd and 4th editions of the WHO classification, PA of the breast is described under benign epithelial hyperplasia, and the ICD-O coding is still 8940/0.
Sato et al[3] suggested that the tumor tissue originated from the myoepithelium, because myoepithelial cells have the potential for mesenchymal differentiation, and the fibrous cells in the mesenchymal components can develop chondrogenic metaplasia and produce mucus[3]. However, some scholars believe that PA of the breast is a variant of ductal adenoma or nodular adenomyoepithelioma[4]. PA originating from the salivary glands mostly has rearrangement of PLAG1 and HMGA2 genes, while PA occurring in the breast usually has no such molecular genetic changes[5,6].
In this case, PA was detected by FISH, and no rearrangement of PLAG1 and HMGA2 genes was observed. Rakha et al[7] considered that PA of the breast differs from PA of the salivary glands in both morphology and genetics. PA of the breast only shows histological features overlapping with PA of the salivary glands, and is often associated with a papillary lesion. The two may not be the same tumor. It is recommended to use the terms PA-like breast tumors to name this tumor[7].
Gao et al[8] reviewed published reports. A total of 85 cases of PA of the breast were retrieved; four of which were malignant (4.7%) but did not mention the histological type of the malignant component[8]. The present case had an infiltrating growth pattern locally, and the infiltrating component had glandular epithelium and myoepithelium, which is consistent with malignant adenomyosis. PA of the breast mostly occurs in women, with only four cases in men, and the male to female ratio is 10:1. The age of onset is 19–85 years, and it often occurs in postmenopausal women. The tumors are mostly located near the areola, indicating that their source may be a large duct. The size of the tumor is 0.6 cm–17.0 cm, and most have a maximum diameter > 2.0 cm. Ultrasonography reveals a mass with unclear borders and uneven internal echo. Mammography shows high-density masses with unclear boundaries and common calcifications. Preoperative examination is very important for auxiliary diagnosis, especially molybdenum[9-11]. Unfortunately, this case lacks mammography target. According to the limited case reports, in patients with PA, including those with malignant components in PA, no axillary lymph node metastasis and distant metastasis have been reported. The prognosis of this patient is also very good. From April 2020 to October 2021, no abnormalities were found. It is suggested that patients should first undergo simple extended excision of breast masses and wait for accurate pathological results. It is important to confirm that the margin is negative. If malignant tissue is found, patients should be treated with modified radical mastectomy. Patients must be followed up after the operation.
Differential diagnosis includes the following: (1) Fibroadenomas and phyllodes tumors, both of which are fibroepithelial tumors, can have mucinous degeneration in the interstitium, and both have ductal epithelial and myoepithelial double-layer structures. They can have a leaf-like structure, lack a cartilage-like matrix, and ductal epithelium does not express CD117; (2) Mucinous breast carcinoma has only one glandular epithelial component and lacks a myoepithelial component. Tumor cells are submerged in the mucus produced by the epithelium; (3) Matrix-producing metaplastic carcinoma of the breast: Cancer cells are atypical, with more frequent mitoses and necrosis, whereas PA cells lack atypia, mitoses are rare, and there is no necrosis. PA has a double-layer cell arrangement structure, but matrix-producing metaplasia carcinoma consists of only one type of epithelial cells showing a clustering tendency. Thick needle biopsy specimens often only penetrate the mucinous interstitium of the cartilage, so special care should be taken. Takahashi reported a case of breast PA diagnosed by thick needle biopsy[12]. He emphasized that the cartilage mucus-like matrix cannot be observed alone, and it must be determined whether there is a double layer of glandular epithelium and myoepithelial cells. Preoperative diagnosis can avoid excessive radical breast cancer surgery; (4) Adenomyoepithelioma has a lack of cartilage mucinous matrix; although it has a double-layer structure, its peripheral myoepithelium often has a significant multilayer arrangement, and nuclear atypia and mitotic figures are more common than in PA; and (5) PA that occurs in breast skin PA that occurs in breast skin may be misdiagnosed as PA that occurs in breast parenchyma due to unknown medical history. Yang et al[13] reported a case of breast skin PA with breast cancer[13]. Therefore, it is necessary to confirm the location of the lesion, and carefully observe whether there is proper breast tissue around and within the lesion.
We report a case of malignant adenomyoepithelioma found in PA, which demonstrates that a breast PA is a mixed tumor of variable morphology with multi-directional differentiation. Therefore, differential diagnosis is particularly important. Because of its rarity, especially in Asia, the biological characteristics of breast PA remain unclear. It is believed that with the deepening of research at the molecular level, understanding of this tumor will be enhanced.
PA of the breast is a mixed tumor with diverse morphology and ability to differentiate in many ways. Because the clinical and imaging manifestations of PA are similar to those of breast cancer, cases are rare and it is easy to misdiagnose it as breast cancer. Given that this is a rare disease, accurate diagnosis through histopathological analysis is important to determine the treatment plan. According to the limited case reports, in patients with PA, including those with malignant components in PA, no axillary lymph node metastasis and distant metastasis have been reported. It is suggested that patients should first undergo extended excision of breast masses followed by pathological examination. It is important to confirm that the surgical margin is negative. If malignant tissue is found, modified radical mastectomy should be performed. Patients must be followed up after the operation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Member of the Chinese Medical Doctor Association Surgery Branch; Member of Anhui Surgery Branch of Chinese Medical Association.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bharati S, Bangladesh; Dalal N, India S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Sheth MT, Hathway D, Petrelli M. Pleomorphic adenoma ("mixed" tumor) of human female breast mimicking carcinoma clinico-radiologically. Cancer. 1978;41:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Smith BH, Taylor HB. The occurrence of bone and cartilage in mammary tumors. Am J Clin Pathol. 1969;51:610-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Sato K, Ueda Y, Shimasaki M, Ozaki M, Nitta N, Chada K, Ishikawa Y, Katsuda S. Pleomorphic adenoma (benign mixed tumor) of the breast: a case report and review of the literature. Pathol Res Pract. 2005;201:333-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Andrew R. Rosen's Breast Pathology, 4th Edition. Adv Anat Pathol. 2014;21:469-000. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 5. | Jakobiec FA, Stagner AM, Eagle RC Jr, Lally SE, Krane JF. Unusual pleomorphic adenoma of the lacrimal Gland: Immunohistochemical demonstration of PLAG1 and HMGA2 oncoproteins. Surv Ophthalmol. 2017;62:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ma S, Zhao D, Liu Y, Rohr J, Zhang F, Ma Y, Gong L, Shi H, Wang Y, Fan L, Qin J, Wang Z, Guo S. Some pleomorphic adenomas of the breast share PLAG1 rearrangements with the analogous tumour of the salivary glands. Histopathology. 2021;79:1030-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Rakha EA, Aleskandarany MA, Samaka RM, Hodi Z, Lee AH, Ellis IO. Pleomorphic adenoma-like tumour of the breast. Histopathology. 2016;68:405-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Gao X, Sun ZG, Guan HW. [Progress in clinicopathologic studies of mammary pleomorphic adenoma]. Zhonghua Bing Li Xue Za Zhi. 2017;46:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Khamparia A, Bharati S, Podder P, Gupta D, Khanna A, Phung TK, Thanh DNH. Diagnosis of breast cancer based on modern mammography using hybrid transfer learning. Multidimens Syst Signal Process. 2021;32:747-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 10. | Bharati S, Rahman MA, Podder P. Breast Cancer Prediction Applying Different Classification Algorithm with Comparative Analysis using WEKA, 2018 4th International Conference on Electrical Engineering and Information & Communication Technology (iCEEiCT) 2018. [cited 10 December 2021]. Available from: https://ieeexplore.ieee.org/document/8628084. |
| 11. | Bharati S, Podder P, Rubaiyat M, Mondal H. Artificial Neural Network Based Breast Cancer Screening: A Comprehensive Review. [cited 10 December 2021]. Available from: https://www.researchgate.net/publication/341851608_Artificial_Neural_Network_Based_Breast_Cancer_Screening_A_Comprehensive_Review. |
| 12. | Takahashi K. Diagnosis of an extremely rare pleomorphic adenoma of the breast with core needle biopsy: A case report. Ann Med Surg (Lond). 2018;36:242-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Yang XX, Wu DD, Mei JH, Wu B, Tao XQ, Liao H, Liu HP, Li HT, Li CQ. A case of breast skin pleomorphic adenoma with breast cancer. Zhongguo Ruxianjibing Zazhi. 2016;10:373-374. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |