Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4640
Peer-review started: November 16, 2021
First decision: December 27, 2021
Revised: January 9, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: May 16, 2022
Processing time: 178 Days and 0 Hours
The contradictory process of coagulation and anticoagulation maintains normal physiological function, and platelets (PLTs) play a key role in hemostasis and bleeding. When severe thrombocytopenia and deep vein thrombosis (DVT) occur simultaneously, the physician will be confronted with a great challenge, especially when interventional thrombectomy fails.
We describe a 52-year-old woman who suffered from myelodysplastic syndrome with severe thrombocytopenia and protein S deficiency with right lower extremity DVT. In this patient, the treatment of DVT was associated with numerous contradictions due to severe thrombocytopenia, especially when interventional thrombectomy was not successful. Fortunately, fondaparinux sodium effectively alleviated the thrombus status of the patient and gradually decreased the D-dimer level. In addition, no increase in bleeding was noted. The application of eltro
This is a contradictory and complex case, and fondaparinux sodium and eltrombopag may represent a good choice for the treatment of DVT in patients with severe thrombocytopenia.
Core Tip: One 52-year-old Chinese female suffered from myelodysplastic syndrome of multilineage dysplasia with severe thrombocytopenia and thrombophilia with deep vein thrombosis. In the basic treatment for underlying diseases, the treatment of thrombosis is full of contradictions because of the low platelets, especially in the situation that the curative effect of surgical thrombectomy was not obvious. Pleasantly, Fondaparinux sodium effectively alleviated the thrombus status, and the D-dimer gradually decreased. In addition, there was not any unexpected bleeding. This patient’s circumference of right lower limb recovered and restored the movement basically finally.
- Citation: Liu WB, Ma JX, Tong HX. Successful treatment in one myelodysplastic syndrome patient with primary thrombocytopenia and secondary deep vein thrombosis: A case report . World J Clin Cases 2022; 10(14): 4640-4647
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4640.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4640
Myelodysplastic syndrome (MDS) is a common hematological malignancy, and its clinical outcome is closely related to the consequences of leukemic evolution or serious clinical events[1]. In all events, thrombosis is a low-risk symptom due to the high frequency of thrombocytopenia and severe anemia, and the rate is similar to that in the general population. However, when pathophysiological changes occur, including vascular complications and the activation of thrombophilia-associated genes, the risk of thrombosis may be increased. As one type of thrombosis, venous thromboembolism (VTE) is considered a multifactorial disease[2] affected by comprehensive clinical signs of interaction from single or multiple genetic, epigenetic and/or acquired predisposing factors[3]. Among them, mutations in these genes eventually lead to the formation of thrombi, including prothrombin, factor V Leiden, protein C (PC) and protein S (PS)[4].
As the typical blood pattern of MDS, thrombocytopenia [platelets (PLTs) < 100 × 109/L] occurs in approximately 40%-65% of MDS patients, and severe thrombocytopenia (PLTs < 20 × 109/L) accounts for approximately 17%[5], which is closely associated with a high risk of bleeding, rather than deep vein thrombosis (DVT). Thus, reports about the combination of severe thrombocytopenia and DVT in MDS patients are limited, and successful treatment is even rarer. Here, we present a case of an MDS patient with complicated comorbid diseases, and this is the first case of successful application of fondaparinux sodium and eltrombopag in the treatment of DVT with severe thrombocytopenia.
A 52-year-old woman was admitted to the hematology department of our hospital in November 2019 with dizziness and fatigue for 8 years and right lower limb swelling and pain for 6 d.
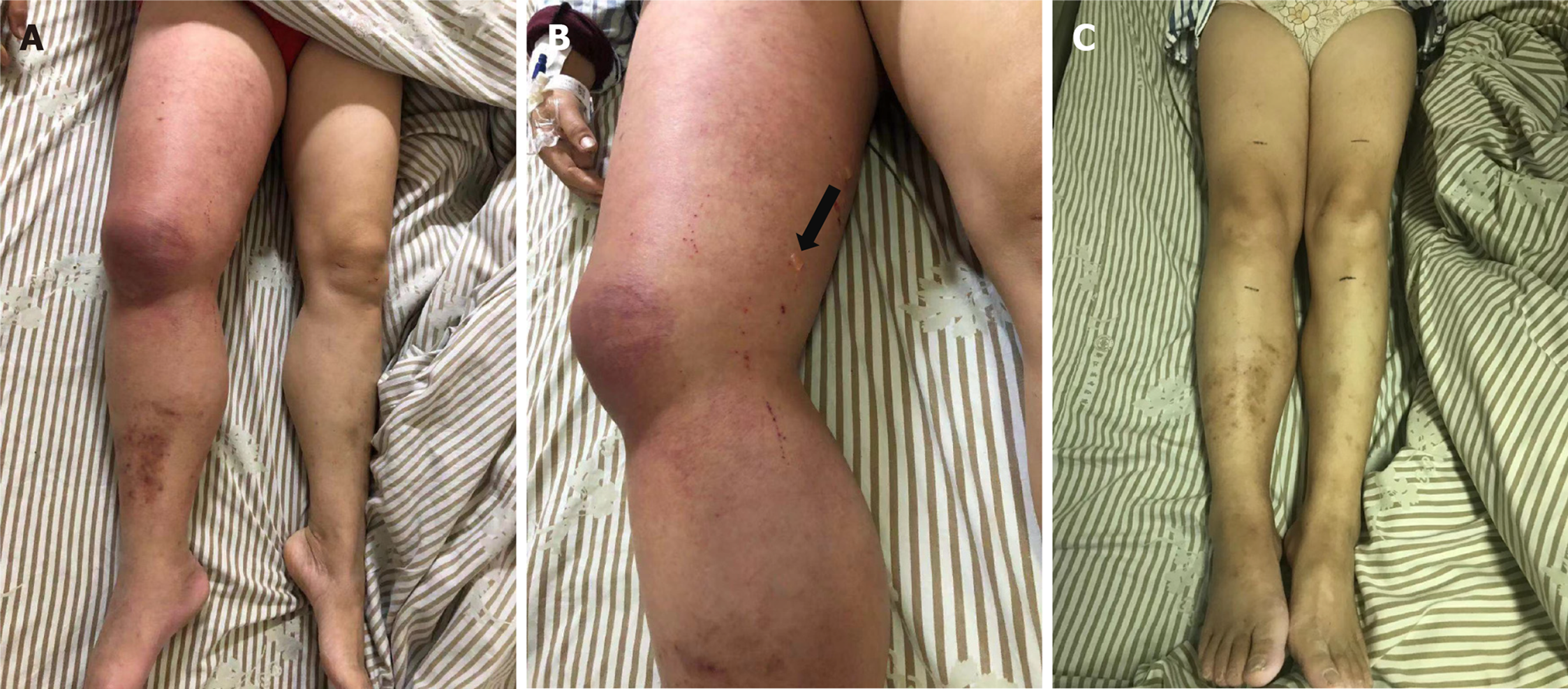
The patient felt dizziness and fatigue in January 2011, and these symptoms worsened after activity and were aggravated gradually without treatment. She first presented at a local hospital in August 2011. Routine blood examination revealed the following: White blood cells (WBCs) 4.0 × 109/L, hemoglobin (Hb) 55 g/L, PLTs 20 × 109/L, and reticulocytes 2%. The bone marrow morphology was characterized by nucleated cells with active proliferation. Erythroid hyperplasia was noted in 48% of erythroid cells. Megaloblastic erythrocytes and 20 megakaryocytes were found. Bone marrow biopsy showed that hematopoietic tissue accounted for approximately 75% of the biopsy. Small megakaryocytes were noted with reticular fiber (+). Chromosome analysis revealed 46 XX. The patient was diagnosed with MDS with refractory anemia and treated with androgen, cyclosporin, and intermittent blood transfusion support. Hb was maintained at 100-120 g/L, and the PLT count was 50-80 × 109/L. However, in 2014, she stopped taking cyclosporine due to renal damage. As she was no longer dependent on blood transfusion, the patient had stopped taking the medicine and her follow-up treatment on her own. In November 2019, the values for the peripheral blood factors decreased as follows: WBC 2.9 × 109/L, Hb 70 g/L, and PLT 20 × 109/L. She began treatment with 200 mg danazol quaque die (qd) and 50 mg thalidomide quaque nocte. Unfortunately, the patient presented with right lower limb swelling that had gradually become aggravated 1 mo later, and the patient gradually lost the ability to exercise. However, blisters were scattered on the skin of the right lower limb, and her temperature increased (Figure 1A and B). The patient underwent an emergency transfer to the hematology department of our hospital on November 25, 2019.
The patient had type 2 diabetes for 5 years, which was controlled well by taking acarbose tablets (50 mg three times a day). She had a history of blood transfusion but no blood transfusion allergy response.
Her parents had died, and she had an older sister with parkinson’s disease and a healthy son. She denied any history of family genetic disease.
On admission, the patient presented with anemia, and occasional bleeding spots were seen on the skin. The skin of the right lower limb was red and swollen with several transparent soybean-sized blisters, and the temperature of the skin was elevated. Her limb showed extreme tenderness with mild concave edema, which resulted in dysmobility of the right lower limb.
The complete blood count analysis revealed pancytopenia (WBC 2.8 × 109/L, neutrophils 1.6 × 109/L, Hb 88 g/L, PLT 15 × 109/L), and her D-dimer was 16.48 mg/L. Screening analysis of anticoagulant proteins demonstrated a markedly low level of PS activity (25%, reference value: 55%-130%), whereas antithrombin (AT) Ⅲ and PC levels were normal. Lower extremity vascular ultrasound revealed extensive acute incomplete thrombosis in the right lower extremities, including the external iliac vein, common femoral vein, superficial femoral vein, deep femoral vein, popliteal vein, and posterior tibial vein of the right lower limb (November 6, 2019). Bone marrow morphology revealed nucleated cells with active proliferation. Primitive cells accounted for 3% of cells, and 12 megakaryocytes were found. Bone marrow biopsy showed that hematopoietic tissue accounted for approximately 70% of the biopsy. Activated nucleated cell hyperplasia was noted, and a small number of mature lymphocytes and plasma cells were observed. The chromosome analysis revealed 46 XX.
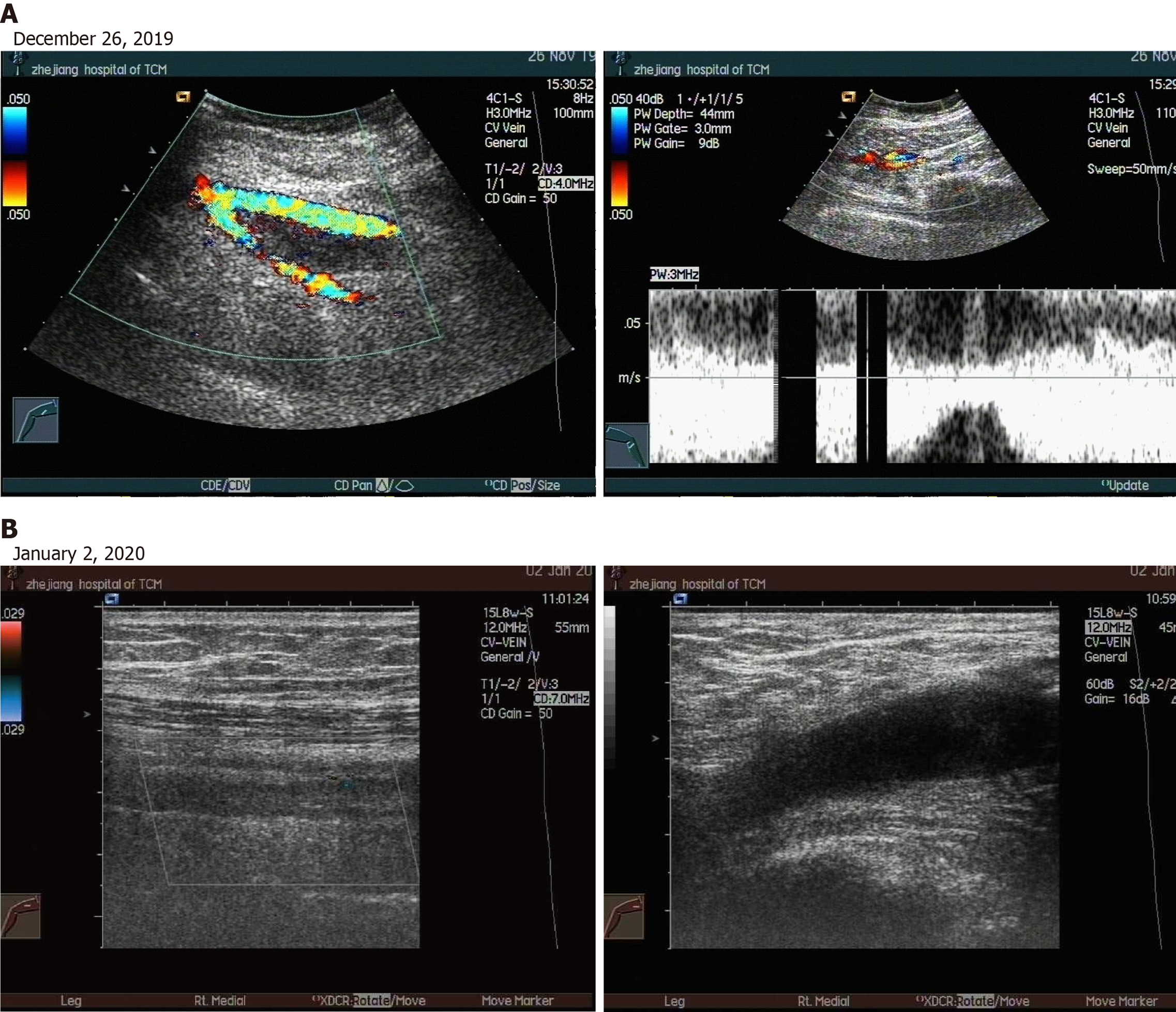
Deep venous ultrasound of the right lower extremity showed that extensive acute incomplete thrombosis in the right lower extremities in November 26, 2019. While, it showed that old thrombosis in the common and superficial femoral veins of the right lower limb in January 2, 2020.
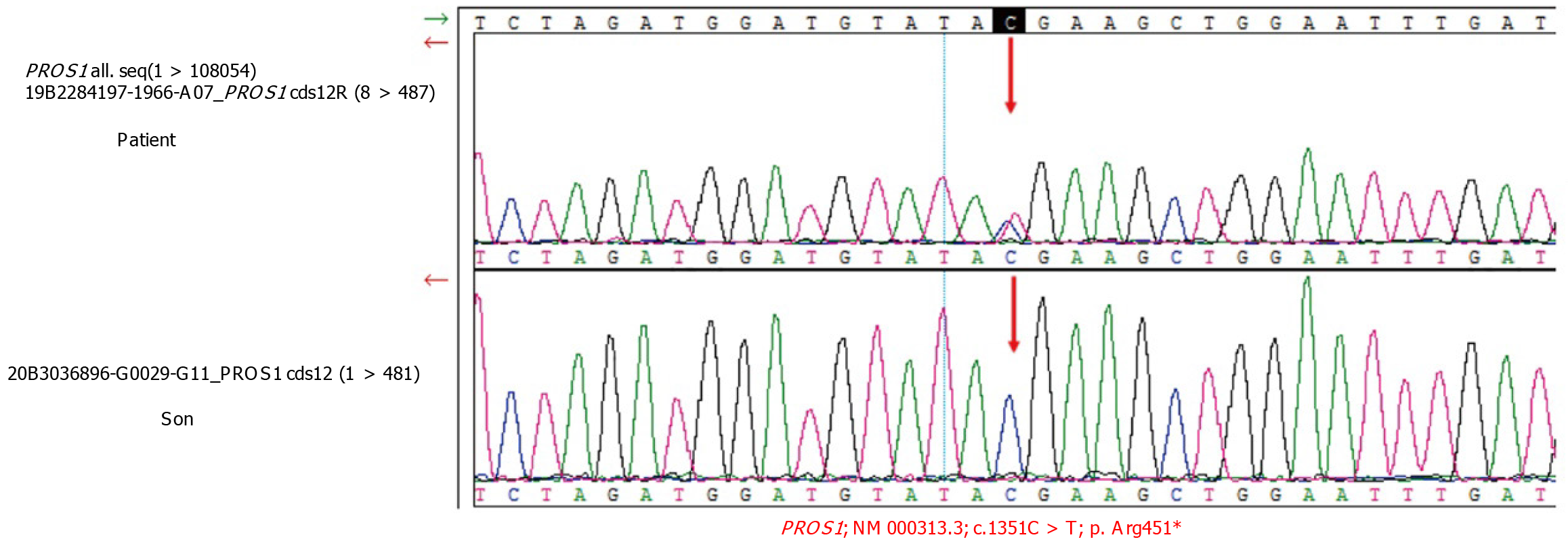
Whole-exome sequencing detection: Figure 2 shows the gene sequencing results of the patient and the patient’s son. A heterozygous mutation of the PROS1 gene was found in the patient (c.1351C > T), but the same mutation was not observed in the patient’s son.
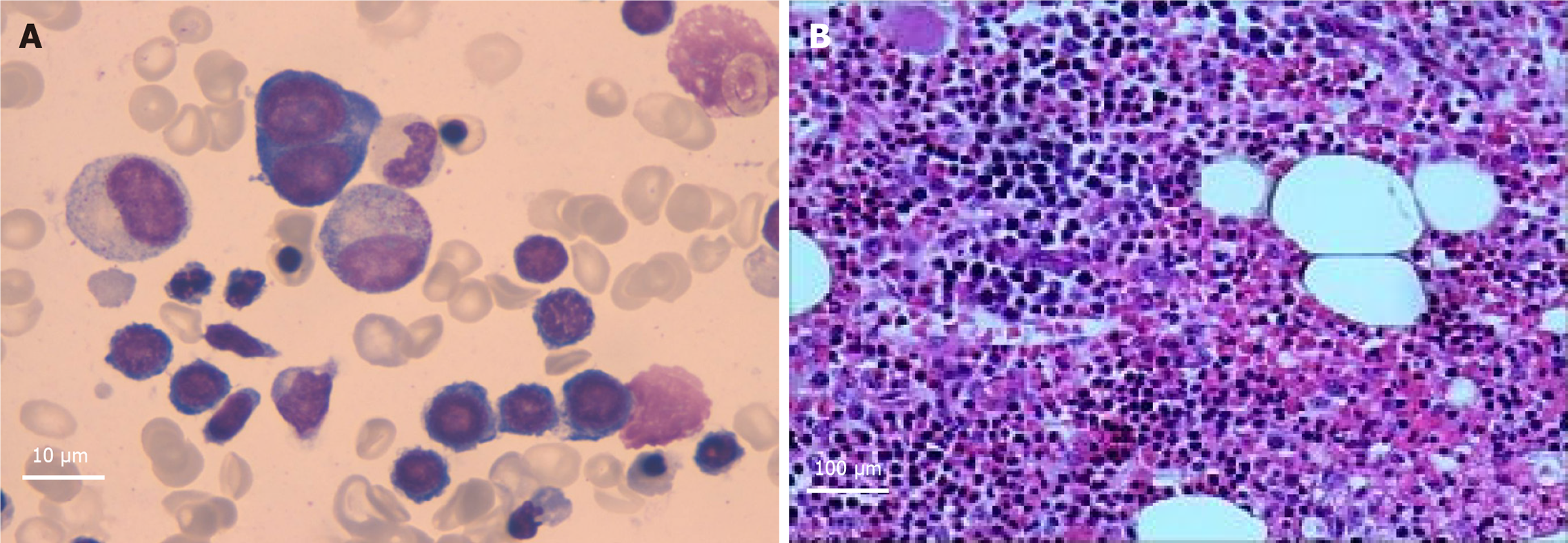
Bone marrow cell examination (December 23, 2019): Obvious proliferation of the erythroid cell line with poor platelet production function was observed in the megakaryocyte line (Figure 3A).
Bone marrow biopsy (December 31, 2019): Bone marrow hematopoiesis of the “posterior iliac spine” (Figure 3B) revealed approximately 70% fat. In addition, active nucleated cell hyperplasia was noted in 30% of cells. Granulocyte proliferation was still active. Granulocytes in the mature stage was slightly reduced. Erythroid hyperplasia was obviously active, mainly in the middle and late stages of erythroblasts, with some cells of different sizes. The number of megakaryocytes was reduced (0-2/high power field), and the morphology was roughly normal. A small number of mature lymphocytes and plasma cells were observed.
The patient was diagnosed with MDS-multilineage dysplasia (MDS-MLD), international prognostic scoring system intermediate Ⅰ-risk group, PS deficiency (PROS1 heterozygous mutation), right lower extremity deep vein thrombosis, and Type 2 diabetes mellitus.
On admission, the patient discontinued treatment with thalidomide and danazole immediately. To improve the clinical condition as quickly as possible, the patient received treatment with deep venous thrombosis aspiration and inferior vena cava filter implantation on November 26, 2019. However, interventional thrombectomy failed. After the surgery, the patient developed chills and fever, which increased to 38.5 degrees. The PLT count dropped to less than 10 × 109/L, and PLT transfusions were necessary. However, the swelling of the right limb did not improve. In addition to anti-infection and transfusion therapy, the patient received anticoagulant therapy with fondaparinux sodium (2.5 mg injection hypodermic qd). Fortunately, the patient’s temperature became normal, and the blisters in the right lower extremity subsided after 1 wk of therapy. In addition, the edema lessened after 2 wk, and swelling of the affected limb subsided to normal after 3 wk. Soon after, the patient regained basic mobility (Figure 1C). On December 26, the patient was administered eltrombopag (50 mg qd) to promote the maturation and differentiation of megakaryocytes and increase the peripheral blood platelet count. On January 2, 2020, vascular ultrasound showed thrombosis in the common femoral vein and superficial femoral vein of the right lower extremity; however, the conditions had improved compared to the first time (Figure 4A and B). More importantly, no serious bleeding occurred during the therapy.
The PLT count of the patient was maintained at 20-40 × 109/L after 2 mo of treatment with eltrombopag. Then, the patient stopped taking eltrombopag and fondaparinux sodium and was switched to 5 mg/d rivaroxaban to prevent DVT. Currently, no swelling, pain or movement disorders are noted in the right lower limb. The latest vascular ultrasound revealed partial old thrombosis without new thrombosis.
The contradictory process of coagulation and anticoagulation maintains normal physiological function. PLTs are pivotal in primary hemostasis and have nonhemostatic properties involved in angiogenesis, tissue repair, inflammation, and metastasis[6]. In most cases, patients suffering from hematological disease with low PLTs suffer from bleeding risk, and the aim to avoid bleeding is the normal strategy employed by clinical physicians to treat these patients[6]. However, we report a rare case of one MDS patient with severe thrombocytopenia and right lower limb DVT, and the patient was treated successfully with fondaparinux sodium and eltrombopag for the first time.
The patient, whose PLT count showed moderate to severe reductions, developed right lower limb DVT 1 mo after taking thalidomide and danazole. Low-dose thalidomide is an effective and safe treatment for patients with low- to intermediate-1-risk MDS, although it inhibits angiogenesis and has complex immunomodulatory effects[7]. However, in patients with multiple myeloma, receiving thalidomide in combination with multiagent chemotherapy and dexamethasone will significantly increase the risk of DVT[8,9]. Thalidomide may act as a procoagulant by significantly increasing the expression of phosphatidylserine and tissue factor and decreasing the expression of endothelial PC receptor, thrombomodulin and activated PC (APC) by a direct inhibitory effect when pretreated with multiple myeloma serum, thereby contributing to thrombogenesis[10]. Thalidomide-related thrombotic events are most common in the deep venous spindle of both limbs, followed by pulmonary embolism and superficial venous thrombosis[11-13]. Although there is not much evidence that danazol, a synthetic androgenic steroid, is related to thrombogenesis in humans, there are still some case reports of cerebral, coronary, and peripheral arterial thrombosis suggesting that danazol may be an independent risk factor for arterial thrombosis that may also contribute to this DVT[14]. In this patient, whole-exome sequencing detection revealed PS deficiency [PROS1+, c.1351C > T (p.Arg451)], which is a heterozygous mutation leading to thrombophilia[15]. The lack of PS, which is a nonenzyme cofactor of APC, reduces the ability of PC to inactivate coagulation factors Va and VIIIa, leading to thrombosis[16]. In addition, we should not overlook the fact that diabetes itself, as an individual risk factor for thromboembolic events, also contributes to the formation of thrombi. Therefore, we concluded that although the occurrence of DVT in this patient may be caused by a variety of factors, thalidomide-induced thrombophilia was the primary cause.
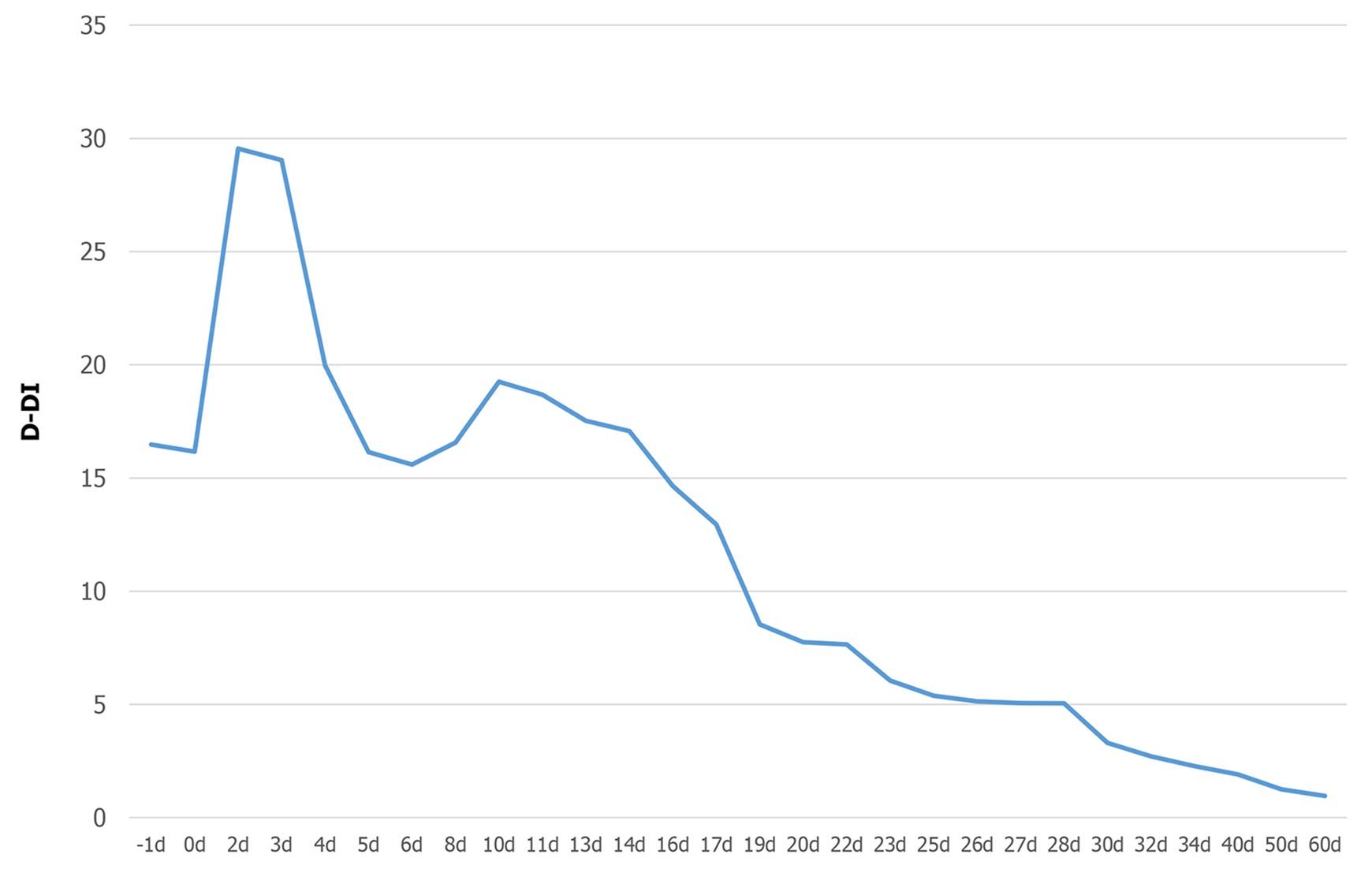
Severe thrombocytopenia with coexisting DVT in patients is quite challenging for physicians. The platelet count of the patient was severely reduced, and DVT continued to deplete the platelet count, which led to an increased risk of fatal bleeding and anticoagulant therapy[17]. The failure of interventional thrombectomy was exacerbated. To better balance bleeding and blood clots, fondaparinux sodium was first selected for anticoagulant therapy. Surprisingly, in this contradictory situation, fondaparinux sodium effectively alleviated the thrombus status, and the D-dimer gradually decreased without any unexpected bleeding (Figure 5). Binding to ATIII, a highly selective factor Xa (FXa) inhibitor, enhances the natural activity of ATIII against FXa, thereby effectively blocking the core steps of the clotting cascade and preventing the formation and development of thrombosis. Studies have shown that fondaparinux sodium is more effective and safer than other anticoagulant drugs when patients suffer from both thrombocytopenia and a high coagulation state. This is similar to the condition noted in this patient, although it was not induced by heparin[18,19]. Then, to increase platelet counts and reduce bleeding risk, we prescribed eltrombopag to promote the maturation and differentiation of megakaryocytes and increase the peripheral blood platelet count. Eltrombopag is an oral thrombopoietin receptor agonist indicated for the treatment of immune thrombocytopenia. Beyond the effect on megakaryopoiesis, the drug also showed a stimulating effect on hematopoietic stem cells with consistent clinical efficacy in aplastic anemia and MDS[20,21]. DVT is also an important rare adverse event for the long-term use of eltrombopag[22]. Thus, the patient discontinued eltrombopag once the platelet count reached the point that she did not need to rely on blood transfusion. In terms of the end result, we succeeded in breaking the patient’s contradictory pathophysiological process with fondaparinux sodium and eltrombopag.
This is the first report of the use of fondaparinux sodium and eltrombopag to successfully treat an MDS patient with severe thrombocytopenia and right lower limb DVT. In addition, no increased risk of bleeding was noted. Furthermore, clinicians should be aware of thrombotic problems in MDS patients taking thalidomide, especially patients with genetic backgrounds for thrombotic susceptibility.
We thank the patient and the patient’s family members for their support and cooperation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Hematology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C; C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gaman MA, Romania; Gessler F, Germany S-Editor: Guo XR L-Editor: A P-Editor: Chen YX
| 1. | Patnaik MM, Tefferi A. Myelodysplastic syndromes with ring sideroblasts (MDS-RS) and MDS/myeloproliferative neoplasm with RS and thrombocytosis (MDS/MPN-RS-T) - "2021 update on diagnosis, risk-stratification, and management". Am J Hematol. 2021;96:379-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Badireddy M, Mudipalli VR. Deep Venous Thrombosis Prophylaxis. StatPearls. Treasure Island (FL): StatPearls Publishing. [DOI] [Full Text] |
| 3. | Preston RJS, O'Sullivan JM, O'Donnell JS. Advances in understanding the molecular mechanisms of venous thrombosis. Br J Haematol. 2019;186:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Colucci G, Tsakiris DA. Thrombophilia Screening: Universal, Selected, or Neither? Clin Appl Thromb Hemost. 2017;23:893-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Basood M, Oster HS, Mittelman M. Thrombocytopenia in Patients with Myelodysplastic Syndromes: Still an Unsolved Problem. Mediterr J Hematol Infect Dis. 2018;10:e2018046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Vinholt PJ. The role of platelets in bleeding in patients with thrombocytopenia and hematological disease. Clin Chem Lab Med. 2019;57:1808-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Chung CY, Lin SF, Chen PM, Chang MC, Kao WY, Chao TY, Hsiao LT, Yen CC, Yang MH, Hwang WS, Lin TL, Chiou TJ, Chang CS. Thalidomide for the treatment of myelodysplastic syndrome in Taiwan: results of a phase II trial. Anticancer Res. 2012;32:3415-3419. [PubMed] |
| 8. | Debbie Jiang MD, Alfred Ian Lee MD. Thrombotic Risk from Chemotherapy and Other Cancer Therapies. Cancer Treat Res. 2019;179:87-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 9. | Kahale LA, Matar CF, Tsolakian I, Hakoum MB, Yosuico VE, Terrenato I, Sperati F, Barba M, Hicks LK, Schünemann H, Akl EA. Antithrombotic therapy for ambulatory patients with multiple myeloma receiving immunomodulatory agents. Cochrane Database Syst Rev. 2021;9:CD014739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Gao Y, Ma G, Liu S, Teng Y, Wang Y, Su Y. Thalidomide and multiple myeloma serum synergistically induce a hemostatic imbalance in endothelial cells in vitro. Thromb Res. 2015;135:1154-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Bujoreanu I, Ferguson M, Saleh H. Chemotherapy associated dural sinus thrombosis presenting as a cerebrospinal fluid leak. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Ay C, Beyer-Westendorf J, Pabinger I. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann Oncol. 2019;30:897-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 13. | Weitz IC. Thrombotic microangiopathy in cancer. Thromb Res. 2018;164 Suppl 1:S103-S105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Alvarado RG, Liu JY, Zwolak RM. Danazol and limb-threatening arterial thrombosis: two case reports. J Vasc Surg. 2001;34:1123-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19696] [Cited by in RCA: 22543] [Article Influence: 2254.3] [Reference Citation Analysis (0)] |
| 16. | Iida H, Nakahara M, Komori K, Fujise M, Wakiyama M, Urata M, Kinoshita S, Tsuda H, Sugimachi K, Hamasaki N. Failure in the detection of aberrant mRNA from the heterozygotic splice site mutant allele for protein S in a patient with protein S deficiency. Thromb Res. 2001;102:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Machin M, Salim S, Tan M, Onida S, Davies AH, Shalhoub J. Surgical and non-surgical approaches in the management of lower limb post-thrombotic syndrome. Expert Rev Cardiovasc Ther. 2021;19:191-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Schindewolf M, Steindl J, Beyer-Westendorf J, Schellong S, Dohmen PM, Brachmann J, Madlener K, Pötzsch B, Klamroth R, Hankowitz J, Banik N, Eberle S, Müller MM, Kropff S, Lindhoff-Last E. Use of Fondaparinux Off-Label or Approved Anticoagulants for Management of Heparin-Induced Thrombocytopenia. J Am Coll Cardiol. 2017;70:2636-2648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Mastroiacovo D, Sala G, Dentali F. The safety of fondaparinux sodium for the treatment of venous thromboembolism. Expert Opin Drug Saf. 2016;15:1259-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Fattizzo B, Levati G, Cassin R, Barcellini W. Eltrombopag in Immune Thrombocytopenia, Aplastic Anemia, and Myelodysplastic Syndrome: From Megakaryopoiesis to Immunomodulation. Drugs. 2019;79:1305-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 21. | Mittelman M, Platzbecker U, Afanasyev B, Grosicki S, Wong RSM, Anagnostopoulos A, Brenner B, Denzlinger C, Rossi G, Nagler A, Garcia-Delgado R, Portella MSO, Zhu Z, Selleslag D. Eltrombopag for advanced myelodysplastic syndromes or acute myeloid leukaemia and severe thrombocytopenia (ASPIRE): a randomised, placebo-controlled, phase 2 trial. Lancet Haematol. 2018;5:e34-e43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 22. | Wong RSM, Saleh MN, Khelif A, Salama A, Portella MSO, Burgess P, Bussel JB. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130:2527-2536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |