Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4601
Peer-review started: October 15, 2021
First decision: December 17, 2021
Revised: December 23, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 16, 2022
Processing time: 210 Days and 1.4 Hours
Cytomegalovirus (CMV) infections in the population are mostly subclinical, inapparent, or latent. However, it is rare in brain tissue. Most reported CMV encephalitis cases were patients with immunodeficiency. The diagnosis and detection rate of CMV encephalitis in patients with normal immune function needs to be further improved.
An 86-year-old male was admitted due to a sudden onset of unconsciousness for 3 h. The patient developed status epilepticus and was relieved after antiepileptic treatment. Encephalitis was considered due to the high signals of diffusion-weighted imaging sequences in the right central region by magnetic resonance imaging. Metagenomic next-generation sequencing (mNGS) of blood and cerebrospinal fluid revealed CMV, with unique reads number being 614 and 1, respectively. Simultaneous quantitative PCR results showed CMV positive in blood samples and negative in cerebrospinal fluid samples. The patient was finally diagnosed as CMV encephalitis with status epilepticus. After the antiviral, hormonal, and γ-globulin pulse therapy, the patient’s condition improved, and he was finally discharged.
mNGS could be a reliable approach for the diagnosis of CMV encephalitis, with high efficiency, sensitivity, and specificity.
Core Tip: Cytomegalovirus infection in brain tissue is rare, and the diagnosis is limited. Here we report a case of cytomegalovirus encephalitis that was diagnosed by metagenomic next-generation sequencing using blood and cerebrospinal fluid samples. This indicated that metagenomic next-generation sequencing could be a reliable approach for the diagnosis of cytomegalovirus encephalitis with high efficiency, sensitivity, and specificity.
- Citation: Xu CQ, Chen XL, Zhang DS, Wang JW, Yuan H, Chen WF, Xia H, Zhang ZY, Peng FH. Diagnosis of cytomegalovirus encephalitis using metagenomic next-generation sequencing of blood and cerebrospinal fluid: A case report. World J Clin Cases 2022; 10(14): 4601-4607
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4601.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4601
Cytomegalovirus (CMV) is a type of herpesvirus[1]. Its gene structure is very similar to herpes simplex virus. Humans are the only host of human CMV, and virus carriers are the most common source of infection[2]. CMV infections are mostly subclinical, inapparent, and latent[1,2]. When the body’s immune function is compromised, CMV can infect multiple organs and systems, manifesting clinical symptoms[3]. The infections of CMV are most common in the lung, followed by liver, kidney, but brain tissues are rarely affected[4,5]. This is different from herpes simplex virus, which is the most common cause of encephalitis. Most of the reported cases of CMV encephalitis had immunodeficiency, such as HIV infection or organ transplantation[6,7]. Studies on CMV patients with normal immune function were few. Its diagnosis and detection rate need to be further improved.
The diagnosis of CMV encephalitis is mainly based on the results of pathogenic examinations. There are no well-defined diagnostic criteria currently. Methods including serology, culture, quantitative PCR (qPCR), antigenemia, histopathology, and CMV-specific T-cell detection are used for the diagnosis, the monitoring of priority treatment, and the antiviral response[8]. However, there are some limitations. For example, the sensitivity of serology and culture is always low, and antigenemia needs enough leukocytes in the samples.
Metagenomic next-generation sequencing (mNGS) has been recently applied more often in pathogen examination of infectious diseases[9]. It can accurately analyze all microorganisms in samples, with results being obtained within 24 h. It could be used as a rapid screening tool for patients with suspected CMV encephalitis.
Here we report a patient who was finally diagnosed with CMV encephalitis with the help of mNGS of the blood and cerebrospinal fluid (CSF) samples. This was confirmed by qPCR of blood samples.
An 86-year-old male was admitted on November 8, 2020 due to sudden onset of unconsciousness for 3 h.
In the early morning of November 8, 2020, the patient suddenly screamed with involuntary tremor of the left upper limb during sleep, out of breath, followed by disturbance of consciousness with eyes tightly closed. The conditions lasted for about half an hour prior to relief. Then, the patient felt dizziness and weakness of the four limbs accompanied by slurred speech. The symptoms were completely relieved in 3-4 h.
The patient had a history of hypertension and diabetes for more than 10 years. Antihypertensive drugs were taken regularly, but no hypoglycemic agent was administered.
The patient had a smoking history for several decades, approximately 10 cigarettes/d. He denied a history of alcohol abuse or other bad habits.
The blood pressure and glycemic control were basically normal.
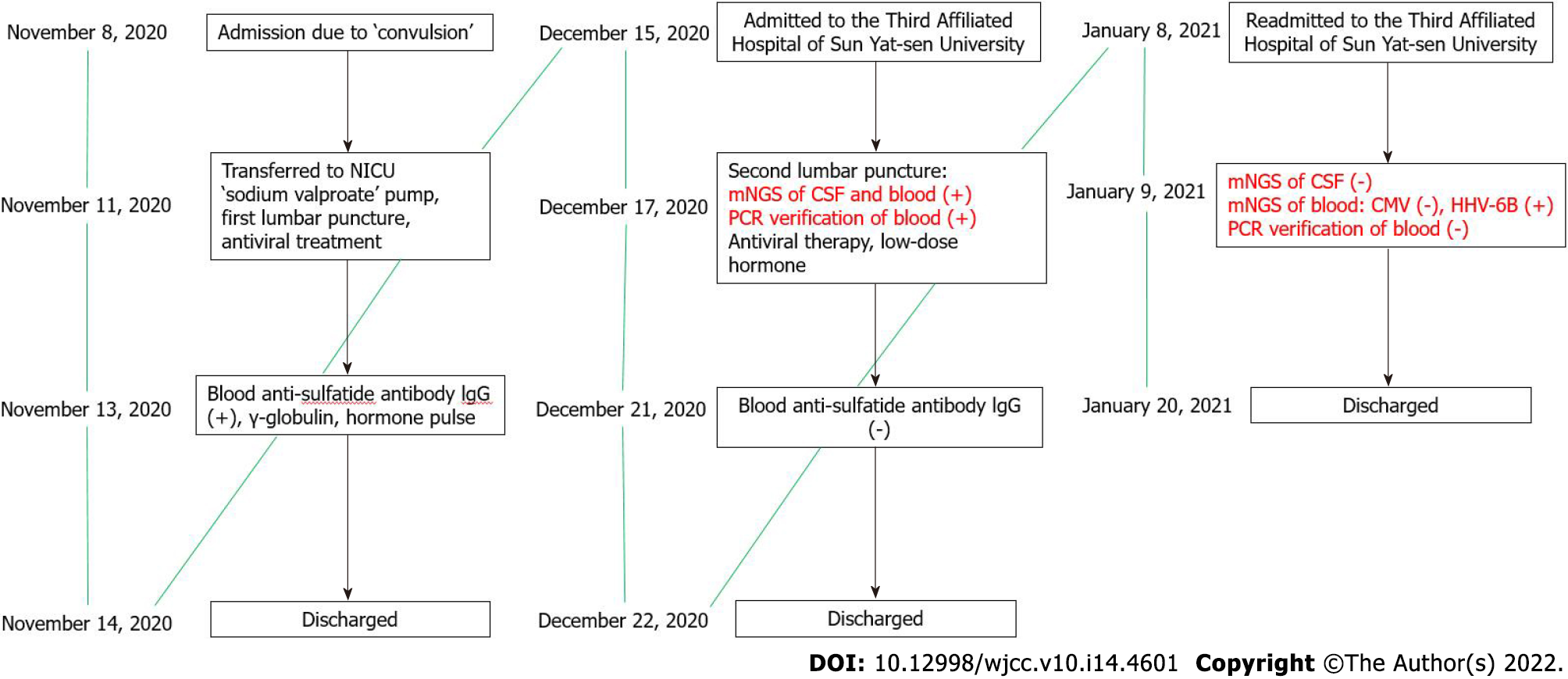
On November 12, a lumbar puncture was performed with the pressure at 250 H2O. CSF routine revealed the nucleated cell counts at 4 × 106/L, chloride at 124.5 mmol/L, CSF pressure at 0.58 ↑g/L, glucose at 3.56 mmol/L, anti-SS-A antibody (+), ANA cytoplasmic granular pattern, ANA titer at 1:100, anti-TPO at 198.8 U/mL, and anti-TG at 128.8 U/mL. Anti-sulfatide antibody IgG was positive. The timeline information of this case was shown in Figure 1.
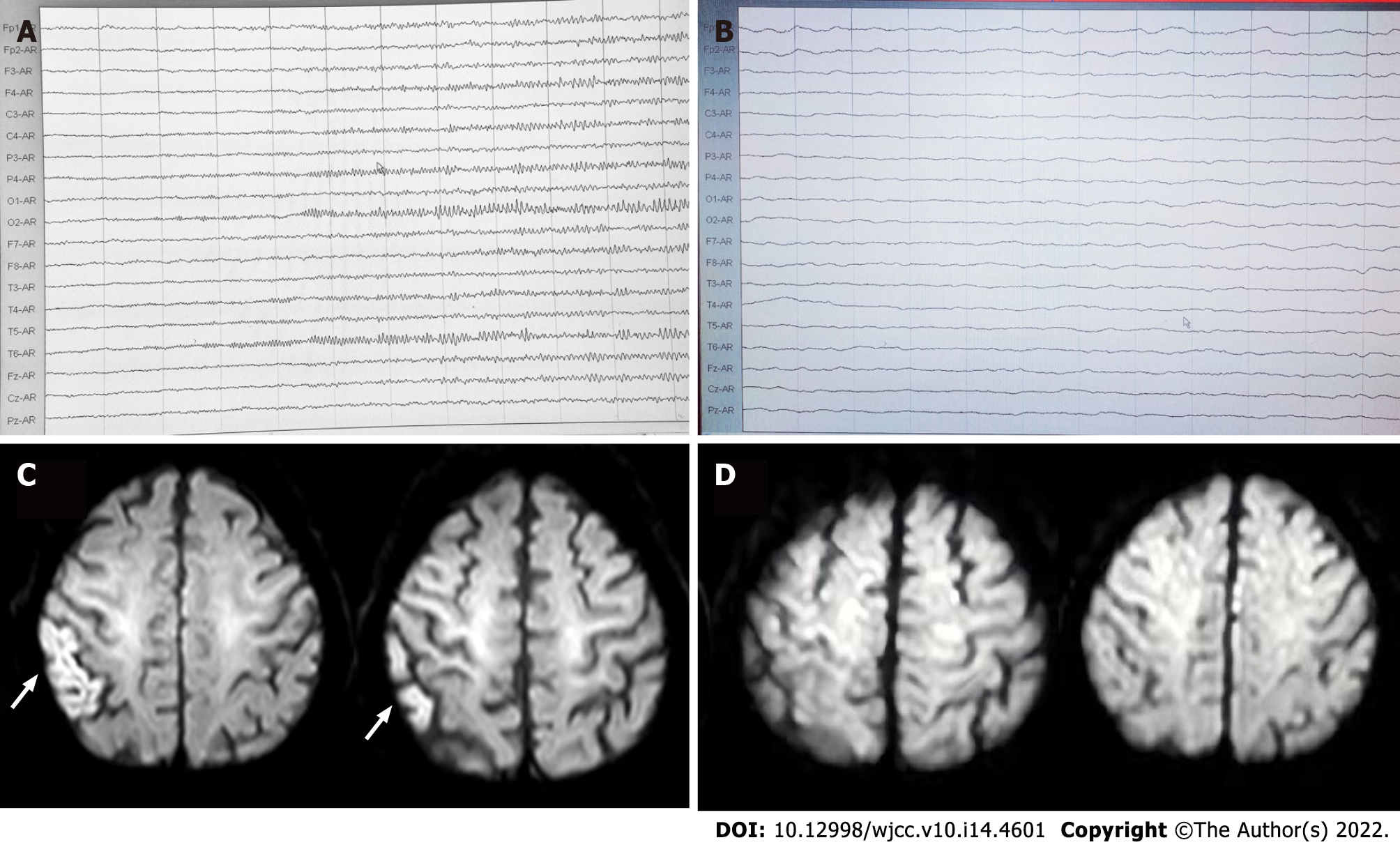
Abnormal electroencephalogram (EEG) was recorded on November 9. Starting from the right hemisphere leads, the amplitude gradually increased and the frequency decreased after appearance of a fast-wave rhythm of 14.0-22.0 Hz, 15-50 μV. Slow-wave rhythm was observed on all leads, and sharp-wave rhythm was observed in the right occipital area, lasting 30-60 s, without obvious clinical seizure or discomfort during this time.
Brain magnetic resonance imaging and magnetic resonance angiography were consistent with subcortical arteriosclerotic encephalopathy and indicated ischemic changes in the right postcentral gyrus and possible early laminar cortical necrosis (Figure 2). Magnetic resonance angiography showed cerebral arteriosclerosis manifestations and reduced branching of the left middle cerebral artery, suggesting poor blood supply. On November 11, video-EEG during epileptic seizures showed low-amplitude fast-wave rhythms at 15.0-22.0 Hz, on all leads, with increasing amplitude and gradually decreasing frequency (Figure 2). After about 30 s, slow-wave activities were observed on all leads, and mixed with a large number of sharp waves and sharp-slow waves, mainly on the right side. Twitching of the right upper limb occurred at this point and recurred at a 4-12 min intervals. This seizure pattern recurred many times, during which the EEG indicated that the symmetrical appearance of 14.0-22.0 Hz, 5-15 μV β-rhythm was the basic rhythm, emitting a large number of 5.5-7.5 Hz low-amplitude θ waves (Figure 2).
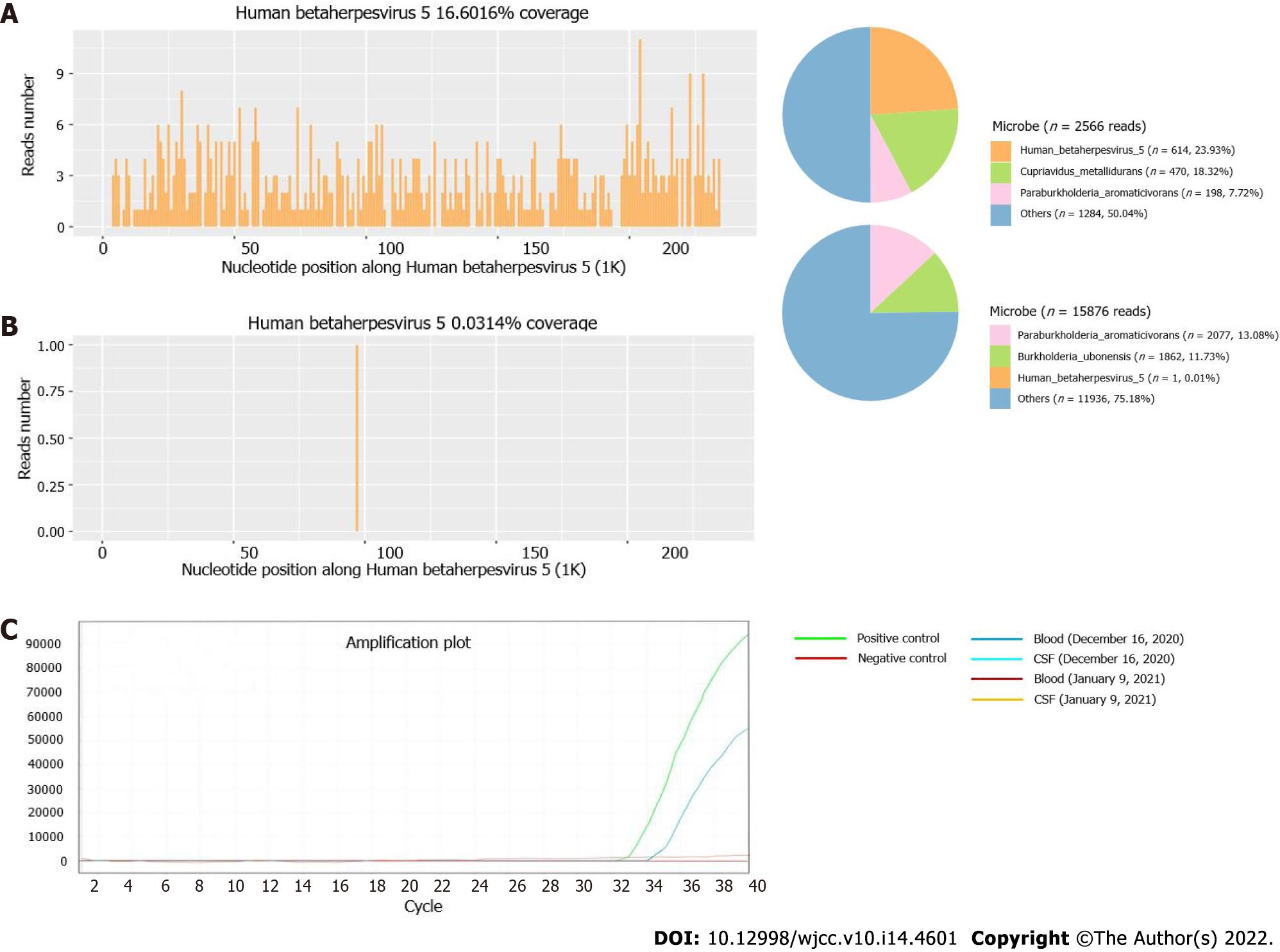
Blood and CSF samples were submitted for PACEseq mNGS detection (Hugobiotech, Beijing, China) on December 16, 2020. CMV was found in CSF samples of 1 unique read and in blood samples of 614 unique reads (Figure 3A and B). Simultaneous qPCR of blood samples confirmed the mNGS results, while it failed to detect CMV in CSF samples (Figure 3C). CMV meningitis with status epilepticus was finally diagnosed in this patient.
The symptoms improved after antiviral, hormonal, antihypertensive, and neurotrophic treatment.
After discharge, the patient regularly took antiviral, low-dose hormone, anti-epileptic, and other medications. He was admitted to the Third Affiliated Hospital of Sun Yat-sen University for the second time on January 8, 2021. Physical examination, paralysis test of the left upper limb, and Romberg’s test showed the same results as before. After admission, the patient completed relevant examinations. Anti-nuclear antibody test of the blood on January 13, 2021 was weakly positive with 1:80 granular pattern, showing anti-SmD1 antibody 118 AU/mL, anti-SS-A antibody 227 AU/mL, and anti-SS-B antibody 202 AU/mL. Salivary gland tissue was submitted for examination. The lobular structure was preserved, with some acinar atrophy. Hyperplasia of interlobular adipose tissue was observed. There were obvious lymphocyte and plasma cell infiltration in the interstitium, and one lymphocyte aggregation focus (approximately > 50/focus) was observed. Combining with clinical findings, these pathological changes were consistent with Sjogren’s syndrome. Blood and CSF samples were submitted for mNGS test again on January 8, 2021. The results of CSF samples were negative, but 1 unique read of human herpes virus 6B was revealed from the blood sample. qPCR did not detect CMV in either the blood or CSF samples. Head magnetic resonance imaging on January 19, 2021 showed various abnormal symptoms, such as multiple ischemia and degeneration foci in the bilateral frontal lobe, infarction and formation of encephalomalacia foci in the left paraventricular area, and cerebral arteriosclerosis. The conditions improved after continued low-dose hormone and anti-epileptic treatment, and the patient was discharged on January 20, 2021.
CMV infection is very common among people. Cellular immunity plays an important role in the defense of infections; when cellular immunity decreases, the incidence of infection and the onset of disease will increase. Some studies showed CMV encephalitis increased at the beginning of organ transplantation and cancer chemotherapy[10]. CMV infections in patients with normal immune function are few.
Intracranial CMV infection can affect the meninges and parenchyma, manifesting different degrees of consciousness disturbance, mental symptoms, seizures, hemiplegia, and sensory disturbances[6]. The epilepsy-type CMV encephalitis always performs non-specifically on EEG, which mainly shows diffused slow waves, accompanied by paroxysmal slow waves and sharp waves[11,12]. In this case, given the history of diabetes, this patient had underlying disease and predisposing factors of immunosenescence. The onset of disease was mainly in the form of epileptic seizure, which subsequently progressed to status epilepticus and accompanied by signs of left side paralysis and unsteady gait. Magnetic resonance imaging indicated high signals on diffusion-weighted imaging sequence of the right postcentral gyrus. mNGS detection of blood and CSF indicated CMV infection, confirmed by qPCR detection using blood samples. The diagnosis was finally confirmed as CMV meningitis of the epilepsy type. After continuously strengthened antiviral and anti-epileptic treatment, the patient’s conditions significantly improved.
The diagnosis of CMV encephalitis was difficult. There is no clear standard currently. Virus culture and brain biopsy are the gold standards for the diagnosis of CMV encephalitis, but they are rarely used in clinical practice[8,13]. Combined with brain biopsy and CSF virus isolation, the sensitivity and specificity of conventional PCR can reach 80% and 90%, respectively[8]. However, PCR always needs a prior hypothesis of the target and can only detect gene sequences of known pathogenic microorganisms. In addition, time is crucial for patients with acute and critical CMV encephalitis. A rapid, accurate, and unbiased diagnostic method is needed. mNGS can quickly and accurately analyze all microorganisms in samples, which has extremely high application value in the diagnosis of infectious diseases, especially for unknown pathogens and those with trace amounts[14]. It has been increasingly applied in diagnosing multiple diseases, including encephalitis and meningitis[15]. mNGS shows a high sensitivity and accuracy in the diagnosis of various pathogens, especially for viruses, difficult-to-cultivation bacteria, and fungi[9].
In this case, the patient’s initial manifestations were epileptic seizures and status epilepticus, accompanied by the signs of left side paresis and unsteady gait. Despite the clear clinical manifestations and imaging evidence of brain parenchymal impairment, the diagnosis of this disease was difficult. Tests including initial CSF routine, biochemistry, ink staining, acid-fast staining smears, cryptococcus capsular antigen detection, and IgG were carried out, all of which only temporarily reduced the possibility of tuberculosis and fungal and bacterial infections. The possibility of special infection was not ruled out. Their values in guiding our clinical treatment were limited. For patients with acute and critical illness of the central nervous system, time is crucial.
It is generally agreed that anti-infective treatment must be initiated immediately as soon as severe central infection is identified. Therefore, rapid and timely diagnosis in central nervous system infection plays a key role for the treatment and prognosis of patients. mNGS was then applied and detected the pathogen as CMV quickly, which was subsequently verified by qPCR. This indicated a great advantage of mNGS in detecting pathogens that are hard to diagnose by conventional methods. Interesting, in blood samples, mNGS detected a total of 614 CMV reads, and qPCR detected CMV at a cycle number of 35. While for CSF samples, mNGS only detected 1 unique read of CMV, but qPCR showed a negative result. The results suggested that mNGS was more likely to be more sensitive than qPCR and could detect trace amounts of pathogens. The patient’s conditions improved after treatments. In the meantime, the negative results of the above reexaminations after improvement of post-treatment symptoms were also consistent with the progression of disease, further supporting the diagnosis of CMV encephalitis.
In this case, mNGS combined with qPCR provided a rapid and reliable diagnosis of CMV encephalitis with high efficiency, sensitivity, and specificity. This indicated that mNGS could be a reliable approach for the diagnosis of CMV encephalitis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Apiratwarakul K, Thailand; Bharati S, Bangladesh S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | Liu J, Jaijyan DK, Tang Q, Zhu H. Promising Cytomegalovirus-Based Vaccine Vector Induces Robust CD8+ T-Cell Response. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Zuhair M, Smit GSA, Wallis G, Jabbar F, Smith C, Devleesschauwer B, Griffiths P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol. 2019;29:e2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 541] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 3. | Emery VC, Sabin CA, Cope AV, Gor D, Hassan-Walker AF, Griffiths PD. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet. 2000;355:2032-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 391] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 4. | Limaye AP, Babu TM, Boeckh M. Progress and Challenges in the Prevention, Diagnosis, and Management of Cytomegalovirus Infection in Transplantation. Clin Microbiol Rev. 2020;34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 5. | Yan CH, Wang Y, Mo XD, Sun YQ, Wang FR, Fu HX, Chen Y, Han TT, Kong J, Cheng YF, Zhang XH, Xu LP, Liu KY, Huang XJ. Incidence, risk factors, and outcomes of cytomegalovirus retinitis after haploidentical hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55:1147-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Arribas JR, Storch GA, Clifford DB, Tselis AC. Cytomegalovirus encephalitis. Ann Intern Med. 1996;125:577-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 134] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Chen SJ, Wang SC, Chen YC. Antiviral Agents as Therapeutic Strategies Against Cytomegalovirus Infections. Viruses. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Razonable RR, Inoue N, Pinninti SG, Boppana SB, Lazzarotto T, Gabrielli L, Simonazzi G, Pellett PE, Schmid DS. Clinical Diagnostic Testing for Human Cytomegalovirus Infections. J Infect Dis. 2020;221:S74-S85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 9. | Chen Y, Feng W, Ye K, Guo L, Xia H, Guan Y, Chai L, Shi W, Zhai C, Wang J, Yan X, Wang Q, Zhang Q, Li C, Liu P, Li M. Application of Metagenomic Next-Generation Sequencing in the Diagnosis of Pulmonary Infectious Pathogens From Bronchoalveolar Lavage Samples. Front Cell Infect Microbiol. 2021;11:541092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 85] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 10. | Liu C, Han Y. Correlation between human cytomegalovirus infection and encephalitis. Journal of Apoplexy and Nervous Diseases. 2015;32: 670-71. |
| 11. | Hua W, Wang Y. Clinical observation of 8 cases of cytomegalovirus encephalitis. Naoyushenjing Jibing Zazhi. 2003;11:379. [DOI] [Full Text] |
| 12. | Feng J, Ge Y, Cui S, Zhang Y, Rao M. Clinical Analysis of Acute Viral Encephalitis in Adult and Curative Effect of Antiviral Drugs. Nervous Diseases and Mental Hygiene. 2002;2:270-71. |
| 13. | Griffiths PD. Diagnosis of cytomegalovirus infection. J Antimicrob Chemother. 1989;23 Suppl E:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20:341-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 909] [Article Influence: 151.5] [Reference Citation Analysis (0)] |
| 15. | Wilson MR, Sample HA, Zorn KC, Arevalo S, Yu G, Neuhaus J, Federman S, Stryke D, Briggs B, Langelier C, Berger A, Douglas V, Josephson SA, Chow FC, Fulton BD, DeRisi JL, Gelfand JM, Naccache SN, Bender J, Dien Bard J, Murkey J, Carlson M, Vespa PM, Vijayan T, Allyn PR, Campeau S, Humphries RM, Klausner JD, Ganzon CD, Memar F, Ocampo NA, Zimmermann LL, Cohen SH, Polage CR, DeBiasi RL, Haller B, Dallas R, Maron G, Hayden R, Messacar K, Dominguez SR, Miller S, Chiu CY. Clinical Metagenomic Sequencing for Diagnosis of Meningitis and Encephalitis. N Engl J Med. 2019;380:2327-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 676] [Article Influence: 112.7] [Reference Citation Analysis (0)] |