Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4580
Peer-review started: October 9, 2021
First decision: January 11, 2022
Revised: January 16, 2022
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 16, 2022
Processing time: 216 Days and 2.5 Hours
Esophagojejunal anastomotic leakage (EJAL) is a serious and potentially crucial complication of total gastrectomy and represents the major cause of postoperative death, with a mortality rate of up to 50%. However, treatment remains challenging and controversial. We report here the case of a patient whose intrathoracic EJAL was successfully treated with computer tomography (CT)-guided negative pressure drainage treatment.
A 69-year-old male patient complained of difficulty swallowing within the last six months. He was diagnosed with esophagogastric junction carcinoma, Siewert II, cT3N0M0 stage II. Total gastrectomy and Roux-en-Y esophagojejunostomy were performed. High fever, left chest pain and dyspnea appeared on postoperative day 5, and EJAL was confirmed by CT, gastroscopy and oral blue-dimethylene tests. Conservative treatment measures were applied immediately, including antibiotics, nasojejunal tubes, and repeated thoracic puncture and drainage under ultrasound guidance. However, without sufficient and effective drainage, the thoracic infection and systemic condition continued to deteriorate. With the cooperation of multiple departments, percutaneous CT-guided drainage (24 Fr 7 mm) in the thoracic cavity was successfully placed near the anastomotic leakage. Because of continuous negative pressure suction, the infection symptoms were effectively controlled and the general situation gradually recovered. Subsequent follow-up examination showed that the patient was in good condition.
Negative pressure drainage via CT may represent an effective minimally invasive approach to treating intrathoracic EJAL.
Core Tip: Treatment for esophagojejunal anastomotic leakage (EJAL) is still challenging. Conservative approaches treat the symptoms but not the root causes, which usually leads to further disease progression. Due to the great trauma associated with surgical treatment, mortality is significantly increased. Endoscopic treatment has a certain failure rate and requires multiple endoscopic operations, which certain patients cannot tolerate. We presented the first intrathoracic EJAL case treated by computed tomography-guided negative pressure drainage, which may represent an effective minimally invasive approach.
- Citation: Jiang ZY, Tao GQ, Zhu YF. Computer tomography-guided negative pressure drainage treatment of intrathoracic esophagojejunal anastomotic leakage: A case report . World J Clin Cases 2022; 10(14): 4580-4585
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4580.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4580
Esophagojejunal anastomotic leakage (EJAL) is a common and serious postoperative complication of total gastrectomy. The reported incidence of EJAL varies between 0.5% and 11.5%, its mortality rate can reach 50%, and it is the major reason for postoperative death after surgery. Intrathoracic anastomotic leakage is associated with significant mortality, and EJAL is associated with high mortality, longer hospital stays and high costs. Moreover, it delays or nullifies the possibility of adjuvant therapy, thereby worsening the patients’ quality of life and survival[1-3].
However, current therapies for EJAL are still inefficient. Therapies range from conservative treatment to aggressive surgical treatment, and the optimal therapy is still controversial. Conservative management may predispose patients to further complications, while surgical treatment presents a high mortality rate. Recent endoscopic treatments show narrow applicability and some potential risks. Thus, a standard strategy for treatment has not been established[4-6]. Here, we present a case of intrathoracic EJAL after total gastrectomy for gastric cancer that was successfully treated with computer tomography (CT)-guided negative pressure drainage treatment, which provided sufficient drainage.
A 69-year-old male patient was admitted to Wuxi People’s Hospital for difficulty swallowing.
Our patient had progressively worsening dysphagia over a period of 6 mo with an acute deterioration over the preceding 2 wk leading to the admission.
Obvious abnormalities were not observed in prior illnesses.
There was no special history and personal history. The patient had no known family history of cancer.
There were no abnormalities in cardiopulmonary or abdominal examinations.
Blood analysis did not reveal increased levels of tumor markers.
Endoscopy was performed and revealed an ulcerative lesion at the gastric cardia. Enhanced abdominal CT indicated that the tumor might invade the muscularis propria and subserosa without enlarged lymph nodes or distant metastases.
The patient was diagnosed with Siewert II esophagogastric junction carcinoma without lymph node metastases. The clinical stage was confirmed as cT3N0M0 stage II (cT1N0M0, 7th edition of UICC TNM Classification of Malignant Tumors). Oral blue-dimethylene test and CT examination were performed when anastomotic leakage was highly suspected after operation.
The patient received total gastrectomy + D2 lymph node dissection. Intestinal reconstruction was performed in the form of Roux-en-Y esophagojejunostomy. Esophagojejunal anastomosis was performed with an end-to-side circular stapler. The circle was removed after the anastomosis was completed, and manual interrupted sutures were added to the seromuscular layer of the anastomosis. The operation duration was 130 min, and 50 mL of blood loss occurred.
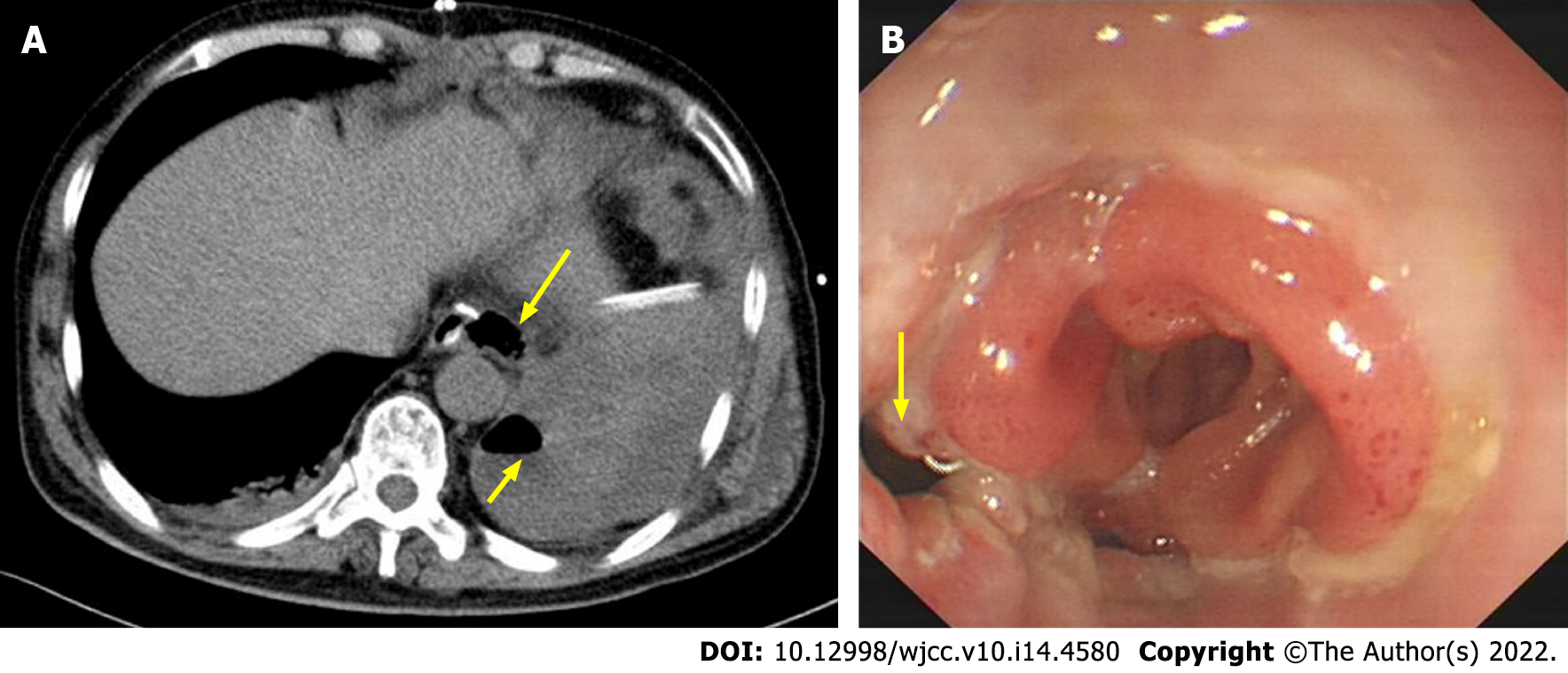
With regular treatments, the clinical manifestation appeared normal within four days after surgery. However, the patient developed fever, left chest pain and dyspnea on postoperative day (POD) 5, including leukocytosis and elevated reactive C protein. We suspected anastomotic leakage and performed a CT examination. CT revealed a large fluid collection containing air around the anastomosis, periesophageal and pulmonary abscesses, bilateral pleural effusion and atelectasis (Figure 1A). Then, percutaneous echo-guided intrathoracic drainage was performed multiple times according to the ultrasound results. Because of its convenient operation, less trauma and good patient tolerance, percutaneous ultrasound puncture has a good drainage effect on postoperative pleural effusion and ascites and early infectious exudate.
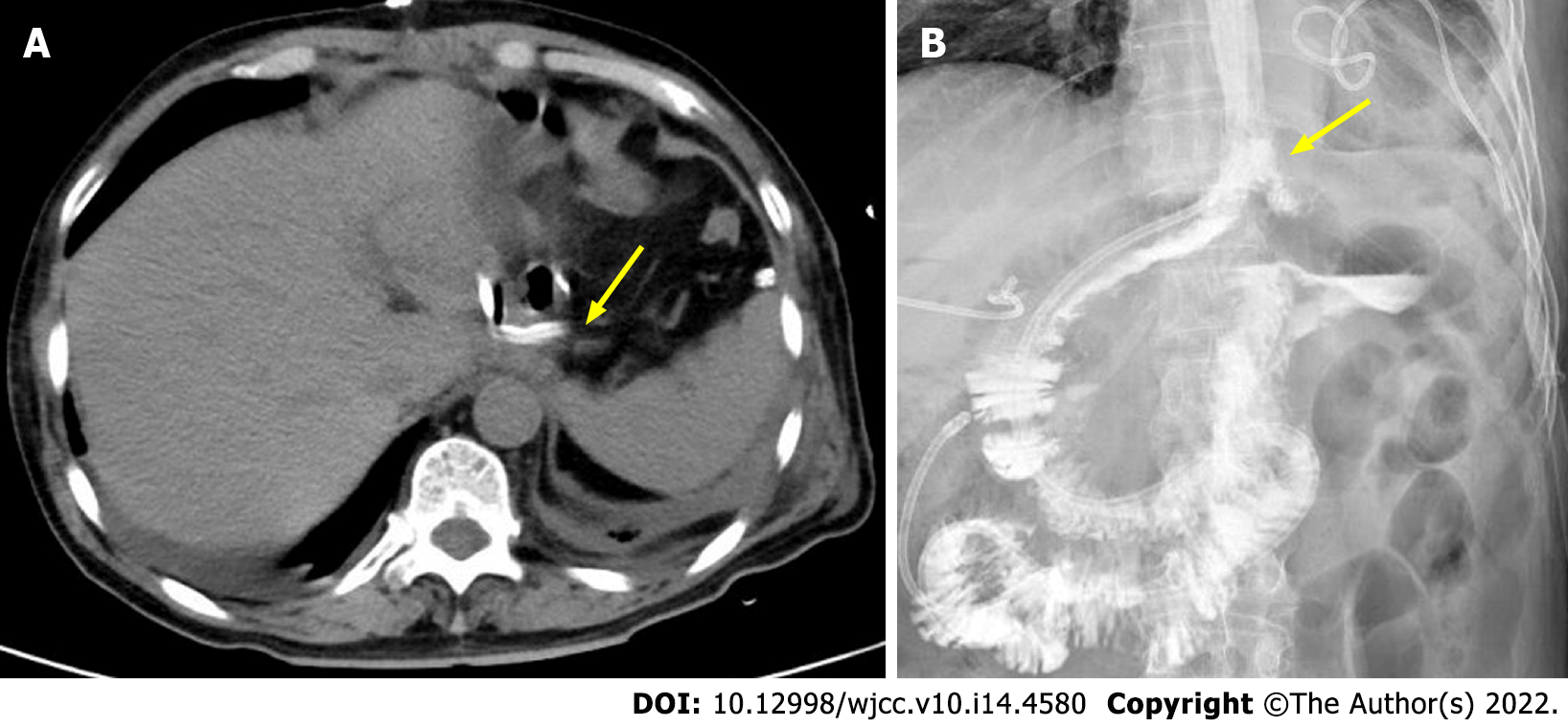
On POD 8, an oral blue-dimethylene test indicated drainage from the chest drain that had been placed next to the anastomosis. Subsequent endoscopy revealed dehiscence of the left lateral wall of the esophagojejunal anastomosis (Figure 1B); thus, a nasointestinal tube was placed simultaneously. Bacterial resistance cultures of ascites, bile and pleural drainage pus were performed. The results showed that human Staphylococcus was infected. According to the drug sensitivity results, Piperacillin sodium, tazobactam and imipenem were given gradually. However, his symptoms did not improve. Due to the small diameter of the chest drain and ineffective drainage, the fluid within the thoracic cavity could not be appropriately discharged. Without effective drainage, sustained mediastinal and chest infection led to deterioration of the general condition. To ameliorate this situation, percutaneous CT-guided drainage (24 Fr 7 mm) in the thoracic cavity with low-pressure suction was performed near the site of anastomosis leakage (Figure 2A). This drain had a significantly enlarged diameter and provided proactive drainage; thus, the bacterial contamination and local edema were decreased while granulation tissue formation was promoted; hence, the chest infection was controlled gradually. The percutaneous drain was maintained for 18 days. Inflammatory indices and clinical conditions improved, and anastomotic leakage on fluoroscopic examination on POD 38 (Figure 2B).
Pathology of the specimen: (esophagogastric junction) adenocarcinoma (poorly differentiated) with neuroendocrine differentiation. The tumor cells had invaded the subserosa. Metastases were not found in the lymph nodes (0/15). Immunohistochemistry: HER2 (+), CGA (partial +), SYN (partial +), SALL4
The patient was discharged on POD 48. The subsequent abdominal CT and all laboratory tests showed that the patient was generally in good condition.
To our knowledge, this is the first report describing the efficacy of negative pressure drainage via CT for intrathoracic EJAL. This simple clinical procedure was performed safely through CT guidance. Due to sufficient drainage, the proposed method reduced the symptoms of systemic infections, especially chest and mediastinal infections, and promoted the improvement of clinical conditions.
EJAL is considered one of most serious complications after total gastrectomy, and it is associated with high mortality. Despite advances in surgical techniques and equipment, the incidence of EJAL remains unchanged, and its treatment remains a challenge. An appropriate strategy should be selected after evaluating many factors, including the anastomotic leakage size, time since surgery, and patient’s general conditions[7-9]. Common interventions for EJAL include conservative treatment, endoscopic treatment, and surgical treatment. Conservative treatment includes fasting, percutaneous drainage, intravenous broad-spectrum antibiotics, nutritional support (enteral or parenteral), and nasojejunal tube insertion. These strategies are basic interventions that treat the symptoms but not the root cause; thus, they usually lead to worse conditions. Surgical treatment includes drainage, repair, or repeat surgery to repair the anastomosis. Because of obvious surgical trauma and anesthetic stress, such treatment is related to a higher mortality rate than other approaches[10,11]. Endoscopic treatment consists of stenting, clipping, endoscopic suturing, and endoscopic vacuum-assisted closure, all of which present specific advantages and disadvantages. Stenting does not fit at every position, and stent migration is a relevant complication of this procedure. Because hemoclips grasp the mucosal layer alone, clipping is only applied to small defects. In addition, the clip reduces the flexibility of the endoscope, and precise access to the leakage may be more difficult[12-14]. Due to the time and costs involved, endoscopic vacuum-assisted closure should be considered carefully. Furthermore, patients often need to undergo multiple endoscopic procedures and experience anesthesia stress, and they may not be able to tolerate the associated physical conditions[15-17].
In our case, the patient had a severe chest and mediastinal infection. Once EJAL was suspected clinically, conservative treatment was performed immediately. Adequate and effective drainage is essential for intrathoracic EJAL. Due to the small diameter and passive drainage, it was difficult to achieve continuous, accurate, and adequate drainage under the guidance of percutaneous ultrasound. Based on clinical experience in the treatment of intra-abdominal intestinal leakage or esophageal leakage, a large-diameter pipe with negative pressure suction was placed at the best position near the leakage. Under the multidisciplinary cooperation of thoracic surgery and radiology, this procedure was completed precisely in one attempt.
The patient tolerated the application of local anesthesia to a small skin area well without obvious discomfort. According to the patient's condition changes, the speed and frequency of negative pressure suction were adjusted accordingly. With the help of sufficient drainage, the patient’s mediastinal infection and thoracic cavity infection were quickly controlled, and his overall condition gradually improved, thereby promoting the healing of EJAL. He ultimately achieved a good clinical outcome.
The causes of anastomotic leakage may be related to the following factors: tumor infiltration leading to esophageal wall edema and poor healing after anastomosis, anastomotic tension, hypoalbuminemia, etc. More samples are needed to evaluate the effectiveness of this method, and intraoperative gastroscopy is recommended to evaluate the anastomotic condition.
EJAL is a dangerous complication, and its treatment remains controversial. Negative pressure drainage via CT may represent an effective minimally invasive approach to treating EJAL that can obviate the need for further life-threatening surgery or long-term conservative management. However, more trials are still required to demonstrate whether it can be recommended as an appropriate treatment for EJAL.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chowdhury D, United Kingdom; Syahputra DA, Indonesia S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Makuuchi R, Irino T, Tanizawa Y, Bando E, Kawamura T, Terashima M. Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg Today. 2019;49:187-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Xing J, Liu M, Qi X, Yu J, Fan Y, Xu K, Gao P, Tan F, Yao Z, Zhang N, Yang H, Zhang C, Cui M, Su X. Risk factors for esophagojejunal anastomotic leakage after curative total gastrectomy combined with D2 lymph node dissection for gastric cancer. J Int Med Res. 2021;49:3000605211000883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Mandarino FV, Bonura GF, Esposito D, Rosati R, Parise P, Fanti L. A large anastomotic leakage after esophageal surgery treated with endoluminal vacuum-assisted closure: a case report. J Surg Case Rep. 2020;2020:rjaa071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Ye HY, Huang WZ, Wu YM, Liang Y, Zheng JM, Jiang HM. Personalized management of anastomotic leak after surgery for esophageal carcinoma. Chin Med Sci J. 2012;27:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Barchi LC, Ramos MFKP, Pereira MA, Dias AR, Ribeiro-Júnior U, Zilberstein B, Cecconello I. Esophagojejunal anastomotic fistula: a major issue after radical total gastrectomy. Updates Surg. 2019;71:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Granata A, Amata M, Traina M. Esophagojejunal anastomotic dehiscence successfully repaired by endoluminal suture and stent positioning: Report of a mini-invasive approach. Dig Endosc. 2018;30:535-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Zhang W, Sun G, Zhang H, Furnee E, Liu Q, Gong H, Sun P, Zhang W. Endoscopic closure of a postoperative rectal anastomotic leakage with hemoclips: A case report. Int J Surg Case Rep. 2021;80:105525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Wang H, Zhang Y, Liu W, Wang J, Liu G, Li C, Ding W. Practice of cervical end-esophageal exteriorization in patients with severe intrathoracic anastomotic leakage after esophagectomy. J Int Med Res. 2018;46:5090-5098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Çetin DA, Gündeş E, Çiyiltepe H, Aday U, Uzun O, Cumhur Değer K, Duman M. Risk factors and laboratory markers used to predict leakage in esophagojejunal anastomotic leakage after total gastrectomy. Turk J Surg. 2019;35:6-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Tsuji T, Saito H, Hayashi K, Kadoya S, Bando H. T-drain esophagostomy under thoracoscopy for intrathoracic esophagogastric anastomotic leakage following esophagectomy for esophagogastric junction cancer: A case report. Int J Surg Case Rep. 2020;73:79-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Gong W, Li J. Combat with esophagojejunal anastomotic leakage after total gastrectomy for gastric cancer: A critical review of the literature. Int J Surg. 2017;47:18-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Galizia G, Napolitano V, Castellano P, Pinto M, Zamboli A, Schettino P, Orditura M, De Vita F, Auricchio A, Mabilia A, Pezzullo A, Lieto E. The Over-The-Scope-Clip (OTSC) system is effective in the treatment of chronic esophagojejunal anastomotic leakage. J Gastrointest Surg. 2012;16:1585-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Schlosser T, Feisthammel J, Gockel I, Mössner J, Hoffmeister A. Endoscopic suturing as a less invasive approach for the treatment of anastomotic leakage after esophagogastrostomy - a case report. Z Gastroenterol. 2018;56:1365-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Hoeppner J, Kulemann B, Seifert G, Marjanovic G, Fischer A, Hopt UT, Richter-Schrag HJ. Covered self-expanding stent treatment for anastomotic leakage: outcomes in esophagogastric and esophagojejunal anastomoses. Surg Endosc. 2014;28:1703-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Pournaras DJ, Hardwick RH, Safranek PM, Sujendran V, Bennett J, Macaulay GD, Hindmarsh A. Endoluminal Vacuum Therapy (E-Vac): A Treatment Option in Oesophagogastric Surgery. World J Surg. 2018;42:2507-2511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 59] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Ward MA, Hassan T, Burdick JS, Leeds SG. Endoscopic vacuum assisted wound closure (EVAC) device to treat esophageal and gastric leaks: assessing time to proficiency and cost. Surg Endosc. 2019;33:3970-3975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Virgilio E, Ceci D, Cavallini M. Surgical Endoscopic Vacuum-assisted Closure Therapy (EVAC) in Treating Anastomotic Leakages After Major Resective Surgery of Esophageal and Gastric Cancer. Anticancer Res. 2018;38:5581-5587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |