Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4502
Peer-review started: September 27, 2021
First decision: December 4, 2021
Revised: December 17, 2021
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 16, 2022
Processing time: 227 Days and 19.2 Hours
Benralizumab is a monoclonal antibody targeting the IL-5 receptor used in the treatment of asthma. The use of benralizumab in other conditions is only emerging and could represent a therapeutic option for other eosinophil-associated diseases. Here, we report the case of a patient suffering from eosinophilic esophagitis and asthma who achieved histological remission of eosinophilic esophagitis (EoE) under benralizumab treatment for his asthma.
Our patient was a 56-year-old white male with a history of eosinophilic eso
Our case shows the effects of therapy with a novel agent not yet approved for this condition but for other diseases, with histological resolution of EoE after treatment. Complete clinical remission was not observed, which exemplifies the complex nature of EoE, its associated psychosomatic burden, and the chronification of the disease. Nevertheless, monoclonal antibodies targeting the Th2 response and, in our case, an IL5 receptor antagonist, achieved complete histological remission, which was not the case with an antibody against IL-5, which was also initiated to treat asthma.
Core Tip: Born in 1965, our patient developed the first symptoms of dyspepsia and food impaction at the age of 28. Eosinophilic esophagitis was diagnosed 19 years later in 2012. The first treatment began in 2013 with oral topical glucocorticoids. With concomitant therapy-resistant asthma, treatment with mepolizumab was initiated in addition to standard asthma therapy in 2016. Because of again suboptimal control of asthma, therapy was switched to benralizumab in 2019. In subsequent gastroscopies, histological remission was observed, but our patient reported pain in the esophagus and further food impaction. This case harbors typical aspects of the complexity of treating eosinophilic esophagitis.
- Citation: Huguenot M, Bruhm AC, Essig M. Histological remission of eosinophilic esophagitis under asthma therapy with IL-5 receptor monoclonal antibody: A case report. World J Clin Cases 2022; 10(14): 4502-4508
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4502.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4502
We report the case of a patient with eosinophilic esophagitis (EoE) with concomitant severe asthma who achieved complete histological resolution of the first after the initiation of therapy with benralizumab for asthma.
Until now, benralizumab has been approved only for severe eosinophilic asthma based on three studies[1-3].
Intermittent dysphagia and poorly controlled asthma.
Born in 1965, our patient developed the first symptoms of dyspepsia and food impaction at the age of 28. The diagnosis of EoE was made 19 years later in 2012 on the basis of symptoms, endoscopy, and histology. We only have part of the histology report at hand and it only mentions slightly active eosinophilic esophagitis, no signs of thrush or virus infection, and no dysplasia. We do not know the eosinophilic count at that time. Celiac disease and inflammatory bowel diseases as other disorders possibly presenting with esophageal eosinophilia were excluded at that time. The first treatment began in 2013 with oral topical glucocorticoids (fluticasone, off-label, and from 2016 onwards switched to budesonide mostly, but intermittent back to fluticasone). Difficulties with swallowing intensified, despite consistent therapy with oral glucocorticoids. The following gastroscopy in December 2014 sowed strictures in the esophagogastric area, which were treated with argon plasma coagulation and dilation with bougies, with reported clinical improvement. The same year, asthma was diagnosed, exhaled NO was 39 ppb and the medication for asthma was as follows: Inhaled combination of formoterol and budesonide, ciclesonide, and, as needed salbutamol. Gastroesophageal reflux disease (GERD) was diagnosed in 2015 via 24-h pH-metry.
Because of therapy-resistant asthma, the first biological treatment with mepolizumab was initiated in addition to standard asthma therapy in 2016 at a dose of 100 mg s.c. monthly. Both as a follow-up and because of increasing dyphagia, the patient underwent another gastroscopy in October 2018. Histology showed 20 eosinophils per high-power field in the distal esophagus and 19 eosinophils per high-power field in the proximal esophagus. Because of suboptimal control of asthma (exhaled NO 34 ppb to that time), the standard asthma therapy was switched to benralizumab between January and March 2019 at a dose of 30 mg s.c. monthly for the first 3 mo and then once every 2 mo until today. In the patient's regular consultations with a pneumologist both before and after treatment with mepolizumab and benralizumab, only partly controlled asthma was described according to GINA guidelines (waking due to asthma, daytime symptoms more than twice a week, use of short acting beta agonist as reliever more than twice a week, and activity limitation due to asthma).
The patient also had recurrent exacerbations of his asthma. The exact number cannot be assessed from the medical history available to us. At least three exacerbations in 2015 and one exacerbation due to an influenza A infection in March 2019 even needed a 4-d hospitalization. For these exacerbations, he received systemic pulse steroid therapy. In September 2019, the patient discontinued oral topical glucocorticoids (budesonide), reporting vertigo.
Delay between symptoms and diagnosis is not uncommon in EoE and correlates with the development of esophageal strictures[4]. Common symptoms of EoE in teenagers and adults are dysphagia, food impaction, abdominal pain, chronic reflux symptoms, heartburn, and spontaneous rupture of the esophagus. Younger children can present nausea, vomiting, feeding difficulties, and growth retardation[5].
The usual delay to diagnosis and the chronic nature of EoE predispose patients to associated psychiatric conditions. A retrospective study between 2002 and 2018 showed that psychiatric comorbidities are common in EoE, with the most common comorbidities being anxiety and depression. Approximately one-third of adults had a diagnosis of a psychiatric condition, and just as many received a prescription medication for a psychiatric illness. The longer the duration of symptoms preceding diagnosis, the more likely that psychiatric comorbidities were found[6]. A recent review article stated the necessity for more research, as there is not even a single prospective study; therefore, the effect of these comorbidities on patients with EoE remains unclear. In addition, anxiety could be driven by limited treatment options[7].
Other comorbidities were moderate obstructive sleep apnea syndrome diagnosed in June 2016, postnasal drip syndrome, hiatal hernia diagnosed in December 2014, ectopic gastric mucosa diagnosed in April 2015, allergic rhinoconjunctivitis, status post prostatectomy for prostatic hyperplasia, status post cholecystectomy, status post multiple vertebral surgeries, and status post left shoulder surgery. Although there is no documented diagnosis for a psychiatric comorbidity, a medical report from December 2019 mentioned treatment with escitalopram and mirtazapin in the patient's medication list, suggesting that the patient might have suffered from depression.
Not applicable.
Histologic examination of esophageal biopsies.
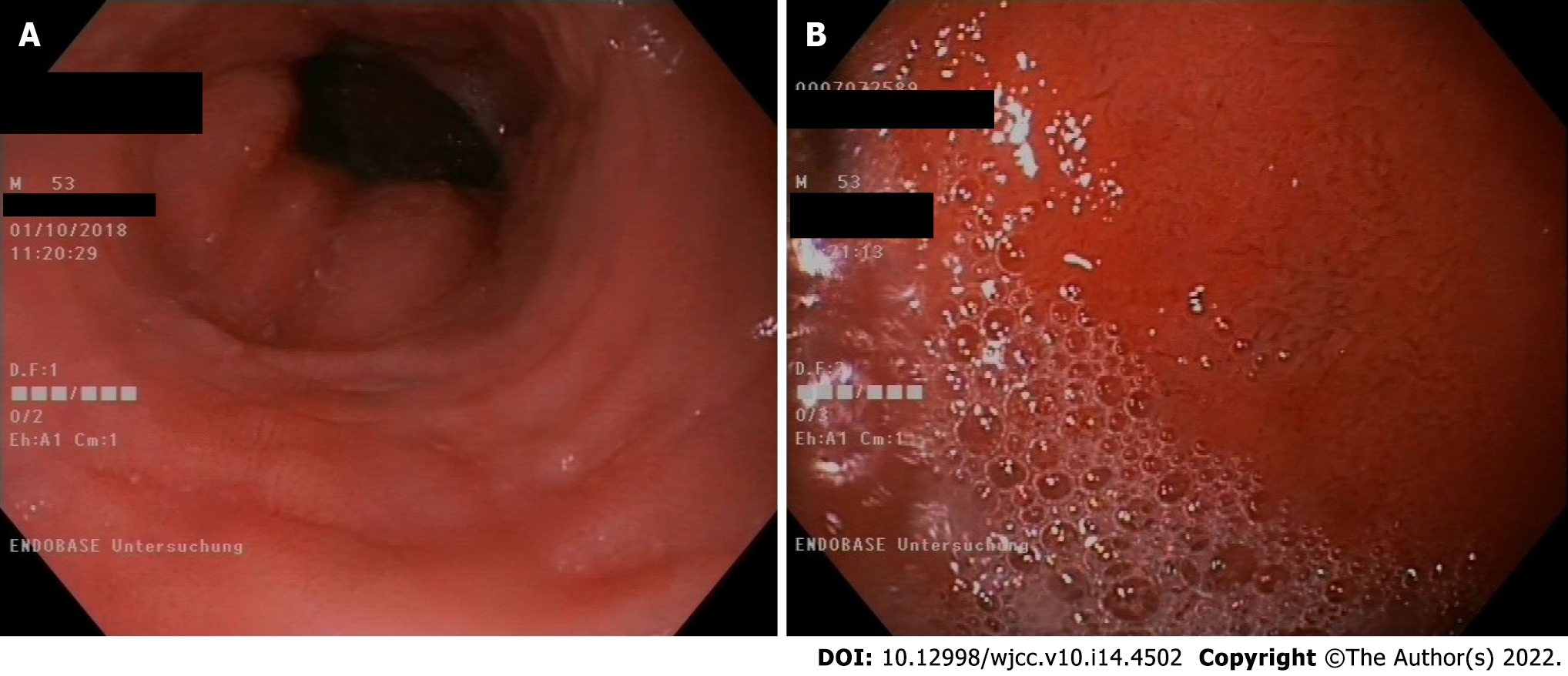
Gastroscopy in May 2019 (Figure 1).
Eosinophilic esophagitis.
For many years, our patient received a proton pump inhibitor (PPI) and budesonide, a swallowed glucocorticoid, according to guidelines, but there was neither clinical nor histological improvement. The dilation of esophageal strictures provided a temporary relief of symptoms. It is difficult to assess the exact diet of our patient, and it is unknown if he practiced dietary restrictions. The patient was on anti-IL5 mepolizumab since October 2016, for approximately 2.5 years, but interestingly, only after treatment with the IL-5 receptor antagonist benralizumab were there no eosinophils found in the esophageal mucosa. This histological remission was observed on May 2019, only approximately 4 mo after treatment began with the IL-5 receptor antagonist. Perhaps a lower concentration of mepolizumab (100 mg), which was the dose that our patient received, was just not enough to reduce the number of eosinophils in tissues. As the patient was under PPIs and topical swallowed glucocorticoids for years and a gastroscopy (October 2018) under this medication showed increased eosinophils, we do not think it likely that this medication with PPI and glucocorticoids alone could have had an impact on the histologic remission. Although they might have contributed to that.
In retrospect, it is difficult to assess the exact medication and the small changes in the medication that the patient received. One problem is that he visited many different specialists in different locations for second opinions. Treatment with systemic glucocorticoids over a longer time than for the exacerbations was not reported. He was under a PPI (esomeprazole) at least since February 2015 and in changing dosages (from esomeprazole 20 mg twice daily to esomeprazole 40 mg twice daily). In March 2019, which was shortly before the gastroscopy in May 2019 (Figure 1) showed a full depletion of eosinophils in the esophagus, it is stated that the dosage was esomeprazole 40 mg twice daily. It is not known whether the patient tried an elimination diet.
This case harbors typical aspects of the complexity of treating EoE and includes a long interval between the initial symptoms and diagnosis, the interaction with other eosinophilic conditions, psychiatric comorbidities or associated symptoms and chronic illnesses and possible new therapeutic approaches.
Although our patient showed objective improvement of his disease under treatment with benralizumab in terms of total depletion of eosinophils in peripheral blood samples as well as in the histological examination, with zero eosinophilic granulocytes (0/high power field), there was limited clinical improvement.
The patient has only partially controlled asthma according to GINA guidelines. He is dependent on inhaled corticosteroids but not systemic corticosteroids. Dyspnea and coughing are troubling at night, but his comorbidity of obstructive sleep apnea syndrome and postnasal drip syndrome likely play a role, in addition to his asthma. He experiences limitations in daily life, cannot perform physical work, and can perform office work only 2 h/d. This goes along with findings that IL-5-receptor targeted therapy leads to fewer exacerbations and lesser steroid dependence but not to many improvements in lung function itself or bronchial hyperreactivity[8]. Indeed, the spirometry results in the case of our patient remained approximately the same.
There also seems to be limited clinical improvement concerning the patient’s gastrointestinal symptoms. Intermittent dysphagia was present when he underwent the last gastroscopy, where the histological evaluation showed no signs of eosinophils under benralizumab treatment. The resolution of esophageal strictures under therapy is still debated, but it is generally assumed that esophageal strictures are not responsive to medical therapy. The subepithelial fibrosis associated with strictures, especially in adults with long-lasting disease, is less reversible by anti-IL-5 therapy.
With the patient’s long history of nearly 30 years of EoE, pain chronification and underlying psychiatric condition are possible.
EoE is a chronic condition affecting both adults and children[4] characterized by symptoms related to esophageal dysfunction and esophageal eosinophilia. Other causes of esophageal eosinophilia, such as GERD, parasitic infections, vasculitis, and Morbus Crohn’s disease, should be excluded[9]. EoE is a chronic antigen-mediated or immune-mediated disease. Most antigens seem to be food-based[9].
The pathogenesis of EoE is multifactorial and not fully understood. Genetic predisposition, environmental factors (microbiome, breastfeeding, and population density), and food antigens play a role. These antigens stimulate Th2 cells, which produce different cytokines, one of which is IL-5, which activates eosinophils, leading to the inflammation of the esophagus[10].
Due to the probable allergic nature of EoE, the avoidance of triggers in food or aeroallergens could cure the disease. Treatment includes both pharmacological and dietary measures. Triggers cannot be identified in every case, and the pharmacological treatment includes PPIs and topical steroids.
Historically, the complex interplay between gastroesophageal reflux and eosinophilic esophagitis has been assumed to represent the same entity.
In previous years, patients with pathological results in pH-metry were categorized as GERD patients, and GERD and EoE were seen as two distinct diseases. Since 2011, a patient who responded to PPI therapy, independent of the pH measurement, was considered to have PPI-responsive EoE (PPI-REE). Now, we know that GERD and PPI-REE are hardly distinguishable from each other (clinically or histologically). The term PPI-REE disappeared from the actual guidelines.
Some cases of EoE respond to a PPI alone, and these cases could represent this associated condition. Up to 40% of children with EoE experience remission under a PPI alone. This subset of patients are considered to have PPI-responsive eosinophilic esophagitis (PPI-REE)[11]. Why this group experiences symptom relief when others do not is unclear. However, GERD may be difficult to rule out because neither the response to PPIs nor the duration of exposure to esophageal acid, measured by means of ambulatory pH monitoring, definitively distinguishes GERD from eosinophilic esophagitis[12].
There is an association of EoE with atopic disorders such as asthma, rhinitis, eczema, and food allergies[9,13].
IL-5 is one of the cytokines responsible for the activation of eosinophils by binding to their IL-5Rα receptors, resulting in subsequent activation of the NF-κB pathway. From a pathophysiological point of view, this activation of the NF-κB pathway causes tissue damage due to endothelial cell damage, changed repair mechanisms, and fibrosis. There are humanized monoclonal antibodies such as mepolizumab and reslizumab that target IL-5, blocking its binding to the receptor. Benralizumab targets the alpha subunit of the IL-5 receptor itself and inhibits the described pathway. Moreover, the antibody benralizumab, through its Fc region, is able to recruit NK cells, mast cells, and basophils and to induce antibody-dependent cellular cytotoxicity.
The result is an entire depletion of eosinophils in the bone marrow and blood and a significant decrease of eosinophils in sputum and tissue[14,15]. With their eosinophil-depleting effect, theoretically, there is a possible application of anti-IL-5 drugs in every disease associated with the pathological presence of eosinophils, namely, eosinophilic asthma, eosinophilic esophagitis, hypereosinophilic syndrome, and eosinophilic granulomatosis with polyangiitis[16].
A systematic review confirmed that anti-IL-5 monoclonal antibodies do not lead to histological depletion of eosinophils or to the improvement of clinical discomfort[17]. One can speculate on the reason for this; perhaps, unlike IL-5 antibodies, IL-5-receptor antibodies can induce cytotoxicity against eosinophils, as described earlier. Other biological drugs targeting the Th2 response, such as anti-IL-13 antibodies, are under evaluation[10].
Most studies had a very small study population size. Only two studies included adults, both with very small sample sizes and with a relatively short follow-up. The most recent study was from 2018, and the others were from 2012 or earlier. First, there was a clear lack of recent studies. Second, there was a lack of studies with a meaningful number of adult participants. Additionally, there have been no clinical studies that examine the effect of IL-5 receptor antagonists in patients with EoE (mepolizumab and reslizumab are IL-5 antagonists).
A multicenter randomized double blind, parallel group, placebo-controlled phase 3 study is recruiting at the moment to investigate the use of benralizumab for eosinophilic esophagitis (MESSINA; ClinicalTrials.gov Identifier: NCT04543409), measuring clinical and histological outcomes.
In our case, it is unclear as well whether the patient's remaining discomforts have a purely somatic cause or are influenced by psychiatric comorbidity. There is a known discrepancy between symptom relief and histological disappearance of eosinophils, as well as a discussion about which of these should be considered to evaluate therapy success. Further studies, one already ongoing, should bring better answers to this question.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Switzerland
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Iovino P, Italy; Kreisel W, Germany; Visaggi P, Italy S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Bleecker ER, FitzGerald JM, Chanez P, Papi A, Weinstein SF, Barker P, Sproule S, Gilmartin G, Aurivillius M, Werkström V, Goldman M; SIROCCO study investigators. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 990] [Article Influence: 110.0] [Reference Citation Analysis (0)] |
| 2. | FitzGerald JM, Bleecker ER, Nair P, Korn S, Ohta K, Lommatzsch M, Ferguson GT, Busse WW, Barker P, Sproule S, Gilmartin G, Werkström V, Aurivillius M, Goldman M; CALIMA study investigators. Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 1009] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 3. | Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, Barker P, Sproule S, Ponnarambil S, Goldman M; ZONDA Trial Investigators. Oral Glucocorticoid-Sparing Effect of Benralizumab in Severe Asthma. N Engl J Med. 2017;376:2448-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 757] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 4. | Schoepfer AM, Safroneeva E, Bussmann C, Kuchen T, Portmann S, Simon HU, Straumann A. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230-6.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 573] [Article Influence: 47.8] [Reference Citation Analysis (1)] |
| 5. | Dellon ES, Hirano I. Epidemiology and Natural History of Eosinophilic Esophagitis. Gastroenterology. 2018;154:319-332.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 564] [Article Influence: 80.6] [Reference Citation Analysis (0)] |
| 6. | Reed CC, Corder SR, Kim E, Sanders E, Tappata M, Eluri S, Dellon ES. Psychiatric Comorbidities and Psychiatric Medication Use Are Highly Prevalent in Patients With Eosinophilic Esophagitis and Associate With Clinical Presentation. Am J Gastroenterol. 2020;115:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Taft TH, Guadagnoli L, Edlynn E. Anxiety and Depression in Eosinophilic Esophagitis: A Scoping Review and Recommendations for Future Research. J Asthma Allergy. 2019;12:389-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Farne HA, Wilson A, Powell C, Bax L, Milan SJ. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Furuta GT, Katzka DA. Eosinophilic Esophagitis. N Engl J Med. 2015;373:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 404] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 10. | Schoepfer AM, Straumann A, Safroneeva E. Pharmacologic Treatment of Eosinophilic Esophagitis: An Update. Gastrointest Endosc Clin N Am. 2018;28:77-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Molina-Infante J, Ferrando-Lamana L, Ripoll C, Hernandez-Alonso M, Mateos JM, Fernandez-Bermejo M, Dueñas C, Fernandez-Gonzalez N, Quintana EM, Gonzalez-Nuñez MA. Esophageal eosinophilic infiltration responds to proton pump inhibition in most adults. Clin Gastroenterol Hepatol. 2011;9:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 279] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 12. | Moawad FJ, Schoepfer AM, Safroneeva E, Ally MR, Chen YJ, Maydonovitch CL, Wong RK. Eosinophilic oesophagitis and proton pump inhibitor-responsive oesophageal eosinophilia have similar clinical, endoscopic and histological findings. Aliment Pharmacol Ther. 2014;39:603-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 13. | Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA; American College of Gastroenterology. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108:679-92; quiz 693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 844] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 14. | Caminati M, Menzella F, Guidolin L, Senna G. Targeting eosinophils: severe asthma and beyond. Drugs Context. 2019;8:212587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Roufosse F. Targeting the Interleukin-5 Pathway for Treatment of Eosinophilic Conditions Other than Asthma. Front Med (Lausanne). 2018;5:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 16. | Hassani M, Koenderman L. Immunological and hematological effects of IL-5(Rα)-targeted therapy: An overview. Allergy. 2018;73:1979-1988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 17. | Sawas T, Dhalla S, Sayyar M, Pasricha PJ, Hernaez R. Systematic review with meta-analysis: pharmacological interventions for eosinophilic oesophagitis. Aliment Pharmacol Ther. 2015;41:797-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |