Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4460
Peer-review started: November 26, 2021
First decision: January 11, 2022
Revised: January 25, 2022
Accepted: March 26, 2022
Article in press: March 26, 2022
Published online: May 16, 2022
Processing time: 168 Days and 7.8 Hours
The liver is one of the most important organs in the human body, with functions such as detoxification, digestion, and blood coagulation. In terms of vascular anatomy, the liver is divided into the left and the right liver by the main portal vein, and there are three hepatic efferent veins (right, middle, and left) and two portal branches. Patients with impaired liver function have increased intrahepatic vascular resistance and splanchnic vasodilation, which may lead to an increase in the portal pressure gradient (PPG) and cause portal hypertension (PHT). In order to measure the increased pressure gradient of portal vein, the hepatic venous pressure gradient (HVPG) can be measured to reflect it in clinical practice. The accuracy of PPG measurements is directly related to patient prognosis.
To analyze the correlation between HVPG of three hepatic veins and PPG in patients with PHT.
From January 2017 to December 2019, 102 patients with PHT who met the inclusion criteria were evaluated during the transjugular intrahepatic portosystemic shunt procedure and analyzed.
The mean HVPG of the middle hepatic vein was 17.47 ± 10.25 mmHg, and the mean HVPG of the right and left hepatic veins was 16.34 ± 7.60 and 16.52 ± 8.15 mmHg, respectively. The average PPG was 26.03 ± 9.24 mmHg. The correlation coefficient and coefficient of determination of the right hepatic vein, middle hepatic vein, and left hepatic vein were 0.15 and 0.02 (P = 0.164); 0.25 and 0.05 (P = 0.013); and 0.14 and 0.02 (P = 0.013), respectively. The mean wedged hepatic vein/venous pressure (WHVP) of the middle and left hepatic veins was similar at 29.71 ± 12.48 and 29.1 ± 10.91 mmHg, respectively, and the mean WHVP of the right hepatic vein was slightly lower at 28.01 ± 8.95 mmHg. The mean portal vein pressure was 34.11 ± 8.56 mmHg. The correlation coefficient and coefficient of determination of the right hepatic vein, middle hepatic vein, and left hepatic vein were 0.26 and 0.07 (P = 0.009); 0.38 and 0.15 (P < 0.001); and 0.26 and 0.07 (P = 0.008), respectively. The average free hepatic venous pressure (FHVP) of the right hepatic vein was lowest at 11.67 ± 5.34 mmHg, and the average FHVP of the middle and left hepatic veins was slightly higher at 12.19 ± 4.88 and 11.67 ± 5.34 mmHg, respectively. The average inferior vena cava pressure was 8.27 ± 4.04 mmHg. The correlation coefficient and coefficient of determination of the right hepatic vein, middle hepatic vein, and left hepatic vein were 0.30 and 0.09 (P = 0.002); 0.18 and 0.03 (P = 0.078); and 0.16 and 0.03 (P = 0.111), respectively.
Measurement of the middle hepatic vein HVPG could better represent PPG. Considering the high success rate of clinical measurement of the right hepatic vein, it can be the second choice.
Core Tip: Portal hypertension (PHT) is a serious complication of various liver diseases, including cirrhosis, with a high mortality rate. To improve its prognosis, methods to accurately measure the magnitude of the increase in portal pressure are needed. This study compared and analyzed the relationship between hepatic venous pressure gradient of three hepatic veins and portal pressure gradient in 102 patients with PHT, aiming to find out the hepatic vein pressure gradient branch that best represents the patients’ actual portal vein pressure gradient in clinic.
- Citation: Wang HY, Song QK, Yue ZD, Wang L, Fan ZH, Wu YF, Dong CB, Zhang Y, Meng MM, Zhang K, Jiang L, Ding HG, Zhang YN, Yang YP, Liu FQ. Correlation of pressure gradient in three hepatic veins with portal pressure gradient. World J Clin Cases 2022; 10(14): 4460-4469
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4460.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4460
The elevated pressure in portal hypertension (PHT) refers to the pressure gradient between the portal vein and the systemic circulation, and is mainly characterized by increased intrahepatic vascular resistance, varicose veins, and bleeding caused by splanchnic vasodilation. The main cause of PHT is liver cirrhosis, which has a high mortality rate as it is a serious complication. In addition, the increased venous pressure gradient in the absence of known liver disease is termed noncirrhotic PHT and is usually caused by vascular liver disease[1]. The clinical symptoms and prognosis of PHT are directly related to the degree of portal pressure, but it is difficult and risky to measure portal pressure directly. The hepatic venous pressure gradient [HVPG = wedged hepatic vein/venous pressure (WHVP) - free hepatic venous pressure (FHVP)] is used in clinical studies as the "gold standard" for indirectly reflecting portal venous pressure (PVP) and is a simple and low-risk technique[2]. Theoretically, HVPG indirectly represents the difference between PVP and inferior vena cava pressure (IVCP) [portal pressure gradient (PPG) = PVP - IVCP]. There are few reports on whether HVPG accurately represents PPG in real-world measurements. In this study, we carried out actual measurements of three hepatic veins related to this issue. From January 2017 to December 2019, 102 patients with PHT who met the enrollment criteria were included in this study and measurements of the three hepatic veins and various other pressures were carried out during the transjugular intrahepatic portosystemic shunt (TIPS) procedure and analyzed.
The pressure of three hepatic veins was measured during the TIPS procedure in 102 patients with PHT who met the inclusion criteria from January 2017 to December 2019 and analyzed. The mean age of the 102 cases was 54.42 ± 12.37 years, of which 67 (mean age 50.28 ± 11.63 years) were male (65.7%) and 35 (mean age 61.87 ± 10.07 years) were female (34.3%). The cause of PHT was hepatitis B cirrhosis in 43 cases, hepatitis C cirrhosis in 8, hepatitis B + C cirrhosis in 1, alcoholic cirrhosis in 12, autoimmune cirrhosis in 7, cholestatic cirrhosis in 6, drug-induced cirrhosis in 2, idiopathic PHT in 5, small hepatic vein occlusion syndrome in 14, and hepatic sinusoidal occlusion syndrome in 4. There were 67 cases of gastrointestinal bleeding, accounting for 68.3%, intractable ascites/pleural ascites in 15 cases, accounting for 14.7%, and gastrointestinal bleeding complicated by intractable ascites in 6 (5.9%). Other conditions included 14 cases of bruising jaundice, severe liver bruising combined with ascites, hepatorenal syndrome, and severe esophagogastric varices. Child-Pugh classification was grade A in 49 cases, grade B in 34, and grade C in 19.
The inclusion criteria were: (1) Patients eligible for TIPS surgery; (2) aged 18-75 years old; (3) TIPS patients scheduled for elective surgery; (4) normal anatomy of the hepatic vein and inferior vena cava; and (5) successful simultaneous measurement of pressure in three hepatic veins.
The exclusion criteria were: (1) Patients with tumors; (2) patients with portal vein thrombosis (generally more than 1/2 of the diameter of the main portal vein); (3) application of drugs affecting portal vein pressure within the previous week; and (4) intraoperative factors affecting the accuracy of manometry, e.g., bile heart reflex and incomplete balloon closure.
The following preoperative tests were conducted: Routine blood, liver and kidney function, ICG-R15 (quantitative liver function test-indocyanine green 15-min retention rate), blood ammonia, blood group, electrocardiogram, coagulation, liver vascular ultrasound, cardiac ultrasound, and abdominal computed tomography and/or magnetic resonance enhancement, appropriate adjustment of coagulation function, platelet count, bilirubin, albumin, and hemoglobin for interventional procedures. The results and risks of the procedure were explained to the patients and their family, and signed consent for the operation protocol was obtained. Medications affecting portal pressure were discontinued for at least 1 week prior to surgery.
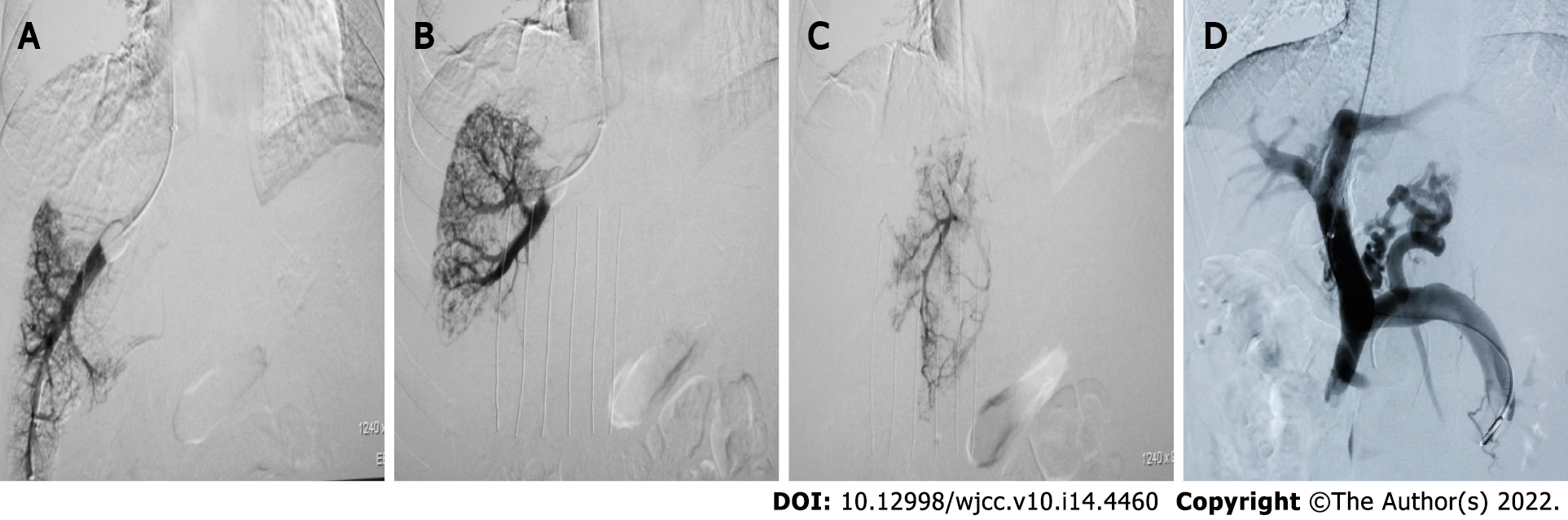
Local routine disinfection drapes were placed. Under local anesthesia, the right internal jugular vein was punctured and the RUPS-100 set (COOK, United States) placed into the right atrium and inferior vena cava to measure pressure. A Fogarty balloon catheter (Edwards, United States) was inserted into the right, middle, and left hepatic veins, respectively, and the balloon catheter tip was placed in the hepatic vein approximately 1-5 cm from the opening of the inferior vena cava. WHVP and FHVP were measured before and after the balloon was dilated to occlude the hepatic vein (5 mL of contrast agent was injected). When the pressure was stable, the value was recorded, the pressure was measured 3 times, the average value was noted, and then the HVPG value was calculated (Figure 1A-C). After measurement, balloon-blocking compression hepatic venography was performed (the total amount of contrast agent was 15 mL, 5 mL/s, pressure 300 psi), and both WHVP and FHVP were measured again after administration of contrast agent. Occlusion of the balloon catheter was observed after the balloon was expanded and the balloon catheter position was adjusted for retesting and imaging if there was poor blockage. The liver parenchyma and portal vein were punctured through the inferior vena cava or hepatic vein. After successful puncture of the portal vein, the pigtail or end-to-side hole catheter was inserted into the splenic vein or superior mesenteric vein for intravenous angiography. Before shunting, pressure in the portal vein was measured (3 measurements, averaged), and the PPG value was calculated (Figure 1D). After that, liver tissue in the pre-shunt channel was obtained, the shunt channel was established, the post-shunt portal trunk pressure was measured (3 measurements, averaged), and the PPG value was calculated. Postoperatively, an indwelling catheter was inserted into the portal vein for at least 48 h and the portal pressure was measured at least 3 times daily. The IVCP and right atrial pressure were measured three times during extubation, respectively, and the average value was taken[3].
SPSS 17.0 software was used for statistical analyses. The differences between PPG and HVPG, WHVP and PVP, and FHVP and IVCP were analyzed using paired t-tests, and the correlations between them were analyzed using Pearson correlation tests to estimate correlations and coefficients of determination. P < 0.05 was considered statistically significant.
The mean HVPG of the three hepatic veins (right, middle, and left) was 16.34 ± 7.60, 17.47 ± 10.25, and 16.52 ± 8.15 mmHg, respectively. Mean PPG was 26.03 ± 9.24 mmHg. By Pearson correlation analysis, the correlation coefficients and coefficients of determination between HVPG and PPG in the right, middle, and left hepatic veins were 0.15 and 0.02 (P = 0.132); 0.25 and 0.05 (P = 0.013); and 0.14 and 0.02 (P = 0.164), respectively (Table 1).
| A-HVPG and PPG | B-WHVP and PVP | C-FHVP and IVCP | |||||||
| Hepatic vein | Right | Middle | Left | Right | Middle | Left | Right | Middle | Left |
| Correlation coefficient | 0.15 | 0.25 | 0.14 | 0.26 | 0.38 | 0.26 | 0.30 | 0.18 | 0.16 |
| Decisive factor | 0.02 | 0.05 | 0.02 | 0.07 | 0.15 | 0.07 | 0.09 | 0.03 | 0.03 |
| P value | 0.164 | 0.013 | 0.013 | 0.009 | < 0.001 | 0.008 | 0.002 | 0.078 | 0.111 |
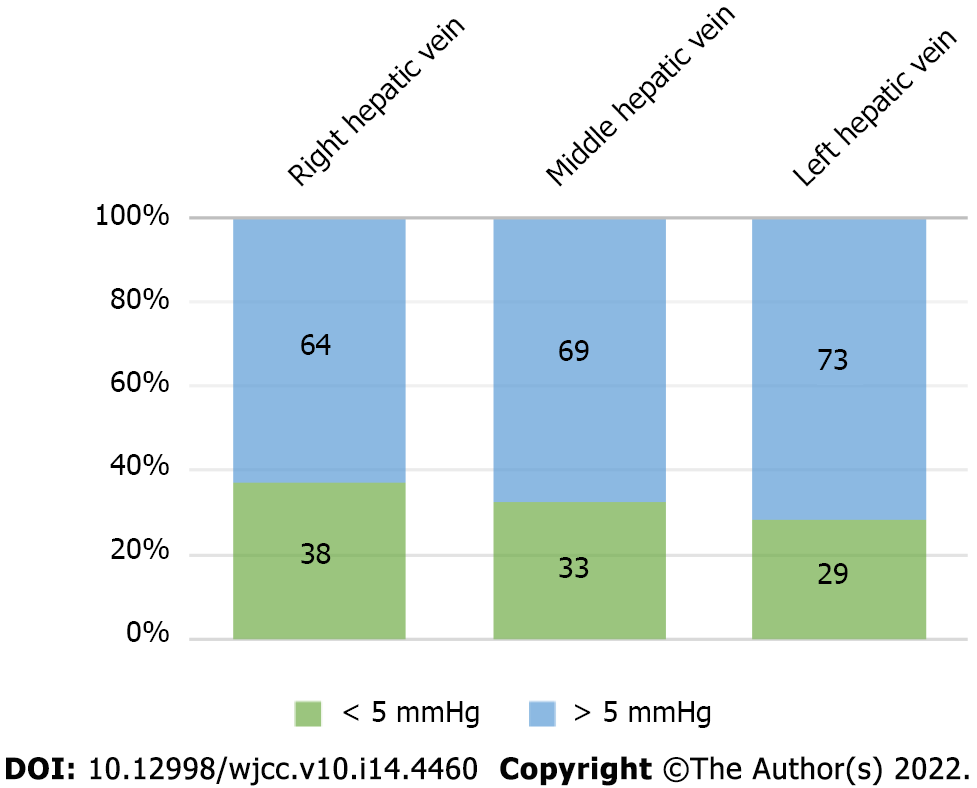
The maximum HVPG was higher than PPG in the right, middle, and left hepatic veins, which was 14 mmHg, 24 mmHg, and 37 mmHg, respectively, and the maximum PPG was higher than HVPG, which was 43.67 mmHg, 43 mmHg, and 44 mmHg, respectively. The difference between HVPG and PPG was within 5 mmHg in 38 cases (37.25%) on the right side of the liver, in 33 (32.35%) in the middle of the liver, and in 29 (28.43%) on the left side of the liver. The difference between HVPG and PPG was more than 5 mmHg in 64 cases on the right side of the liver, accounting for 62.75%, in 69 in the middle of the liver, accounting for 67.65%, and in 73 on the left side of the liver, accounting for 71.57% (Figure 2).
The average WHVP of the right hepatic vein was 28.01 ± 8.95 mmHg, the average WHVP of the middle hepatic vein was 29.71 ± 12.48 mmHg, and the average WHVP of the left hepatic vein was 29.1 ± 10.91 mmHg. The average PVP was 34.11 ± 8.56 mmHg. Following Pearson correlation analysis, the correlation coefficients and coefficients of determination between WHVP and PVP for the right, middle, and left hepatic veins were 0.26 and 0.07 (P = 0.009); 0.38 and 0.15 (P < 0.001); and 0.26 and 0.07 (P = 0.008), respectively (Table 1).
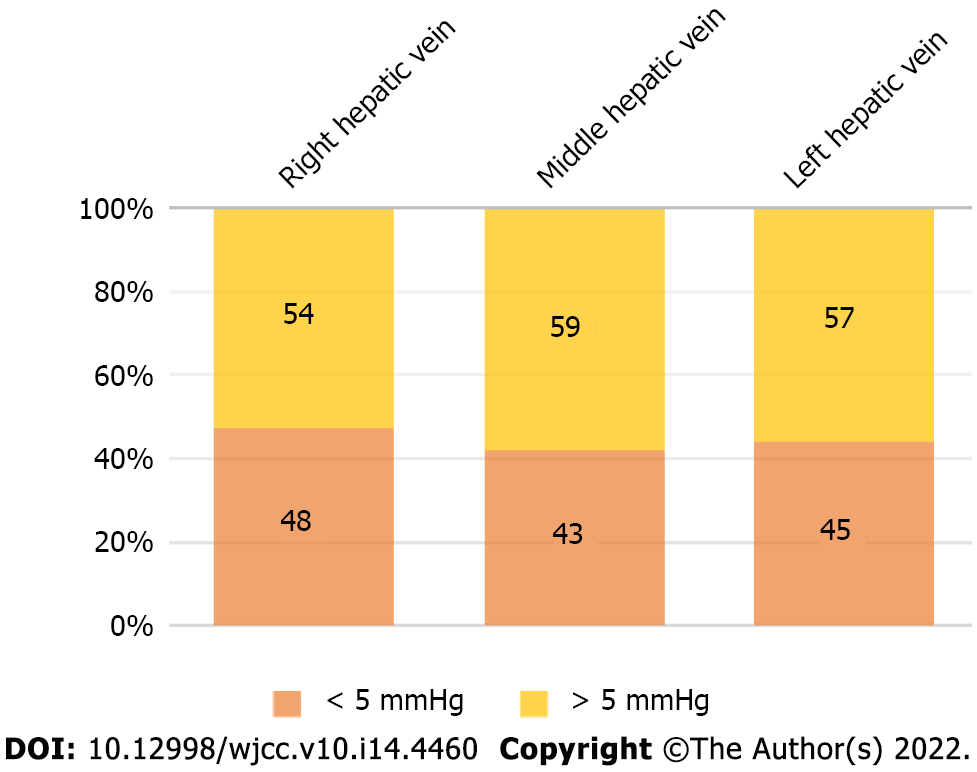
The maximum WHVP was higher than PVP in the right liver, middle liver, and left liver and was 14 mmHg, 28 mmHg, and 40 mmHg, respectively, and the maximum PVP was higher than WHVP, which was 43.67 mmHg, 32 mmHg, and 40.67 mmHg, respectively. The difference between WHVP and PVP was within 5 mmHg in 48 cases on the right side of the liver, accounting for 47.06%, in 43 in the middle of the liver, accounting for 42.16%, and in 45 on the left side of the liver, accounting for 44.12%. The difference between WHVP and PVP was more than 5 mmHg in 54 cases on the right side of the liver, accounting for 52.94%, in 59 in the middle of the liver, accounting for 57.84%, and in 57 on the left side of the liver, accounting for 55.88% (Figure 3).
The average FHVP was 11.67 ± 5.34 mmHg for the right hepatic vein, 12.19 ± 4.88 mmHg for the middle hepatic vein, and 12.64 ± 4.99 mmHg for the left hepatic vein. Average IVCP was 8.27 ± 4.04 mmHg. The correlation coefficients and coefficients of determination between the right hepatic, middle hepatic, and left hepatic venous FHVP and IVCP were 0.30 and 0.09 (P = 0.002); 0.18 and 0.03 (P = 0.078); and 0.16 and 0.03 (P = 0.111), respectively (Table 1).
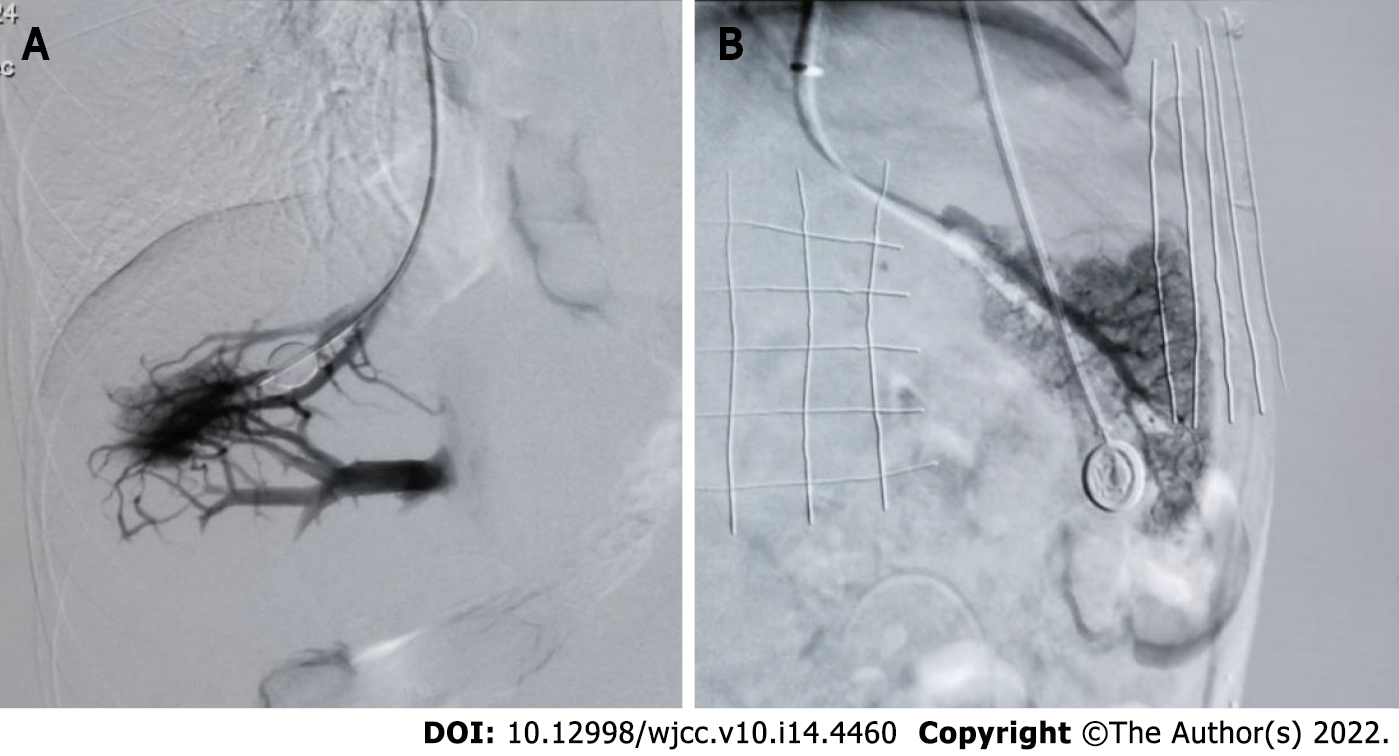
There were 16 (15.7%), 18 (17.6%), and 22 cases (21.6%) with collaterals in the right, middle, and left hepatic vein angiography of the balloon blocked liver, respectively (Figure 4A). The average HVPG was (8.02 ± 6.58) mmHg, (14.36 ± 6.65) mmHg, and (12.09 ± 5.36) mmHg. The average PPG was (26.13 ± 9.97) mmHg, (29.67 ± 7.96) mmHg, and (29.68 ± 8.77) mmHg (P < 0.001); the average WHVP was (19.81 ± 8.18) mmHg, (24.62 ± 6.26) mmHg, and (23.26 ± 6.77) mmHg. The average PVP was (35.56 ± 9.86) mmHg, (37.72 ± 7.79) mmHg, and (37.86 ± 8.40) mmHg (P < 0.001). The average FHVP was (11.79 ± 4.46) mmHg, (10.26 ± 3.07) mmHg, and (11.17 ± 4.20) mmHg, and the average IVCP was (9.44 ± 2.68) mmHg, (8.06 ± 3.23) mmHg, and (8.18 ± 3.19) mmHg, respectively, and the differences were statistically significant (P < 0.001) (Table 2).
| Hepatic vein | HVPG | PPG | WHVP | PVP | FHVP | IVCP |
| Right (mmHg) | 8.02 ± 6.58 | 26.13 ± 9.97 | 19.81 ± 8.18 | 35.56 ± 9.86 | 11.79 ± 4.46 | 9.44 ± 2.68 |
| Middle (mmHg) | 14.36 ± 6.65 | 29.67 ± 7.96 | 24.62 ± 6.26 | 37.72 ± 7.79 | 10.26 ± 3.07 | 8.06 ± 3.23 |
| Left (mmHg) | 12.09 ± 5.36 | 29.68 ± 8.77 | 23.26 ± 6.77 | 37.86 ± 8.40 | 11.17 ± 4.20 | 8.18 ± 3.19 |
Balloon occlusion of the right, middle, and left hepatic veins without collaterals (Figure 4B) was seen in 86 (84.3%), 84 (82.4%), and 80 cases (78.4%), respectively. The average HVPG was (18.64 ± 6.33) mmHg, (18.68 ± 8.47) mmHg, and (18.34 ± 7.21) mmHg. The average PPG was (25.33 ± 8.90) mmHg, (24.55 ± 9.03) mmHg, and (24.29 ± 8.80) mmHg (P < 0.001); the average WHVP was (30.14 ± 7.52) mmHg, (30.74 ± 10.10) mmHg, and (30.75 ± 9.45) mmHg. The average PVP was (33.60 ± 8.06) mmHg, (33.10 ± 8.28) mmHg, and (32.83 ± 8.05) mmHg (P < 0.001); the average FHVP was (11.50 ± 4.87) mmHg, (12.06 ± 4.21) mmHg, and (12.40 ± 4.29) mmHg, and the average IVCP was (8.28 ± 3.93) mmHg, (8.55 ± 3.90) mmHg, and (8.54 ± 3.94) mmHg, respectively, and the differences were statistically significant (P < 0.001) (Table 3).
| Hepatic vein | HVPG | PPG | WHVP | PVP | FHVP | IVCP |
| Right (mmHg) | 18.64 ± 6.33 | 25.33 ± 8.90 | 30.14 ± 7.52 | 33.60 ± 8.06 | 11.50 ± 4.87 | 8.28 ± 3.93 |
| Middle (mmHg) | 18.68 ± 8.47 | 24.55 ± 9.03 | 30.74 ± 10.10 | 33.10 ± 8.28 | 12.06 ± 4.21 | 8.55 ± 3.90 |
| Left (mmHg) | 18.34 ± 7.21 | 24.29 ± 8.80 | 30.75 ± 9.45 | 32.83 ± 8.05 | 12.40 ± 4.29 | 8.54 ± 3.94 |
The liver has a dual blood supply, with blood from the hepatic artery and portal vein entering the hepatic sinusoids and returning to the hepatic vein. Under normal conditions, the hemodynamics are in equilibrium, and generally the PVP is higher than or equal to the hepatic sinusoidal pressure, and WHVP represents the hepatic sinusoidal pressure. Generally FHVP is 0.5-1.0 mmHg higher than IVCP[4,5]. Normally HVPG is approximately equal to PPG and indirectly represents the perfusion pressure in the portal vein. Patients with PHT, especially cirrhotic PHT, have significant changes in liver tissue, blood vessels, and other structures, resulting in changes in local and body hemodynamics, the main features of which are visceral vasodilation and increased total blood volume[6]. Therefore, whether the changes in various vascular pressures in patients with PHT are consistent with normal hepatic vascular pressures has been shown to be controversial[7]. Studies have suggested that PPG is more meaningful than PVP in predicting the prognosis of PHT[4,5,7,8]. The importance of HVPG in diagnosing the etiology of PHT[9,10], in predicting the prognosis of chronic liver disease[10], in predicting gastrointestinal bleeding[11], in determining patient prognosis[8], in determining the efficacy of drugs[12], in the development of primary hepatocellular carcinoma, and in surgical prognosis[13-15] has been reported in the literature, based on the theoretical HVPG as the "gold standard" indirectly representing PPG or separate studies on HVPG.
Limited reports show a correlation between WHVP and PVP in patients with hepatitis cirrhosis and alcoholic cirrhosis, with fewer national studies[4,7,16,17]. A poor correlation between WHVP and PVP has been reported for large nodular cirrhosis[18]. The reasons for WHVP being lower than PVP are related to hepatic vein collateral shunts. The reason for the higher WHVP than PVP is unclear and may be related to reverse hepatic flow, opening of the accessory umbilical vein, portal anastomotic branch[16,17], and gastrorenal shunt[19]. The above study focused on data measured in a single hepatic vein (right hepatic vein). Non-cirrhotic PHT is generally acute or subacute, and is usually caused by vascular liver diseases such as hepatic sinusoidal obstruction syndrome[20], idiopathic PHT[1], and Budd-Chiari syndrome. The main clinical manifestations are ascites and variceal hemorrhage. Moreover, in contrast to cirrhotic PHT, the collateral circulation is not established[20]. HVPG, as a standard for measuring portal pressure, can accurately measure sinus PHT; however, it has been reported that for patients with a presinusoidal type of PHT, if the balloon is inflated below the vein-to-vein shunt, the measured HVPG can still accurately represent portal pressure[1].
The present study measured three hepatic veins in the same patient and showed that the correlation between WHVP and PVP was poor in all three hepatic veins, the right hepatic vein was similar to the left hepatic vein, and the middle hepatic vein was slightly better. The correlation between HVPG and PPG was poor in all three hepatic veins, the middle hepatic vein was better than the other two types, and the right hepatic vein and the left hepatic vein were similar. The mean WHVP with hepatic vein collateral branches was significantly lower than the mean PVP, suggesting that hepatic vein collateral branches severely affect and underestimate WHVP. The mean HVPG of the three hepatic veins was also significantly lower than the mean PPG. The mean WHVP without hepatic vein collateral branches and the mean PVP of the three hepatic veins were also lower than the mean PVP. The mean HVPG of the three hepatic veins was also lower than the mean PPG; the correlation between the FHVP and IVCP of the right hepatic vein was better, and the literature reported better stability of FHVP and IVCP[16,17,19]. During the measurement of WHVP, FHVP, IVCP, PVP, HVPG, and PPG, some drugs or measurement methods may affect the accuracy of monitoring results, such as non-selective beta-blockers, which have an effect on PVP[21], propofol deep sedation has a huge effect on the patient's PPG[22], the bile heart reflex during TIPS as well as the position and thickness of the measurement catheter may affect the accuracy of PVP, and the position of the catheter and the thickness of the hepatic vein wall during the measurement of WHVP and FHVP may have some influence on the pressure measurement results[4,5]. The effects of the preoperative application of growth inhibitors and their analogs, posterior pituitary hormones, and terlipressin on manometry are unclear. In this study, the unity of subjective factors was particularly emphasized, including preoperative treatment, balloon occlusion method to determine WHVP, measurement site, patient's respiratory activity, drug application, etc., to exclude various factors that affect the accuracy of pressure measurement.
In summary, the results of this study show that the correlations between WHVP and PVP, as well as between HVPG and PPG are poor in all three hepatic veins, but they are both highest in the middle hepatic vein with a basic compliance rate (within 5 mmHg difference) of 47.06% and 37.25%, respectively. The measurement of pressure in the middle hepatic vein could better represent the pressure in the left and right hepatic veins. However, in practice, the right hepatic vein is relatively thicker in its course and has a higher success rate. In addition, hepatic vein collateral branches are an important cause of inaccuracy. The correlations between WHVP and PVP as well as between HVPG and PPG are also poor in patients without hepatic vein collateral branches. And the reasons for the generation of WHVP over PVP and HVPG over PPG are unclear. These issues need to be studied in depth.
The prognosis of portal hypertension (PHT) with high mortality is directly related to the accuracy of the measured portal pressure.
To improve the prognosis of PHT.
To identify which of the three hepatic veins that can more accurately represent portal pressure.
The pressure in three hepatic veins in 102 patients with PHT of different etiologies was measured and compared with their mean portal pressure.
Correlation of portal pressure gradient (PPG) and hepatic venous pressure gradient is higher in the middle hepatic vein.
The measurement of pressure in the middle hepatic vein could better represent PPG than the pressure in the left and right hepatic veins.
Considering the high success rate of clinical measurement of the right hepatic vein, it can be the second choice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Hepatology Professional Committee of Chinese Research Hospital Association; Portal Hypertension Expert Committee; Interventional Expert Committee of the Department of Oncology, Capital Medical University; Beijing Interventional Medicine Branch of Chinese Medical Association; Beijing Medical Association; Chinese Society of Clinical Oncology (CSCO) Interventional Radiology Expert Committee; Interventional Physician Branch of Chinese Medical Doctor Association.
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): E
P-Reviewer: Kim DJ, South Korea; Marra P, Italy; Tripathi D, United Kingdom; Yoshida H, Japan S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Strauss E, Valla D. Non-cirrhotic portal hypertension--concept, diagnosis and clinical management. Clin Res Hepatol Gastroenterol. 2014;38:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 2. | de Franchis R; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2294] [Article Influence: 229.4] [Reference Citation Analysis (3)] |
| 3. | Chinese Portal Hypertension Diagnosis and Monitoring Study Group (CHESS); Minimally Invasive Intervention Collaborative Group, Chinese Society of Gastroenterology; Emergency Intervention Committee, Chinese College of Interventionalists; Hepatobiliary Diseases Collaborative Group, Chinese Society of Gastroenterology; Spleen and Portal Hypertension Group, Chinese Society of Surgery; Fatty Liver and Alcoholic Liver Disease Group, Chineses Society of Hepatology; Chinese Research Hospital Association for the Study of the Liver; Hepatobiliary and Pancreatic Diseases Prevention and Control Committee, Chinese Preventive Medicine Association; Chinese Society of Digital Medicine; Chinese Society of Clinical Epidemiology and Evidence Based Medicine. . [Consensus on clinical application of hepatic venous pressure gradient in China (2018)]. Zhonghua Gan Zang Bing Za Zhi. 2018;26:801-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 4. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 5. | Parikh S. Hepatic venous pressure gradient: worth another look? Dig Dis Sci. 2009;54:1178-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Gunarathne LS, Rajapaksha H, Shackel N, Angus PW, Herath CB. Cirrhotic portal hypertension: From pathophysiology to novel therapeutics. World J Gastroenterol. 2020;26:6111-6140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (6)] |
| 7. | Zhang M, Zhuge YZ, Zou XP, Zhang F, Peng CY, He QB, Li ZL. Correlation between wedge hepatic vein pressure and portal vein pressure in 22 patients with liver cirrhosis. Zhonghua Xiaohua Zazhi. 2016;36: 554-558. [RCA] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Merkel C, Montagnese S. Hepatic venous pressure gradient measurement in clinical hepatology. Dig Liver Dis. 2011;43:762-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Bosch J, Garcia-Pagán JC, Berzigotti A, Abraldes JG. Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis. 2006;26:348-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 10. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS, Bosch J; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 747] [Cited by in RCA: 811] [Article Influence: 45.1] [Reference Citation Analysis (1)] |
| 11. | Suk KT, Kim CH, Park SH, Sung HT, Choi JY, Han KH, Hong SH, Kim DY, Yoon JH, Kim YS, Baik GH, Kim JB, Kim DJ. Comparison of hepatic venous pressure gradient and two models of end-stage liver disease for predicting the survival in patients with decompensated liver cirrhosis. J Clin Gastroenterol. 2012;46:880-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Rincón D, Lo Iacono O, Tejedor M, Hernando A, Ripoll C, Catalina MV, Salcedo M, Matilla A, Senosiain M, Clemente G, Molinero LM, Albillos A, Bañares R. Prognostic value of hepatic venous pressure gradient in patients with compensated chronic hepatitis C-related cirrhosis. Scand J Gastroenterol. 2013;48:487-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D, Matloff DS; Portal Hypertension Collaborative Group. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923-928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 336] [Cited by in RCA: 292] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 14. | Figueras J. Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis (Br J Surg 2012; 99: 855-863). Br J Surg. 2012;99:863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Hidaka M, Takatsuki M, Soyama A, Tanaka T, Muraoka I, Hara T, Kuroki T, Kanematsu T, Eguchi S. Intraoperative portal venous pressure and long-term outcome after curative resection for hepatocellular carcinoma. Br J Surg. 2012;99:1284-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Perelló A, Escorsell A, Bru C, Gilabert R, Moitinho E, García-Pagán JC, Bosch J. Wedged hepatic venous pressure adequately reflects portal pressure in hepatitis C virus-related cirrhosis. Hepatology. 1999;30:1393-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Deplano A, Migaleddu V, Pischedda A, Garrucciu G, Gregu G, Multinu C, Piredda M, Tocco A, Urigo F, Cossu PA, Solinas A. Portohepatic gradient and portal hemodynamics in patients with cirrhosis due to hepatitis C virus infection. Dig Dis Sci. 1999;44:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Pomier-Layrargues G, Kusielewicz D, Willems B, Villeneuve JP, Marleau D, Côté J, Huet PM. Presinusoidal portal hypertension in non-alcoholic cirrhosis. Hepatology. 1985;5:415-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Osada Y, Kanazawa H, Narahara Y, Mamiya Y, Nakatsuka K, Sakamoto C. Wedged hepatic venous pressure does not reflect portal pressure in patients with cirrhosis and hepatic veno-venous communications. Dig Dis Sci. 2008;53:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Zhang W, Peng C, Zhang S, Huang S, Shen S, Xu G, Zhang F, Xiao J, Zhang M, Zhuge Y, Wang L, Zou X, Lv Y. EUS-guided portal pressure gradient measurement in patients with acute or subacute portal hypertension. Gastrointest Endosc. 2021;93:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 21. | Valla D, Bercoff E, Menu Y, Bataille C, Lebrec D. Discrepancy between wedged hepatic venous pressure and portal venous pressure after acute propranolol administration in patients with alcoholic cirrhosis. Gastroenterology. 1984;86:1400-1403. [PubMed] [DOI] [Full Text] |
| 22. | Reverter E, Blasi A, Abraldes JG, Martínez-Palli G, Seijo S, Turon F, Berzigotti A, Balust J, Bosch J, García-Pagán JC. Impact of deep sedation on the accuracy of hepatic and portal venous pressure measurements in patients with cirrhosis. Liver Int. 2014;34:16-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |