Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4357
Peer-review started: February 22, 2021
First decision: September 28, 2021
Revised: October 19, 2021
Accepted: March 24, 2022
Article in press: March 24, 2022
Published online: May 16, 2022
Processing time: 444 Days and 21.9 Hours
Robotic pancreaticoduodenectomy (RPD) can achieve similar surgical results to open and PD; however, RPD has a long learning curve and operation time (OT). To address this issue, we have summarized a surgical path to shorten the surgical learning curve and OT.
To investigate the effective learning curve of a “G”-shaped surgical approach in RPD for patients.
A total of 60 patients, who received “G”-shaped RPD (GRPD) by a single surgeon in the First Hospital of Shanxi Medical University from May 2017 to April 2020, were included in this study. The OT, demographic data, intraoperative blood loss, complications, hospitalization time, and pathological results were recorded, and the cumulative sum (CUSUM) analysis was performed to evaluate the learning curve for GRPD.
According to the CUSUM analysis, the learning curve for GRPD was grouped into two phases: The early and late phases. The OT was 480 ± 81.65 min vs 331 ± 76.54 min, hospitalization time was 22 ± 4.53 d vs 17 ± 6.08 d, and blood loss was 308 ± 54.78 mL vs 169.2 ± 35.33 mL in the respective groups. Complications, including pancreatic fistula, bile leakage, reoperation rate, postoperative death, and delayed gastric emptying, were significantly decreased after this surgical technique.
GRPD can improve the learning curve and operative time, providing a new method for shortening the RPD learning curve.
Core Tip: Robotic pancreaticoduodenectomy (RPD) can achieve similar surgical results to open and PD; however, RPD has a long learning curve and operation time. A total of 60 patients, who received “G”-shaped RPD (GRPD) by a single surgeon in the First Hospital of Shanxi Medical University from May 2017 to April 2020, were included in this study. GRPD can improve the learning curve and operative time, providing a new method for shortening the RPD learning curve.
- Citation: Wei ZG, Liang CJ, Du Y, Zhang YP, Liu Y. Learning curve for a surgeon in robotic pancreaticoduodenectomy through a “G”-shaped approach: A cumulative sum analysis. World J Clin Cases 2022; 10(14): 4357-4367
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4357.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4357
Pancreaticoduodenectomy (PD) is one of the most difficult abdominal surgical procedures[1], as it involves a large range of resections and there is a need to complete the tumor-free, vascular skeletal, gastrointestinal, pancreaticojejunostomy, and biliary anastomosis during the operation. Therefore, the rate of complications in PD is high. In recent years, laparoscopic techniques have been widely used. Since the first report of laparoscopic PD (LPD) in 1994[2], an increasing number of people have investigated its postoperative efficacy, but the mortality rate was as high as 30% in early reports[3]. With the improvement in perioperative management and surgical skills, the mortality rate decreased significantly to 2% in large sample center reports, but the incidence of complications, such as pancreatic fistula, bleeding, and delayed gastric emptying, was more than 30%. During the treatment of tumors around the head of the pancreas or ampulla, the mortality associated with LPD was almost five times higher than that after open surgery, and there was no significant difference in the recovery time between the two groups. Furthermore, a meta-analysis comparing open PD (OPD) and LPD indicated that LPD has no advantage over OPD[4], and new research[5] on LPD suggested that even when LPD is conducted by a well-trained pancreatic surgeon who has performed at least 20 surgeries, there is an increased risk and no significant benefit.
The robot system has developed rapidly in recent years. Compared with the laparoscopic system, the robot system has a tremor filter system, a 3D high-definition zoom lens, and 7 free arms. As a result, robot-assisted surgery is a complicated operation that is associated with a fine operation and anatomical difficulty. In 2003, Giulianotti et al[6] reported for the first time that PD was performed using the Da Vinci robotic surgery system, and this procedure has since made great progress. A meta-analysis[7] showed that robotic PD (RPD) is associated with fewer intraoperative abdominal hemorrhages and a shorter postoperative hospital stay compared to OPD, although the operation time (OT) is longer.
However, RPD is a very complicated operation, and surgeons are worried about its long learning curve. Zhang et al[8] reported that RPD was a safe and effective operation in 100 cases of RPD surgery, and the learning curve was ultimately completed after 40 cases. The last 60 cases that underwent RPD had significantly less OT, hospitalization time, intraoperative bleeding, and fewer perioperative complications, such as hemorrhage, pancreatic fistula, reoperation rate, and delayed gastric emptying, compared to the first 40 cases. Napoli et al[9] studied the perioperative data of 70 patients treated with RPD and found that the OT was significantly shortened after 33 cases. Boone et al[10] found that after completing 20 RPD procedures, the learning curve improved significantly in terms of bleeding volume and conversion to open surgery; after 40 cases, the pancreatic surgery rate was decreased; and after 80 cases, the OT was shortened. However, the overall learning curve is still much longer than that of OPD. Moreover, the sample size of RPD surgery in single-center studies is small, and some physicians may not have any experience in robotic surgery in the early stage. There is an increase in the length of the learning curve; therefore, it should be considered whether this difficulty can be overcome through relatively simple surgical paths. Thus, the current study aims to analyze the learning curve of the G-path in RPD and provide some new methods for shortening the RPD learning curve.
A total of 60 patients who underwent a “G”-shaped surgical approach for RPD from May 2017 to April 2020 in the First Hospital of Shanxi Medical University were included in this study. Patients with obstructive jaundice were given intervention measures to improve their liver function before surgical treatment. Patients were included in this study if they met the following criteria: (1) Patients diagnosed with a benign or low-malignant tumor of the pancreatic head, early ampullary and periampullary tumor, pancreatic head cancer with T1/T2 stage (less than 3 cm in diameter), common bile duct carcinoma, duodenal papillary carcinoma based on abdominal computed tomography or magnetic resonance cholangiopancreatography examination; (2) Patients without mesenteric vascular invasion; (3) Patients without distant metastasis; (4) American Society of Anesthesiologists grade I/II/ III; and (5) Patients who could tolerate the surgery. Patients were excluded if one of the following conditions was present: (1) Distant metastasis (including celiac trunk and/or abdominal aorta); (2) Tumor encasement of the superior mesenteric artery (SMA) or celiac artery; (3) SMA/photovoltaic occlusion; (4) Surrounded aorta or inferior vena cava invasion; and (5) SMA invasion below the transverse mesocolon. This study was approved by the ethics committee of the First Hospital of Shanxi Medical University. The patients had a good understanding of the study, and they signed the informed consent form.
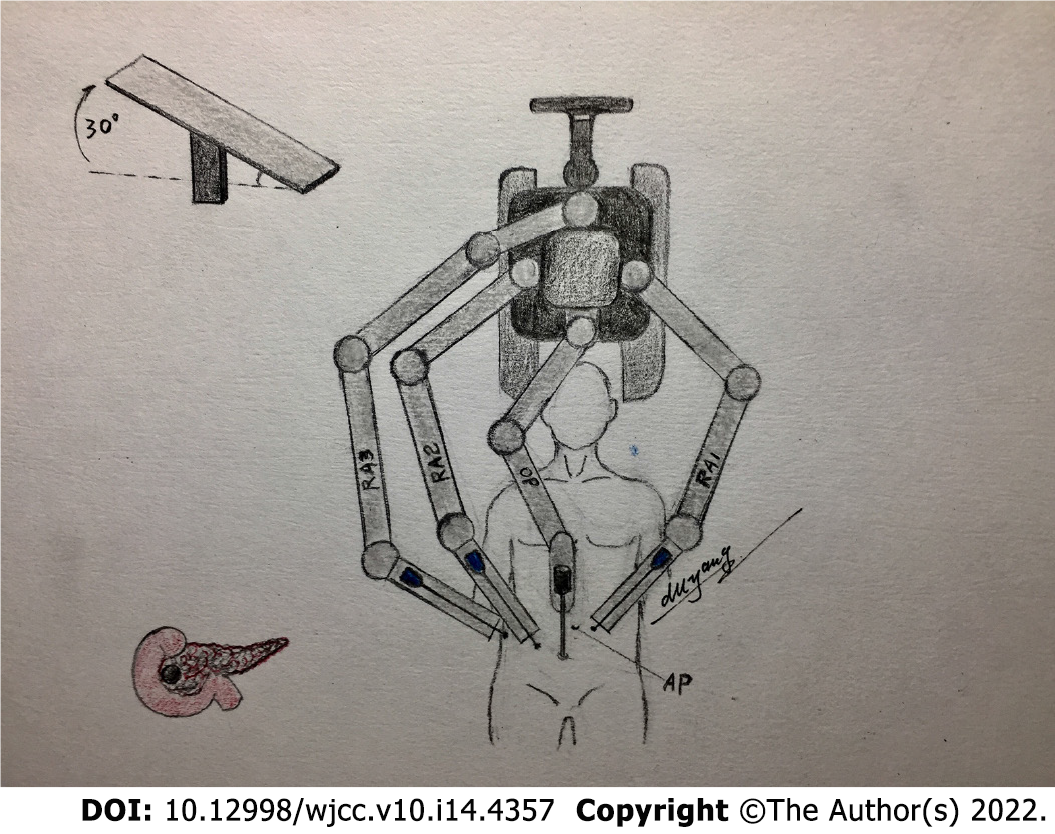
After anesthesia and tracheal cannulation, the patients were placed in the supine small position and their legs were placed in a split position. “Five trocars” in the shape of the letter “V” were used for the surgical incision, and the position of the trocars and patients are shown in Figures 1 and 2, respectively. The surgical assistant stood on the left side of the patient. A laparoscope was placed into the abdominal cavity through an incision at the inferior navel ring edge, with a pneumoperitoneum of 12 mmHg pressure (1 mmHg = 0.133 kPa). The remaining trocars were also placed in their appropriate positions under direct vision according to Figure 1, and the #1, #2, and #3 robotic arms were connected to the laparoscopic instrument. Laparoscopy was conventionally used to probe the liver, abdominal cavity, omentum majus, and other organs to identify whether there was a tumor, knot, or metastasis.
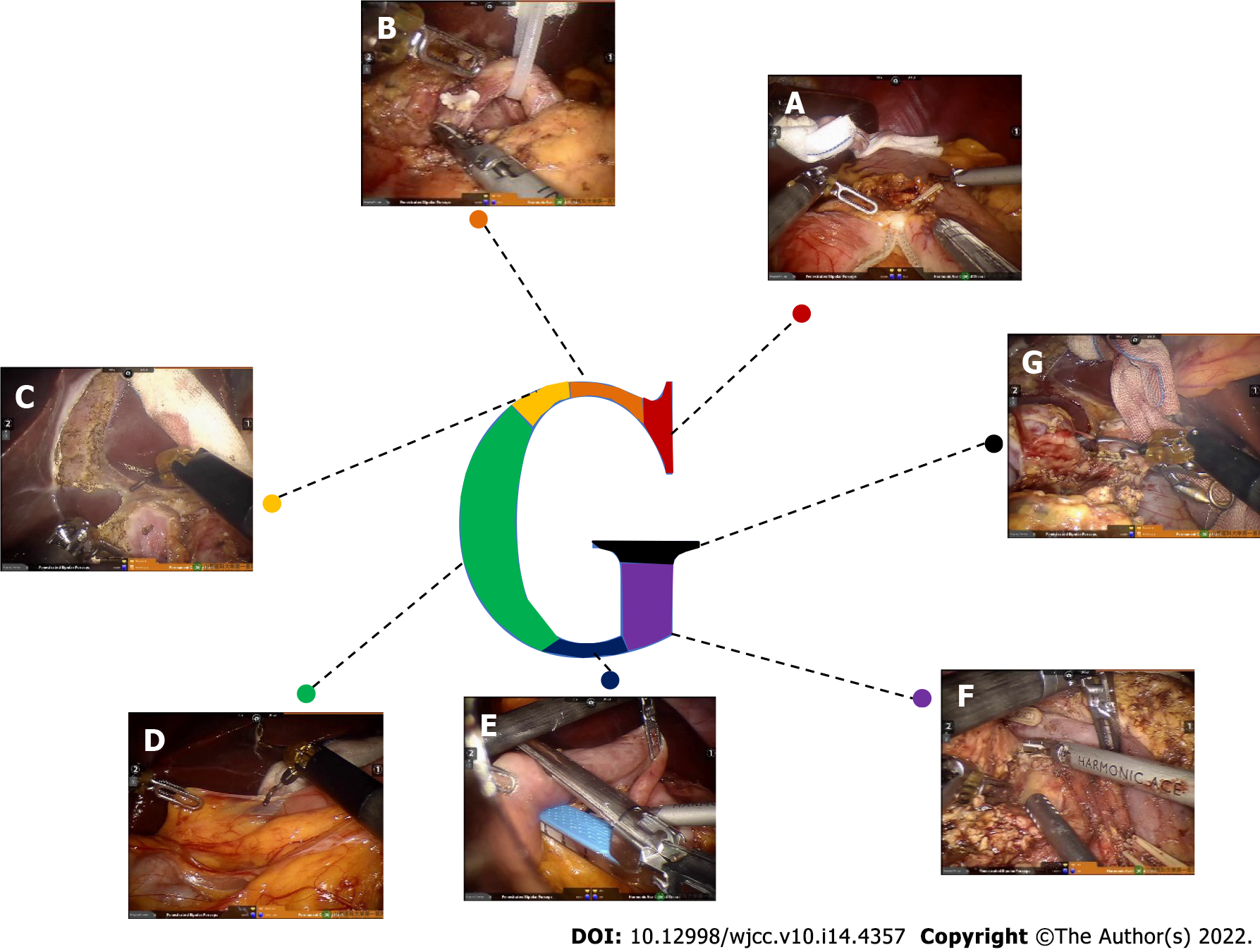
Step 1 (Figure 3A): The omentum and transverse ligament were isolated using an intestinal or stomach clamp and dissected using an ultrasonic knife. The dorsal pancreatic membrane was carefully exposed, the right gastroepiploic vein and artery were ligated, the small omentum was freed, and the gastric antrum was lifted and cut off with the Endo-GiA cutting closure. The partial stomach was isolated for the gastrojejunal anastomosis later.
Step 2 (Figure 3B): The gastric antrum was turned to the left side to expose the common hepatic artery, hepatic artery, hepatoduodenal ligament, and gastroduodenal artery. The surrounding loose tissues and lymph nodes were thoroughly cleaned. Then, the common hepatic artery was suspended, the gastroduodenal artery was ligated, and 8 pairs of lymph nodes near the proximal end of the common hepatic artery were cleaned. The portal vein in the posterior part was exposed, and 12 pairs of lymph nodes on the left front of the hepatoduodenal ligament were cleaned along the hepatic artery and the common bile duct. The superior mesenteric vein was separated and exposed along the inferior margin of the pancreas.
Step 3 (Figure 3C): The cystic duct and the common bile duct were isolated using an ultrasonic scalpel, the cystic duct and the cystic artery were closed and isolated, and the gallbladder was dissected from the gallbladder bed. The bile duct was freed and temporarily suspended with a vascular sling to reduce bile contamination.
Step 4 (Figure 3D): The Kocher incision was performed, and the inferior vena cava and the left renal vein were exposed. The ascending part of the duodenum was completely freed at the posterior part of the vascular roots in the mesentery.
Step 5 (Figure 3E): The jejunum was cut off at a distance of 15 cm from the ligament of Treitz by a linear cutting closure. An ultrasonic scalpel was used to dissect the jejunum and the duodenum to free the proximal jejunum completely and push the freed proximal jejunum to the right side.
Step 6 (Figure 3F): The superior mesenteric vein was separated along the inferior margin of the pancreas under direct vision. The small branch of the vein originating from the superior mesenteric vein and portal vein was carefully clamped until the upper edge of the pancreas, and the pancreatic neck tunnel was successfully established. The pancreatic neck was transected on the left side of the superior mesenteric vein, and the pancreas section was carefully stopped. Subsequently, the duodenal end was pulled to the upper right, the superior mesenteric vein and the portal vein were suspended, and the SMA was fully exposed. The pancreatic neck was isolated on the left side of the superior mesenteric vein or portal vein, and the pancreatic duct was cut off with scissors. An ultrasound knife was used to stop bleeding at the cut end of the pancreas. Furthermore, the ascending part of the duodenum and proximal jejunum were pulled from the rear side of the superior mesenteric vein root to the upper right, and then the duodenal end was pulled to the upper right to increase the distance from the pancreatic sulcus to the superior mesenteric vein and portal vein. The superior mesenteric vein was suspended and pulled to the left of the SMA with a vascular band. The SMA and its right branches were then double dissected from the bottom up with biological clips. The pancreatic uncinate process membrane was detached along the right side of the SMA to ensure complete removal of the uncinate process.
Step 7 (Figure 3G): The common hepatic duct above the confluence of the cystic duct and the common bile duct was transected to clearly expose the portal vein. Then, lymph node dissection was conducted on the right and rear sides of the hepatoduodenal ligament and behind the common liver artery, and, using this simple and complicated way to remove each tissue, the shape achieved was a G-type.
Accordingly, this method was called the “G-shaped” approach, and the surgical images are shown in Figure 3. The anastomosis technique was basically the same as that in a previously published article by Liu et al[11], and the method of pancreaticojejunos to my was explained in our previous article.
Nutritional support is the most important strategy during postoperative care after RPD. A conventional gastric tube was placed in the patients instead of a jejunal nutrition tube. Patients were given a small liquid diet 2-3 d after surgery and a normal diet 5-7 d after surgery. Intravenous nutrition was performed in patients who were unable to eat. The stomach tube was pulled out without a gastrointestinal emptying obstacle. Pancreatic fistula was diagnosed when the fluid amylase level in drainage output was more than three times the serum amylase level after the third postoperative day[12].
OT; blood loss; complications, including pancreatic fistula, bile leakage, gastric fistula, delayed gastric emptying, wound healing, 30-d reoperation, and 30-d mortality; and hospitalization time were observed.
The learning curve was constructed based on the results of cumulative sum (CUSUM) analysis, and this method has been widely used to determine the learning curve to explore different stages of the learning process. The difference between the OT of each patient and the average OT was calculated by chronological order, and then the difference in the first patient was accumulated to the next patient to obtain the CUSUM OT. Finally, the CUSUM OT was calculated as zero. The calculation formula was CUSUM OT = ∑ni=1 (xi - μ), where xi represents an individual OT, and μ represents the mean overall OT.
The SPSS 21.0 statistics software was used to calculate the results of the χ2 test, t test, and Fisher’s exact test. Mean ± SD deviation was used for continuous variables, the difference between two groups was assessed by Student’s t test, and χ2 test or Fisher’s exact test was used to compare the categorical variables. When the P value was < 0.05, the result was considered statistically significant.
The characteristics of all patients are shown in Table 1. The patients enrolled in this study included 25 females and 35 males with an average age of 57 years. According to imaging, pathological, and other laboratory examinations, 13 patients were diagnosed with pancreatic cancer, 5 patients were diagnosed with a solid pseudopapillary tumor, 22 patients were diagnosed with cholangiocarcinoma, 15 patients were diagnosed with ampullary cancer, 2 patients were diagnosed with a neuroendocrine tumor, 2 patients were diagnosed with intraductal papillary mucinous neoplasia, and 1 patient was diagnosed with chronic pancreatitis.
| Variable | Total | Early (n = 16) | Late (n = 44) | P value |
| Gender (male/female) | 35/25 | 8/8 | 27/17 | |
| Age | 57.32 ± 1.58 | 55.74 ± 3.45 | 57.89 ± 1.77 | 0.55 |
| ASA score | ||||
| 1 | 42 | 13 | 29 | |
| 2 | 18 | 3 | 15 | |
| 3 | 0 | 0 | 0 | |
| Preoperative | ||||
| BMI | 22.91 ± 0.38 | 22.99 ± 0.86 | 22.88 ± 0.42 | 0.90 |
| CA 19-9 (U/mL) | 311.4 ± 81.24 | 313.4 ± 148.6 | 337.9 ± 97.66 | 0.89 |
| ALT | 184.1 ± 22.78 | 103.0 ± 28.08 | 213.6 ± 28.20 | 0.03 |
| AST | 123.1 ± 13.19 | 89.79 ± 24.12 | 135.5 ± 15.45 | 0.12 |
| Total bilirubin | 186.3 ± 17.08 | 94.45 ± 30.06 | 218.3 ± 18.29 | 0.001 |
| Direct bilirubin | 110.9 ± 11.33 | 45.11 ± 16.31 | 1340 ± 12.32 | 0.0003 |
| Pathological parameters | 0.53 | |||
| Pancreatic cancer | 13 | 2 | 11 | |
| Solid pseudopapillary tumor | 5 | 0 | 5 | |
| Cholangiocarcinoma | 22 | 6 | 16 | |
| Ampulla cancer | 15 | 7 | 8 | |
| Neuroendocrine tumor | 2 | 0 | 2 | |
| IPMN | 2 | 1 | 1 | |
| Chronic pancreatitis | 1 | 0 | 1 |
The intraoperative and postoperative outcomes are summarized in Table 2. The OT ranged from 340 to 498 min with an average OT of 378.55 ± 94.30 min, and the mean estimated blood loss was 223 mL. A total of 39 patients developed complications, which included pancreatic fistula (n = 17, 28.3%), bile leakage (n = 4, 6.67%), delayed gastric emptying (n = 16, 26.67%), and wound healing (n = 1, 1.67%). Only one patient could not be diagnosed accurately by preoperative imaging examination. Grade B pancreatic leakage occurred in six patients after surgery. Fasting, somatostatin injection, drainage, nutritional support, and other intervention measures were implemented in a timely manner until the patient was discharged. No secondary surgery, postoperative bleeding, or other serious complications occurred in any patients. Postoperative pathology confirmed that all patients underwent R0 resection. During the follow-up period, none of the patients died and the survival rate was 100%.
| Variable | Total | Early (n = 16) | Late (n = 44) | P value |
| Operative time (min) | 378.55 ± 94.30 | 480 ± 81.65 | 331 ± 76.54 | < 0.01 |
| Estimated blood loss (mL) | 223.3 ± 31.94 | 308 ± 54.78 | 169.2 ± 35.33 | < 0.01 |
| Conversion to OPD | 3 (5%) | 2 (12.5%) | 1 (2.27%) | 0.11 |
| Postoperative stay (d) | 19 ± 6.09 | 22 ± 4.53 | 17 ± 6.08 | < 0.01 |
| Overall complication | ||||
| Pancreatic fistula | 17 (28.3%) | 6 (37.5%) | 11 (25%) | 0.34 |
| Grade A | 11 (18.3%) | 3 (18.75%) | 8 (18.2%) | 0.95 |
| Grade B | 6 (10%) | 3 (18.75%) | 3 (6.82%) | 0.17 |
| Grade C | 0 | 0 | 0 | |
| Bile leakage | 4 (6.67%) | 3 (18.75%) | 1 (2.27%) | 0.024 |
| Gastric fistula | 0 | 0 | 0 | |
| Delayed gastric emptying | 16 (26.67%) | 8 (50%) | 8 (18.18%) | 0.01 |
| Wound healing | 1 | 0 | 1 | |
| 30-d reoperation | 0 | 0 | 0 | |
| 30-d mortality | 0 | 0 | 0 | |
| Postoperative hospital stay (d) | 18.8 ± 11.8 | 22.5 ± 14.5 | 17.5 ± 9.3 | < 0.01 |
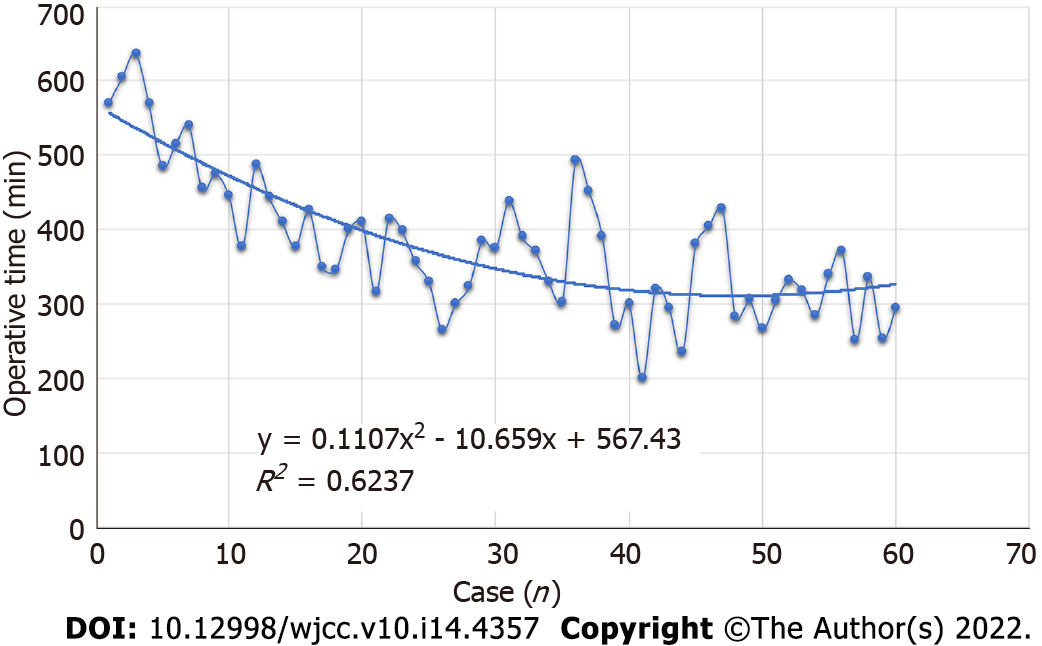
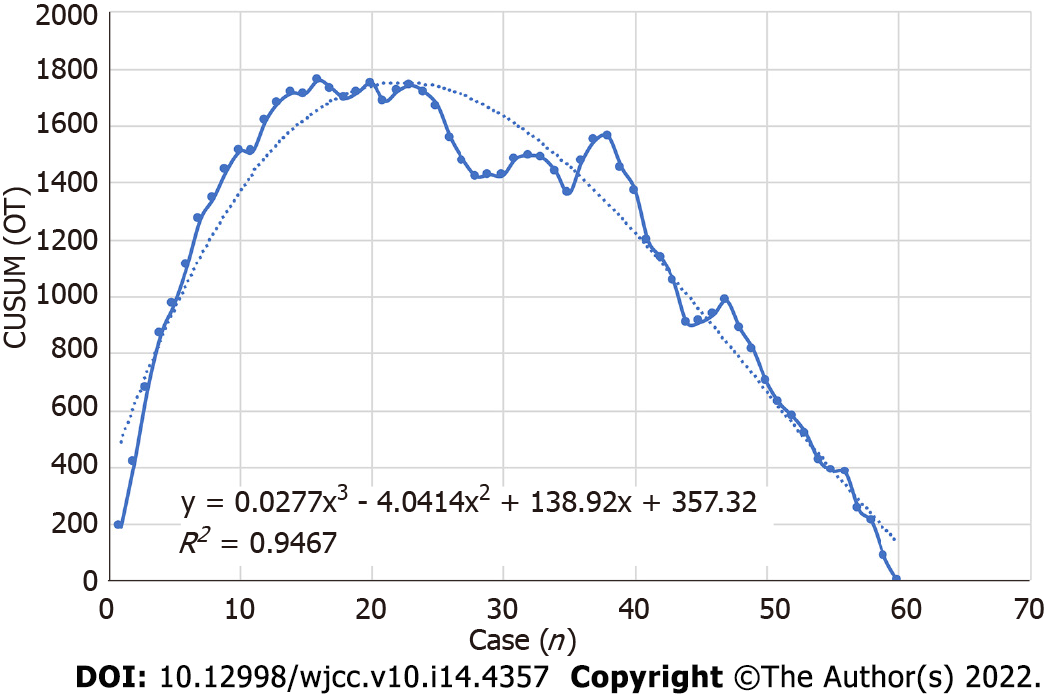
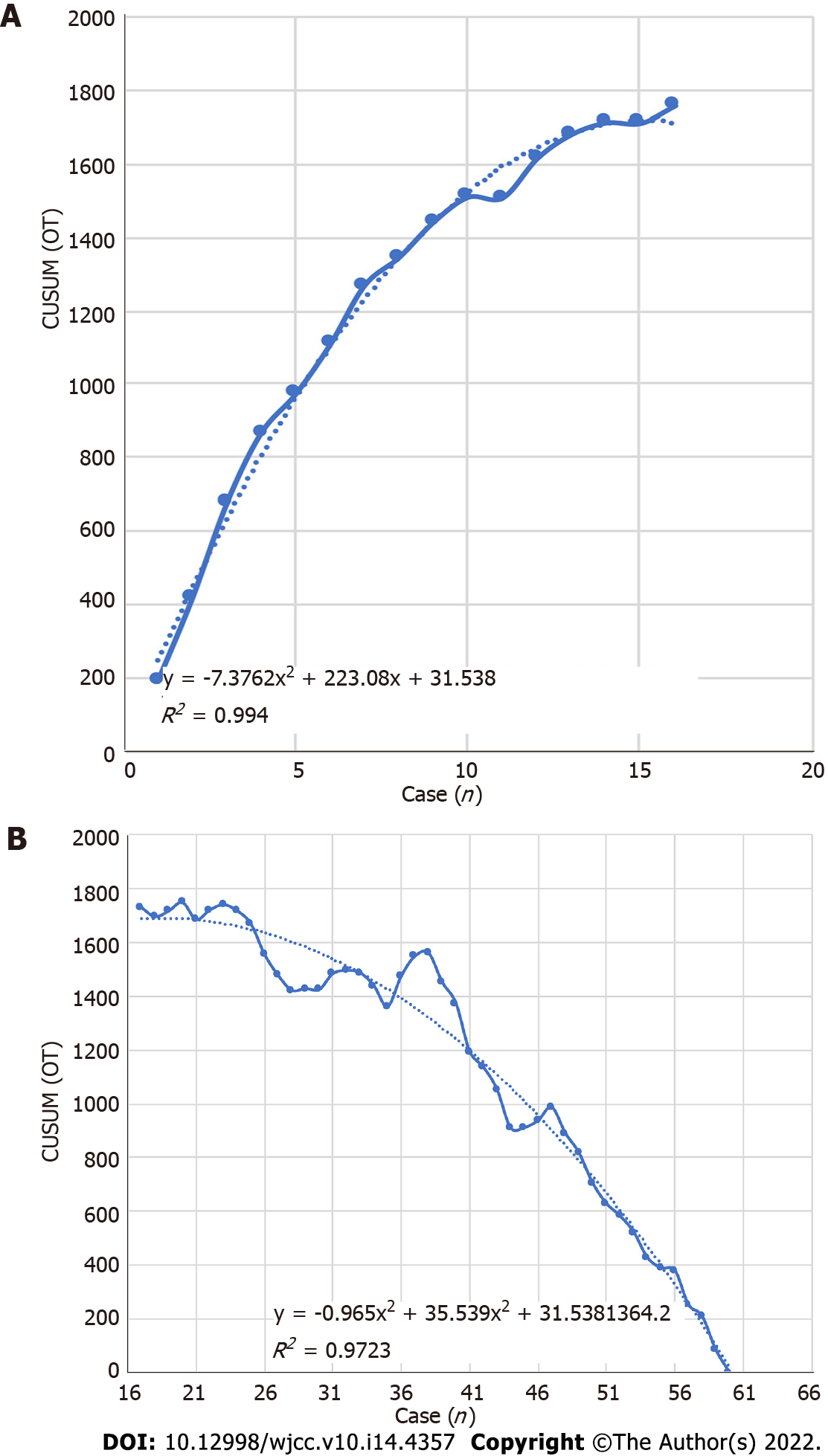
All perioperative outcomes were analyzed in this study, and a graph of OT was plotted according to the order of each patient (Figure 4), where the OT gradually shortened as the number of cases increased. Two distinct phases of the learning process were found according to the CUSUM analysis learning curve (Figure 5), and there was a significant reduction in the OT after the first 16 patients (480 min vs 331 min, P < 0.01). The two slopes, which included the upward slope (y = -7.3762x2 + 223.08x + 31.538, R2 = 0.994) and the downward slope (y = -0.965x2 + 35.539x + 1364.2, R2 = 0.9723), are shown in Figure 6, and the results showed shorter operative times after the first 16 patients.
A comparison of the operative and postoperative outcomes between the two phases was performed, and the results are summarized in Table 2. The operative time (mean, 480 min vs 331 min, P < 0.01), estimated blood loss (mean, 308 mL vs 169.2 mL, P < 0.01), postoperative stay (mean, 22 d vs 17 d, P < 0.01), bile leakage (P = 0.024), and delayed gastric emptying (P = 0.01) were significantly lower after the first 16 patients (P < 0.05), and there were no significant differences in conversion to OPD, pancreatic fistula, gastric fistula, wound healing, 30-d reoperation, or 30-d mortality.
Despite being first reported by Gagner and Pomp[2] in 1994, LPD is still not a widely accepted surgical procedure due to its complex dissection and digestive tract reconstruction. Concerns regarding LPD application were focused on the long operative time, requirement for advanced laparoscopic skills, and lack of advantages compared with OPD[13]. Studies also showed that LPD has an advantage in improving perioperative inflammation and the quality of life of patients[14]. However, recent studies indicated that LPD shows no advantage over OPD. With the rapid development of science and technology, robotic surgery has gradually become a hot trend[15-19].
Zhang et al[16] demonstrated that RPD has a shorter learning curve compared to LPD. Several studies have focused on the learning curve of RPD, although the learning curve is still long. Therefore, the RPD surgical approach needs to constantly be improved to overcome the existing shortcomings. Compared with the other study, the learning curve could be completed after 16 patients in our study, and after 16 patients, the outcomes of RPD included less operative time, estimated blood loss, postoperative stay, bile leakage, and delayed gastric emptying compared to that in previous reports[8-10]. Postoperative pathology confirmed that all cases included in our study achieved R0 resection. There were no cases of conversion or death, which indicated that the prognosis may be better.
Currently, the standard surgical approach (Kocher incision approach) and the arterial priority approach are the main ways of performing minimally invasive PD[16]. The traditional anterior approach is initiated by freeing the descending duodenum. However, this intestinal segment is found deep in the retroperitoneum, in which the transverse colon and its mesentery are covered and cannot be easily reached. This operation requires highly skilled operators. The arterial priority approach can determine whether the artery is involved to avoid palliative resection. Puntambekar et al[18] reported on the SMA approach-based LPD in 38 cases, in which the tumor was resected completely and the negative margin rate was over 94.74%. However, in early patients with ampullary and periampullary tumors, preoperative imaging diagnosis can determine the exact relationship between the tumor and blood vessel. There is no need for repeated confirmation. Moreover, this surgical approach also requires a high level of skill. For young doctors or surgical beginners, it is difficult to directly and accurately identify and isolate the mesenteric blood vessels.
The “G”-shaped surgical approach proposed in this study is conducive to a free Kocher incision. The treatment of the uncinate process and the superior mesenteric vessels is considered the last step of the process, which makes it easy for beginners to master the technique. Studies have shown that patients with ampullary carcinoma had a high metastasis rate at No. 13, 14, 8, and 12 group lymph nodes[18]. In “G”-shaped RPD, the stomach was isolated first and turned to the left to reveal the common hepatic artery and hepatoduodenal ligament, which was conducive for a three-dimensional lymph node dissection of the 8a and 8p lymph nodes. After lymph node cleansing, the common hepatic artery was suspended to isolate the hepatic artery and gastroduodenal artery. The hepatoduodenal ligament was divided into three parts consisting of the left front, left posterior, and right sides, and then cleansing was completed. In addition, the small branches imported to the portal vein system were managed. Good exposure of the upper edge of the pancreatic portal vein was conducive for establishing the posterior pancreatic tunnel. In some patients, the establishment of the posterior pancreatic tunnel was more difficult due to inflammation caused by tumor compression. In these patients, the forcible establishment of the posterior pancreatic tunnel might lead to a portal vein and superior mesenteric vein tear. To avoid uncontrollable bleeding caused by blood vessel tear, we recommend isolating the upper and lower edges of the pancreas first instead of creating a posterior pancreatic tunnel forcibly. We isolated the neck of the pancreas to expose the superior mesenteric vein. We pulled and suspended the superior mesenteric vein to the left with a vessel band and dragged the superior mesenteric vein to the left of the SMA. Then, we isolated the pancreatic tissue and branch of the blood vessels close to the blood vessel wall to completely resect the whole pancreatic membrane. Maximum retention of nerve tissues on the left side of the SMA is essential to avoid postoperative refractory diarrhea. This approach is based on the “periphery to center, easy to difficult, small vessel ligation first, and large vessel ligation last” principle to reduce intraoperative bleeding throughout the process. It might also avoid the spread of tumor cells, reduce abdominal harassment, and accelerate the postoperative recovery of intestinal function, although this requires further research.
There are some limitations in this study that need to be addressed. Firstly, the conclusion was verified in a single surgical center; hence, more cases from other surgical centers are needed to support the conclusion. Secondly, in this study, we chose patients diagnosed with ampullary and periampullary cancer, pancreatic head cancer less than 3 cm in diameter, and benign and low malignant tumors without vascular invasion. A tumor with a diameter greater than 3 cm was not included in this study, as it increased the difficulty of laparoscopic resection and anastomosis. Thus, this modified technique for RPD was only suitable for benign or low-grade malignant tumors. Thirdly, we preoperatively screened the patients with predictable resection through imaging and other data. Therefore, we did not explore the relationship between the pancreas and mesenteric arteries and veins during surgery. Although the situation observed during surgery was consistent with the preoperative assessment, there may be some inconsistencies as the number of cases increase. This may be a drawback of this modified technique for RPD. As such, more detailed preoperative and postoperative assessments are necessary.
The “G”-shaped surgical approach is effective, and this approach can shorten the surgical learning curve.
Robotic pancreaticoduodenectomy (RPD) can achieve similar surgical results to open and PD; however, RPD has a long learning curve and operation time (OT).
To address this issue, we have summarized a surgical path to shorten the surgical learning curve and OT.
This study aimed to investigate the efficacy and learning curve of a “G”-shaped surgical approach in RPD.
A total of 60 patients, who received “G”-shaped RPD (GRPD) by a single surgeon in the First Hospital of Shanxi Medical University from May 2017 to April 2020, were included in this study. OT, demographic data, intraoperative blood loss, complications, hospitalization time, and pathological results were recorded, and the cumulative sum (CUSUM) analysis was performed to evaluate the learning curve for GRPD.
According to the CUSUM analysis, the learning curve for GRPD was grouped into two phases (early and late phases). The OT was 480 ± 81.65 min vs 331 ± 76.54 min, hospitalization time was 22 ± 4.53 d vs 17 ± 6.08 d, and blood loss was 308 ± 54.78 mL vs 169.2 ± 35.33 mL in the respective groups. The complications, including pancreatic fistula, bile leakage, reoperation rate, postoperative death, and delayed gastric emptying, were significantly decreased after this surgical technique.
GRPD can improve the learning curve and operative time, and this will provide a new method for shortening the RPD learning curve.
GRPD can improve the learning curve and operative time, and this will provide a new method for shortening the RPD learning curve.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Silano F, Brazil; Yamanaka K, Japan S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
| 1. | Torres OJ, Moraes Junior JM, Moraes AM, Torres CC, Oliveira AT. Performance of Laparoscopic Pancreatoduodenectomy for Solid Pseudopapillary Tumor of Pancreas. Am J Case Rep. 2016;17:894-898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 2. | Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 659] [Article Influence: 21.3] [Reference Citation Analysis (1)] |
| 3. | Nakamura M, Nakashima H. Laparoscopic distal pancreatectomy and pancreatoduodenectomy: is it worthwhile? J Hepatobiliary Pancreat Sci. 2013;20:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Ausania F, Landi F, Martínez-Pérez A, Fondevila C. A meta-analysis of randomized controlled trials comparing laparoscopic vs open pancreaticoduodenectomy. HPB (Oxford). 2019;21:1613-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | van Hilst J, de Rooij T, Bosscha K, Brinkman DJ, van Dieren S, Dijkgraaf MG, Gerhards MF, de Hingh IH, Karsten TM, Lips DJ, Luyer MD, Busch OR, Festen S, Besselink MG; Dutch Pancreatic Cancer Group. Laparoscopic vs open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019;4:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 409] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 6. | Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 772] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 7. | Zhao W, Liu C, Li S, Geng D, Feng Y, Sun M. Safety and efficacy for robot-assisted vs open pancreaticoduodenectomy and distal pancreatectomy: A systematic review and meta-analysis. Surg Oncol. 2018;27:468-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Zhang T, Zhao ZM, Gao YX, Lau WY, Liu R. The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high-volume pancreatic center. Surg Endosc. 2019;33:2927-2933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Napoli N, Kauffmann EF, Palmeri M, Miccoli M, Costa F, Vistoli F, Amorese G, Boggi U. The Learning Curve in Robotic Pancreaticoduodenectomy. Dig Surg. 2016;33:299-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 10. | Boone BA, Zenati M, Hogg ME, Steve J, Moser AJ, Bartlett DL, Zeh HJ, Zureikat AH. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg. 2015;150:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 235] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 11. | Liu Q, Zhao Z, Zhang X, Zhao G, Tan X, Gao Y, Lau WY, Liu R. Robotic pancreaticoduodenectomy in elderly and younger patients: A retrospective cohort study. Int J Surg. 2020;81:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 12. | Zhu HY, Cui WP, Zhang XN, Dong Y, Wei ZG. Application of “double-U” three-step pancreaticjejunostomy in robotic pancreaticoduodenectomy. Chin J Open Proc Gen Surg. 2020;14 (4):374-377. [DOI] [Full Text] |
| 13. | Pratt WB, Callery MP, Vollmer CM Jr. Risk prediction for development of pancreatic fistula using the ISGPF classification scheme. World J Surg. 2008;32:419-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 14. | Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 15. | Feng Q, Xin Z, Qiu J, Xu M. Laparoscopic vs. Open Pancreaticoduodenectomy After Learning Curve: A Systematic Review and Meta-Analysis of Single-Center Studies. Front Surg. 2021;8:715083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Zhang Y, Hong D, Zhang C, Hu Z. Total laparoscopic vs robot-assisted laparoscopic pancreaticoduodenectomy. Biosci Trends. 2018;12:484-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Nagakawa Y, Hosokawa Y, Sahara Y, Takishita C, Nakajima T, Hijikata Y, Tago T, Kasuya K, Tsuchida A. A Novel "Artery First" Approach Allowing Safe Resection in Laparoscopic Pancreaticoduodenectomy: The Uncinate Process First Approach. Hepatogastroenterology. 2015;62:1037-1040. [PubMed] |
| 18. | Puntambekar S, Kenawadekar R, Pandit A, Nadkarni A, Bhat NA, Joshi S, Joshi GA. Laparoscopic Supracolic Pancreaticoduodenectomy: A Novel Technique For Complete Uncinate Process Resection. Hepatogastroenterology. 2014;61:1118-1123. [PubMed] |
| 19. | Fujita T, Konishi M, Gotohda N, Takahashi S, Nakagohri T, Kojima M, Kinoshita T. Invasive micropapillary carcinoma of the ampulla of Vater with extensive lymph node metastasis: Report of a case. Surg Today. 2010;40:1197-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |