Published online May 16, 2022. doi: 10.12998/wjcc.v10.i14.4348
Peer-review started: October 11, 2021
First decision: November 15, 2021
Revised: January 13, 2022
Accepted: March 25, 2022
Article in press: March 25, 2022
Published online: May 16, 2022
Processing time: 213 Days and 12.4 Hours
Determination of the mesenchymal stem cells is one of the greatest and most exciting achievements that tissue engineering and regenerative medicine have achieved. Adipose-derived mesenchymal stem cells (AD-MSC) are easily isolated and cultured for a long time before losing their stem cell characteristics, which are self-renewal and pluripotency. AD-MSC are mesenchymal stem cells that have pluripotent lineage characteristics. They are easily accessible, and the fraction of stem cells in the adipose tissue lysates is highest among all other sources of mesenchymal stem cells. It is also HLA-DR negative and can be transplanted allogenically without the need for immunosuppression. These advantages have popularized its use in many fields including plastic reconstructive surgery. However, in the field of hepatology and liver transplantation, the progress is slower. AD-MSC have the potential to modulate inflammation, ameliorate ischemia-reperfusion injury, and support liver and biliary tract regeneration. These are very important for the treatment of various hepatobiliary diseases. Furthermore, the anti-inflammatory potential of these cells has paramount importance in the treatment of sepsis. We need alternative therapeutic approaches to treat end-stage liver failure. AD-MSC can provide a means of therapy to bridge to definitive therapeutic alternatives such as liver transplantation. Here we propose to review theoretic applications of AD-MSC in the treatment of hepatobiliary diseases and sepsis.
Core Tip: Adipose-derived mesenchymal stem cells (AD-MSC) are mesenchymal stem cells that have pluripotent lineage characteristics. They are easily accessible, and the fraction of stem cells in the adipose tissue lysates is the highest among all other sources of mesenchymal stem cells. It is also HLA-DR negative and can be transplanted allogenically without the need for immunosuppression. We clearly need alternative therapeutic approaches to treat end-stage liver failure. AD-MSC can provide a means of bridge therapy to definitive therapeutic alternatives such as liver transplantation. We review the theoretic applications of AD-MSC in the treatment of hepatobiliary diseases.
- Citation: Satilmis B, Cicek GS, Cicek E, Akbulut S, Sahin TT, Yilmaz S. Adipose-derived stem cells in the treatment of hepatobiliary diseases and sepsis. World J Clin Cases 2022; 10(14): 4348-4356
- URL: https://www.wjgnet.com/2307-8960/full/v10/i14/4348.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i14.4348
Stem cell therapy provides limitless therapeutic options in the field of medicine, which is the direct result of the achievements obtained by the field of regenerative medicine. The therapeutic applications are early in its stages, and the clinical trials are ongoing[1]. Ideal stem cells should have the ability of self-renewal, multilineage capacity, and easily isolated, and cultivation conditions should be simple[2,3]. Mesenchymal stem cells (MSC) have been used abundantly for this purpose[3,4]. MSC have an intricate cell biology and are amenable to being utilized in tissue engineering. They secrete various potent growth factors and cytokines, they have pluripotent differentiation capabilities, and they are abundant in the body including the bone marrow, oral cavity, and adipose tissue[5].
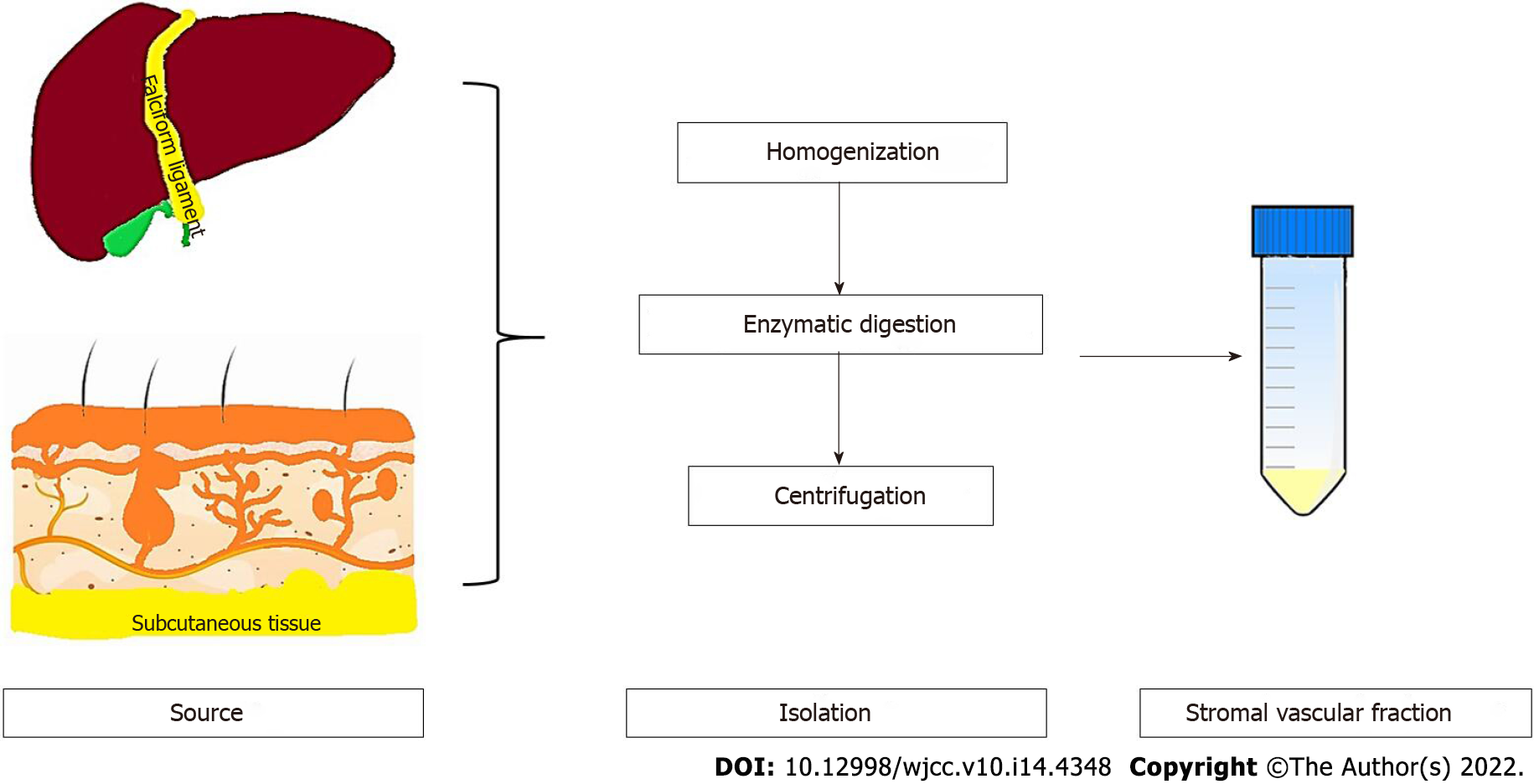
In 2001, Zuk et al[6] isolated and defined the stromal vascular fraction of adipose tissue. The stromal vascular fraction contains a mixture of erythrocytes, fibromyoblasts, endothelial cells, smooth muscle cells, pericytes of vascular origin, and fat cells. The stromal vascular fraction can be cultured, forming fibroblast-like cells that are adherent to the culture flask. These cells were originally named pre-adipocytes[7,8]. However, it has been shown that these cells have mesodermal multipotent differentiation ability, and they are currently called adipose-derived MSC (AD-MSC)[7]. Currently, advancements in tissue engineering and regenerative medicine have shown that these cells can differentiate into cells and tissues of endodermal, mesodermal, and ectodermal origin[1,2]. There are some advantages to using AD-MSC in regenerative medicine. The most important one is the abundance of stem cells in the adipose tissue[9,10]. Adipose tissue contains at least 100 times higher amounts of stem cells when compared to other sources such as the bone marrow[1,11]. Furthermore, the isolation procedure is very simple and efficient[1,2,6,7,12]. Obtaining the fat tissue from individuals is very easy. The proliferative capacity and durability of AD-MSC exceed MSC obtained from other sources[2,13-15].
The isolation procedure is very simple and has been done for a long time[16]. It includes mechanical disruption of the tissue followed by enzymatic digestion and ultracentrifugation (Figure 1). It is cultured in standard cell culture media without the need of a special culture media. During the standard culture of eukaryotic cells 10% (v/v), fetal bovine serum is used. This can lead to certain problems such as immune reactivity and transmission of zoonotic infections[17]. For this reason, platelet-rich plasma can be used as an alternative to fetal bovine serum. Platelet-rich plasma has also been shown to enhance AD-MSC growth in vitro[18].
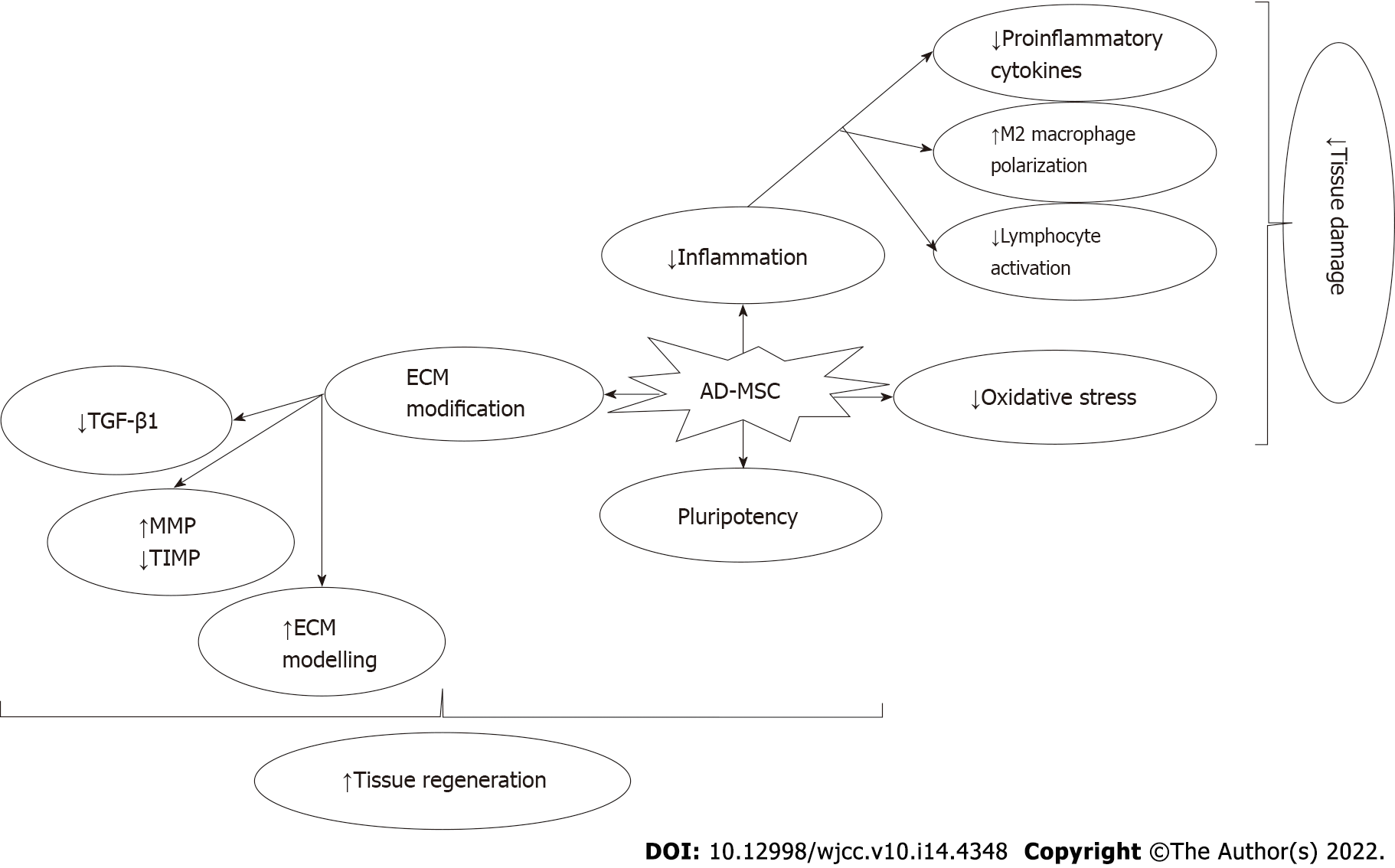
There is a uniform pattern of surface marker expression for AD-MSC, which is the presence of CD90, CD73, CD105, and CD44[19,20]. Expression of CD34 and CD49d is highly reserved for AD-MSC and is absent in other MSC types[21]. The secretion profile of AD-MSC includes a wide range of cytokines, chemokines, and growth factors. The effects of the secreted factors are paracrine in their activity. There are factors that promote angiogenesis such as fibroblast growth factor 2, vascular endothelial growth factor, hepatocyte growth factor, and insulin-like growth factor 1[22]. Also, matrix metalloproteinase-3 and matrix metalloproteinase-9 contribute to their proangiogenic activity[23]. Their effect on the system is usually induction of immunoregulatory type changes promoting tissue injury and angiogenesis. The factors that are responsible for the immune effects of AD-MSC are macrophage-colony stimulating factor, granulocyte-colony stimulating factor, interleukin (IL) 6, tumor necrosis factor, and prostaglandin E2[23]. Therefore, there is T helper type 2 polarization of CD4-positive T cells and M2 polarization of the macrophages. All these changes reduce inflammation and increase the wound healing capacity of the tissues[24,25].
In acute and chronic liver failure, the regenerative capacity of the liver is overwhelmed by the noxious stimuli[26,27]. Therefore, regeneration or repair of the liver is very complicated due to the presence of a variety of parenchymal cells[28]. These cells include the hepatocytes, cholangiocytes, hepatic stellate cells, and immune cells including the Kupffer cells, natural killer cells, natural killer T cells, and eosinophils[28,29].
In summary, through the paracrine effects of the AD-MSC-derived cytokines, chemokines, and growth factors, AD-MSC stimulate angiogenesis, exert antiapoptotic effects, and recruit other MSC and progenitor cells to the site of injury[27,30,31]. In addition, they stimulate the proliferation and differentiation of the wide range of cells present in the site of injury. They also reduce the reactive oxygen species in the microenvironment and reduce reactive oxygen species-mediated injury to the tissues[27,30,31]. One unique feature of MSC is their ability to fuse with parenchymal cells in the injury site to promote intercellular interactions and exchange cellular macromolecules through the intercellular nanochannels that are formed[27,30-32].
The aim of the present study was to summarize the current literature in terms of AD-MSC in the cellular therapy for hepatic and biliary regeneration. Also, we briefly summarize the role of AD-MSC in the treatment of septic conditions. We hope this will help the readers to grasp the potential of AD-MSC in the treatment of hepatobiliary diseases.
The immune regulatory and antiapoptotic effects of AD-MSC aid regeneration of the liver and help healing of liver injury caused by viral infections, toxins, and genetic diseases[33,34]. Studies have shown that AD-MSC express liver specific markers even if they are not targeted in vitro[35]. There are many sources for AD-MSC, and each have different biological behavior. Liver falciform ligament-derived AD-MSC show higher proliferative capacity and higher embryonic stem cell capabilities[36]. Falciform ligament is readily available during liver surgery and can be used to enhance healing of the tissue following liver surgery. Surgeons have been using falciform ligament flaps to support anastomosis or to fill a gap in the liver following resection for a long time[37]. This may be attributed to the enhanced healing capacity of the stem cells present in the falciform ligament.
Experimental studies are abundant showing reduced inflammation, support of hepatic regeneration, and normalization of metabolic derangements in liver failure experimental models. We briefly summarize some of the cornerstone experiments that are present in the literature. Experimental studies have shown that the condition of the host determined the type of differentiation of the AD-MSC. In an experimental model of acute liver failure, it has been shown that AD-MSC showed increased expression of specific markers for hepatocytes[38]. AD-MSC have been shown to be amenable to in vitro targeting to hepatocytes, which can later be used to treat an experimental model of acute liver failure[39]. Transplantation of AD-MSC 24 h before 70% hepatectomy model in rats ameliorated hepatic dysfunction and improved liver regeneration by normalizing the metabolic processes in the liver[40]. Banas et al[41] have also reported their results in a carbon tetrachloride treated acute liver failure model. They have shown that treatment with AD-MSC that were preconditioned in vitro ameliorated the liver failure and normalized liver function tests in animals in the treatment arm[41]. AD-MSC given as treatment after the development of acute liver failure have also been shown to be effective in improving liver regeneration and functions[42]. Preconditioning of AD-MSC has resulted in development of functional liver tissue (liver bud) in experimental models[43]. AD-MSC were shown to significantly inhibit the proliferation and activation of hematopoietic stem cells and promote the programmed cell death of hematopoietic stem cells thereby reducing hepatic fibrosis in experimental models[44,45].
AD-MSC can also be used together with nanoparticle technology to increase the engraftment rates and enhance the efficacy of the AD-MSC in reversal of liver injury and liver fibrosis[43]. Furthermore, the use of liver bioscaffolds has been shown to support the growth of neonatal multilineage progenitor cells into fully functional liver tissue[46]. If stem cells are used during the recellularization process, the results seem to be better when comparted to primary parenchymal cells such as the hepatocytes[43]. Apart from supporting the regenerative process, AD-MSC reduce the ischemia and reperfusion injury during liver surgery and diseases. It has been shown that AD-MSC reduce ischemia reperfusion injury in liver by reduction of various inflammatory cytokines such as IL-1β, IL-6, and tumor necrosis factor[47]. Furthermore, AD-MSC secrete counter regulatory cytokines such as IL-10 and secrete factors such as hepatocyte growth factor and cyclin D1, which are effective in hepatic regeneration[47]. The effects of AD-MSC include immune regulation, reduction of oxidative and inflammatory tissue destruction, and regeneration of the parenchymal cells. The proposed mechanisms of action of AD-MSC are summarized in Figure 2. The clinical trials so far have been successful and have shown a good safety profile of AD-MSC in humans. It prevented acute-on-chronic liver failure and improved liver functions in patients with cirrhosis with various etiologies[48-50]. The summary of the preclinical studies and clinical trials are summarized in Supplementary Table 1 and 2.
Liver resection and transplantation are among the definitive treatments of life-threatening chronic liver disease and primary/secondary liver tumors. The most frequent complication following liver disease is the biliary complications[51,52]. Some of these complications may even cause mortality in the patients. Stenosis is one of the biliary complications that are observed following hepatobiliary surgery. In major surgeries like living donor liver transplantation, it has been reported to affect 10%-30% of the patients[53]. Treatment of this complication requires repeated procedures, restorative operations, and frequent prolonged hospitalizations.
The majority of the complications are due to ischemia and reperfusion injury. Ischemia reperfusion injury has adverse effects on both hepatic and biliary regeneration[54,55]. Zhu et al[55] reported that warm ischemia times exceeding 20 min were associated with biliary complication and biliary epithelial damage in an experimental model. The cholangiocytes have pluripotent differentiation potential, but it is overwhelmed during ischemia and reperfusion injury[54,55]. MSC therapy may be an alternative or adjunct to conventional therapies for biliary complications. AD-MSC are preferred alternatives for they are easily accessible, and they promote anti-inflammatory mechanisms and regeneration in the tissue, which may be beneficial for biliary regeneration[55,56]. There are limited studies regarding the versatility of AD-MSC in biliary regeneration[47,57]. Abraham et al[57] showed that AD-MSC sheets were effective in preventing biliary strictures in duct-to-duct anastomoses. However, the studies were limited regarding the role of AD-MSC in biliary regeneration. Further studies will provide innovative therapeutic options for biliary complications.
Sepsis is an overwhelming inflammatory response to invading microorganisms. The severity of the disease depends on the virulence of the microbial pathogen, amount of toxins secreted by the pathogen, and the physiologic status of the host. The recovery from sepsis depends on the balance between the proinflammatory cytokines and anti-inflammatory mechanisms that counterbalance inflammation[58]. Tumor necrosis factor-α3 and IL-6 are the potent proinflammatory cytokines that have a major role during the pathogenesis of sepsis[59,60]. As our understanding of the physiopathology increases, alternative immunomodulatory therapies are being developed and investigated for clinical use[61].
Currently, MSC are being used for the treatment of sepsis. The majority of these are isolated from the bone marrow, which is not an easy process[62]. AD-MSC have been shown to be effective in endotoxemia-induced sepsis models in rats by reducing apoptosis and the rate of multi-organ failure[63]. The anti-inflammatory action of MSC can be a direct effect through cell-to-cell interaction or may be through paracrine effects of the secreted mediators or secretion of exosomes/microvesicles to the inflammatory microenvironment[62]. MSC have been shown to reduce proinflammatory cytokines and increase cytokines such as IL-10 and induce a regulatory phenotype in the immune cells[64]. Through this mechanism, MSC reduce the amount of macrophage and neutrophil infiltration in target organs such as the lungs, kidneys, and the liver, thus reducing the risk of multiple organ failure[62,65-67]. MSC also increase the phagocytic activity of monocytes in circulation and reduce the effective microbial concentrations[68].
In sepsis or viral pneumonia such as the one seen in coronavirus disease 2019, traditional therapeutic options were insufficient[69]. Bone marrow-derived MSC played an important role for both reduction of inflammatory damage in end-organs and in clearance of microbial agents from the circulation of the patients. The studies regarding the role of AD-MSC in sepsis are not enough, and further studies are needed. The proposed mechanism of action of AD-MSC in hepatobiliary diseases and sepsis are summarized in Figure 2.
There are many unknown points regarding the role of AD-MSC in the treatment of hepatobiliary diseases. However, the results of preclinical studies and limited clinical trials are promising. It seems to be a good alternative treatment to bridge acute or acute-on-chronic liver failure until a definitive liver transplantation can be performed. Furthermore, it may promote the wound-healing process preventing many complications in the biliary tract following major liver surgeries.
The utility of AD-MSC in the treatment of hepatobiliary disease and sepsis is relatively new. Brief reports are showing the efficacy of AD-MSC in controlling inflammation and regenerating parenchymal tissue. However, there is not a firmly established protocol. The dose and dosing intervals of the allogenic AD-MSC transplantation requires further research for establishing universal protocols. Furthermore, the role of targeted or genetically modified AD-MSC are unknown. Bioscaffolds may also provide modeling of the tissue and providing precursors for the liver and biliary tract. Combination of AD-MSC with nanoparticles for potentiating the anti-inflammatory response will be an important area of research in the future. Therefore, further research is needed to guide physicians for future innovative clinical applications.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Various Associations: American Association of Cancer Research, 233259; American College of Surgeons, 3223715.
Specialty type: Transplantation
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodrigues AT, Brazil; Sheykhhasan M, Iran S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | Chu DT, Nguyen Thi Phuong T, Tien NLB, Tran DK, Minh LB, Thanh VV, Gia Anh P, Pham VH, Thi Nga V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Zhang J, Liu Y, Chen Y, Yuan L, Liu H, Wang J, Liu Q, Zhang Y. Adipose-Derived Stem Cells: Current Applications and Future Directions in the Regeneration of Multiple Tissues. Stem Cells Int. 2020;2020:8810813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 3. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12664] [Article Influence: 703.6] [Reference Citation Analysis (2)] |
| 4. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 948] [Article Influence: 158.0] [Reference Citation Analysis (35)] |
| 5. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1244] [Article Influence: 207.3] [Reference Citation Analysis (0)] |
| 6. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5758] [Article Influence: 239.9] [Reference Citation Analysis (0)] |
| 7. | Zhu M, Heydarkhan-Hagvall S, Hedrick M, Benhaim P, Zuk P. Manual isolation of adipose-derived stem cells from human lipoaspirates. J Vis Exp. 2013;e50585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 804] [Cited by in RCA: 744] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 9. | Semon JA, Maness C, Zhang X, Sharkey SA, Beuttler MM, Shah FS, Pandey AC, Gimble JM, Zhang S, Scruggs BA, Strong AL, Strong TA, Bunnell BA. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res Ther. 2014;5:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Mazini L, Rochette L, Amine M, Malka G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 267] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 11. | Chu DT, Tao Y, Son LH, Le DH. Cell source, differentiation, functional stimulation, and potential application of human thermogenic adipocytes in vitro. J Physiol Biochem. 2016;73:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Djouad F, Bouffi C, Ghannam S, Noël D, Jorgensen C. Mesenchymal stem cells: innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol. 2009;5:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 13. | Chen YJ, Liu HY, Chang YT, Cheng YH, Mersmann HJ, Kuo WH, Ding ST. Isolation and Differentiation of Adipose-Derived Stem Cells from Porcine Subcutaneous Adipose Tissues. J Vis Exp. 2016;e53886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Dai R, Wang Z, Samanipour R, Koo KI, Kim K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016;2016:6737345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 15. | Gu X, Li C, Yin F, Yang G. Adipose-derived stem cells in articular cartilage regeneration: current concepts and optimization strategies. Histol Histopathol. 2018;33:639-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Rodbell M. Metabolism of isolated fat cells. i. effects of hormones on glucose metabolism and lipolysis. J Biol Chem. 1964;239:375-380. [PubMed] |
| 17. | Laitinen A, Oja S, Kilpinen L, Kaartinen T, Möller J, Laitinen S, Korhonen M, Nystedt J. A robust and reproducible animal serum-free culture method for clinical-grade bone marrow-derived mesenchymal stromal cells. Cytotechnology. 2016;68:891-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Atashi F, Jaconi ME, Pittet-Cuénod B, Modarressi A. Autologous platelet-rich plasma: a biological supplement to enhance adipose-derived mesenchymal stem cell expansion. Tissue Eng Part C Methods. 2015;21:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Mildmay-White A, Khan W. Cell Surface Markers on Adipose-Derived Stem Cells: A Systematic Review. Curr Stem Cell Res Ther. 2017;12:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Samadi P, Saki S, Manoochehri H, Sheykhhasan M. Therapeutic Applications of Mesenchymal Stem Cells: A Comprehensive Review. Curr Stem Cell Res Ther. 2021;16:323-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (3)] |
| 21. | Kocan B, Maziarz A, Tabarkiewicz J, Ochiya T, Banaś-Ząbczyk A. Trophic Activity and Phenotype of Adipose Tissue-Derived Mesenchymal Stem Cells as a Background of Their Regenerative Potential. Stem Cells Int. 2017;2017:1653254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 65] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Combellack EJ, Jessop ZM, Naderi N, Griffin M, Dobbs T, Ibrahim A, Evans S, Burnell S, Doak SH, Whitaker IS. Adipose regeneration and implications for breast reconstruction: update and the future. Gland Surg. 2016;5:227-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 23. | Gokce A, Abd Elmageed ZY, Lasker GF, Bouljihad M, Kim H, Trost LW, Kadowitz PJ, Abdel-Mageed AB, Sikka SC, Hellstrom WJ. Adipose tissue-derived stem cell therapy for prevention and treatment of erectile dysfunction in a rat model of Peyronie's disease. Andrology. 2014;2:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Schon HT, Weiskirchen R. Immunomodulatory effects of transforming growth factor-β in the liver. Hepatobiliary Surg Nutr. 2014;3:386-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 50] [Reference Citation Analysis (0)] |
| 25. | Kokai LE, Marra K, Rubin JP. Adipose stem cells: biology and clinical applications for tissue repair and regeneration. Transl Res. 2014;163:399-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 208] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 26. | Yang X, He C, Zhu L, Zhao W, Li S, Xia C, Xu C. Comparative Analysis of Regulatory Role of Notch Signaling Pathway in 8 Types Liver Cell During Liver Regeneration. Biochem Genet. 2019;57:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Mok PL, Leong CF, Cheong SK. Cellular mechanisms of emerging applications of mesenchymal stem cells. Malays J Pathol. 2013;35:17-32. [PubMed] |
| 28. | D'Ambrosio DN, Walewski JL, Clugston RD, Berk PD, Rippe RA, Blaner WS. Distinct populations of hepatic stellate cells in the mouse liver have different capacities for retinoid and lipid storage. PLoS One. 2011;6:e24993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Ding BS, Nolan DJ, Butler JM, James D, Babazadeh AO, Rosenwaks Z, Mittal V, Kobayashi H, Shido K, Lyden D, Sato TN, Rabbany SY, Rafii S. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468:310-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 640] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 30. | Nowak WN, Taha H, Kachamakova-Trojanowska N, Stępniewski J, Markiewicz JA, Kusienicka A, Szade K, Szade A, Bukowska-Strakova K, Hajduk K, Klóska D, Kopacz A, Grochot-Przęczek A, Barthenheier K, Cauvin C, Dulak J, Józkowicz A. Murine Bone Marrow Mesenchymal Stromal Cells Respond Efficiently to Oxidative Stress Despite the Low Level of Heme Oxygenases 1 and 2. Antioxid Redox Signal. 2018;29:111-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Polymeri A, Giannobile WV, Kaigler D. Bone Marrow Stromal Stem Cells in Tissue Engineering and Regenerative Medicine. Horm Metab Res. 2016;48:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 32. | Chan YW, So C, Yau KL, Chiu KC, Wang X, Chan FL, Tsang SY. Adipose-derived stem cells and cancer cells fuse to generate cancer stem cell-like cells with increased tumorigenicity. J Cell Physiol. 2020;235:6794-6807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 33. | Berardis S, Lombard C, Evraerts J, El Taghdouini A, Rosseels V, Sancho-Bru P, Lozano JJ, van Grunsven L, Sokal E, Najimi M. Gene expression profiling and secretome analysis differentiate adult-derived human liver stem/progenitor cells and human hepatic stellate cells. PLoS One. 2014;9:e86137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Liang H, Ding X, Yu Y, Zhang H, Wang L, Kan Q, Ma S, Guan F, Sun T. Adipose-derived mesenchymal stem cells ameliorate acute liver injury in rat model of CLP induced-sepsis via sTNFR1. Exp Cell Res. 2019;383:111465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Zemel R, Bachmetov L, Ad-El D, Abraham A, Tur-Kaspa R. Expression of liver-specific markers in naïve adipose-derived mesenchymal stem cells. Liver Int. 2009;29:1326-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Lee SW, Chong JU, Min SO, Bak SY, Kim KS. Are Adipose-Derived Stem Cells From Liver Falciform Ligaments Another Possible Source of Mesenchymal Stem Cells? Cell Transplant. 2017;26:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Ozmen MM, Coskun F, Ziraman I. Falciform ligament in the management of the residual cavity for liver hydatidosis: new surgical technique. World J Surg. 2006;30:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Hu C, Zhou N, Li J, Shi D, Cao H, Li L. Porcine Adipose-Derived Mesenchymal Stem Cells Retain Their Stem Cell Characteristics and Cell Activities While Enhancing the Expression of Liver-Specific Genes after Acute Liver Failure. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Liang L, Ma T, Chen W, Hu J, Bai X, Li J, Liang T. Therapeutic potential and related signal pathway of adipose-derived stem cell transplantation for rat liver injury. Hepatol Res. 2009;39:822-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Tautenhahn HM, Brückner S, Baumann S, Winkler S, Otto W, von Bergen M, Bartels M, Christ B. Attenuation of Postoperative Acute Liver Failure by Mesenchymal Stem Cell Treatment Due to Metabolic Implications. Ann Surg. 2016;263:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Banas A, Teratani T, Yamamoto Y, Tokuhara M, Takeshita F, Osaki M, Kato T, Okochi H, Ochiya T. Rapid hepatic fate specification of adipose-derived stem cells and their therapeutic potential for liver failure. J Gastroenterol Hepatol. 2009;24:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 42. | Zare H, Jamshidi S, Dehghan MM, Saheli M, Piryaei A. Bone marrow or adipose tissue mesenchymal stem cells: Comparison of the therapeutic potentials in mice model of acute liver failure. J Cell Biochem. 2018;119:5834-5842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Zhang Y, Chen XM, Sun DL. Effects of coencapsulation of hepatocytes with adipose-derived stem cells in the treatment of rats with acute-on-chronic liver failure. Int J Artif Organs. 2014;37:133-141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Hao T, Chen J, Zhi S, Zhang Q, Chen G, Yu F. Comparison of bone marrow-vs. adipose tissue-derived mesenchymal stem cells for attenuating liver fibrosis. Exp Ther Med. 2017;14:5956-5964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Nguyen NH, Le T Van, Do HQ, Ngo DQ, Le HM, Truong NH. Comparative treatment efficiency of adipose and bone marrow derived allogenic mesenchymal stem cell transplantation in mouse models of liver fibrosis. Biomed Res Ther. 2017;4:1374. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 46. | Hassanein W, Cimeno A, Werdesheim A, Buckingham B, Harrison J, Uluer MC, Khalifeh A, Rivera-Pratt C, Klepfer S, Woodall JD, Dhru U, Bromberg E, Parsell D, Drachenberg C, Barth RN, LaMattina JC. Liver Scaffolds Support Survival and Metabolic Function of Multilineage Neonatal Allogenic Cells. Tissue Eng Part A. 2018;24:786-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Ge Y, Zhang Q, Jiao Z, Li H, Bai G, Wang H. Adipose-derived stem cells reduce liver oxidative stress and autophagy induced by ischemia-reperfusion and hepatectomy injury in swine. Life Sci. 2018;214:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 48. | Götze T, Krueger M, Meutsch J, Dörfel M, Born S, Sowa JP, Canbay A. Three Cases of Alcohol-Induced Acute-On-Chronic Liver Failure With Successful Support by Adipose-Derived Stem Cells. Clin Transl Gastroenterol. 2019;10:e00095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 49. | Huang KC, Chuang MH, Lin ZS, Lin YC, Chen CH, Chang CL, Huang PC, Syu WS, Chiou TW, Hong ZH, Tsai YC, Harn HJ, Lin PC, Lin SZ. Transplantation with GXHPC1 for Liver Cirrhosis: Phase 1 Trial. Cell Transplant. 2019;28:100S-111S. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Sakai Y, Takamura M, Seki A, Sunagozaka H, Terashima T, Komura T, Yamato M, Miyazawa M, Kawaguchi K, Nasti A, Mochida H, Usui S, Otani N, Ochiya T, Wada T, Honda M, Kaneko S. Phase I clinical study of liver regenerative therapy for cirrhosis by intrahepatic arterial infusion of freshly isolated autologous adipose tissue-derived stromal/stem (regenerative) cell. Regen Ther. 2017;6:52-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Chang JH, Lee IS, Choi JY, Yoon SK, Kim DG, You YK, Chun HJ, Lee DK, Choi MG, Chung IS. Biliary Stricture after Adult Right-Lobe Living-Donor Liver Transplantation with Duct-to-Duct Anastomosis: Long-Term Outcome and Its Related Factors after Endoscopic Treatment. Gut Liver. 2010;4:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 52. | Park JK, Yang JI, Lee JK, Park JK, Lee KH, Lee KT, Joh JW, Kwon CHD, Kim JM. Long-term Outcome of Endoscopic Retrograde Biliary Drainage of Biliary Stricture Following Living Donor Liver Transplantation. Gut Liver. 2020;14:125-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (37)] |
| 53. | Wojcicki M, Milkiewicz P, Silva M. Biliary tract complications after liver transplantation: a review. Dig Surg. 2008;25:245-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 54. | Saidi RF, Rajeshkumar B, Shariftabrizi A, Bogdanov AA, Zheng S, Dresser K, Walter O. Human adipose-derived mesenchymal stem cells attenuate liver ischemia-reperfusion injury and promote liver regeneration. Surgery. 2014;156:1225-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Zhu XH, Pan JP, Wu YF, Ding YT. Establishment of a rat liver transplantation model with prolonged biliary warm ischemia time. World J Gastroenterol. 2012;18:7194-7200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Baglio SR, Pegtel DM, Baldini N. Mesenchymal stem cell secreted vesicles provide novel opportunities in (stem) cell-free therapy. Front Physiol. 2012;3:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 402] [Article Influence: 30.9] [Reference Citation Analysis (1)] |
| 57. | Abraham E, Wunderink R, Silverman H, Perl TM, Nasraway S, Levy H, Bone R, Wenzel RP, Balk R, Allred R. Efficacy and safety of monoclonal antibody to human tumor necrosis factor alpha in patients with sepsis syndrome. A randomized, controlled, double-blind, multicenter clinical trial. TNF-alpha MAb Sepsis Study Group. JAMA. 1995;273:934-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 58. | Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1114] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 59. | Huang M, Cai S, Su J. The Pathogenesis of Sepsis and Potential Therapeutic Targets. Int J Mol Sci. 2019;20:5376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 481] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 60. | Mira JP, Cariou A, Grall F, Delclaux C, Losser MR, Heshmati F, Cheval C, Monchi M, Teboul JL, Riché F, Leleu G, Arbibe L, Mignon A, Delpech M, Dhainaut JF. Association of TNF2, a TNF-alpha promoter polymorphism, with septic shock susceptibility and mortality: a multicenter study. JAMA. 1999;282:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 516] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 61. | Cribbs SK, Matthay MA, Martin GS. Stem cells in sepsis and acute lung injury. Crit Care Med. 2010;38:2379-2385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Keane C, Jerkic M, Laffey JG. Stem Cell-based Therapies for Sepsis. Anesthesiology. 2017;127:1017-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 63. | Hu Y, Qin C, Zheng G, Lai D, Tao H, Zhang Y, Qiu G, Ge M, Huang L, Chen L, Cheng B, Shu Q, Xu J. Mesenchymal Stem Cell-Educated Macrophages Ameliorate LPS-Induced Systemic Response. Mediators Inflamm. 2016;2016:3735452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 64. | Sun J, Sun X, Chen J, Liao X, He Y, Wang J, Chen R, Hu S, Qiu C. microRNA-27b shuttled by mesenchymal stem cell-derived exosomes prevents sepsis by targeting JMJD3 and downregulating NF-κB signaling pathway. Stem Cell Res Ther. 2021;12:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 65. | Chen J, Li C, Liang Z, Li Y, Zhao Z, Qiu T, Hao H, Niu R, Chen L. Human mesenchymal stromal cells small extracellular vesicles attenuate sepsis-induced acute lung injury in a mouse model: the role of oxidative stress and the mitogen-activated protein kinase/nuclear factor kappa B pathway. Cytotherapy. 2021;23:918-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 66. | Sheng M, Lin Y, Xu D, Tian Y, Zhan Y, Li C, Farmer DG, Kupiec-Weglinski JW, Ke B. CD47-Mediated Hedgehog/SMO/GLI1 Signaling Promotes Mesenchymal Stem Cell Immunomodulation in Mouse Liver Inflammation. Hepatology. 2021;74:1560-1577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 67. | Luo CJ, Zhang FJ, Zhang L, Geng YQ, Li QG, Hong Q, Fu B, Zhu F, Cui SY, Feng Z, Sun XF, Chen XM. Mesenchymal stem cells ameliorate sepsis-associated acute kidney injury in mice. Shock. 2014;41:123-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 68. | Krasnodembskaya A, Samarani G, Song Y, Zhuo H, Su X, Lee JW, Gupta N, Petrini M, Matthay MA. Human mesenchymal stem cells reduce mortality and bacteremia in gram-negative sepsis in mice in part by enhancing the phagocytic activity of blood monocytes. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1003-L1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 69. | Wang L, Li Y, Xu M, Deng Z, Zhao Y, Yang M, Liu Y, Yuan R, Sun Y, Zhang H, Wang H, Qian Z, Kang H. Regulation of Inflammatory Cytokine Storms by Mesenchymal Stem Cells. Front Immunol. 2021;12:726909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |