Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4249
Peer-review started: October 2, 2021
First decision: October 22, 2021
Revised: October 29, 2021
Accepted: March 15, 2022
Article in press: March 15, 2022
Published online: May 6, 2022
Processing time: 210 Days and 1.4 Hours
The coexistence of meningioma and other intracranial primary benign tumors is rare, especially in non-neurofibromatosis type 2, and there is limited guidance for the management of such patients. Here, we report a series of 5 patients with concomitant meningioma and other intracranial benign tumors, including subependymoma and pituitary adenoma.
Five non-neurofibromatosis type 2 patients with simultaneous occurrence of meningioma and other intracranial benign tumors were retrospectively reviewed. The patients had no history of previous irradiation. The clinical features, pre- and postoperative imaging, surgical procedure and pathological findings were extracted from electronic medical records. There were 4 female patients (80%) and 1 male patient (20%). The mean age was 42.8 years (range: 29-52 years). The coexisting tumors included subependymoma in 1 case (20%) and pituitary adenoma in 4 cases (80%). The most common clinical symptom was headache (3/5, 60%). Four patients (80%) underwent craniotomy. One patient (20%) underwent transsphenoidal surgery followed by transcranial operation. All tumor diagnoses were confirmed by histopathological examination. The mean follow-up was 38.8 mo (range: 23-96 mo), and all 5 patients were in a stable condition at the last follow-up.
The simultaneous occurrence of meningioma and other intracranial benign tumors is a rare clinical event. Histological examination is necessary for the accurate diagnosis. Neurosurgeons should select the appropriate surgical strategy according to the clinical features of each patient, which may provide a more favorable prognosis for individual patients.
Core Tip: The simultaneous occurrence of meningioma and other intracranial primary benign tumors is rare, especially in non-neurofibromatosis type 2, and there is limited guidance for the management of such patients. In this study, we report a series of 5 patients with coexistence of meningioma and other intracranial benign tumors, including subependymoma and pituitary adenoma.
- Citation: Hu TH, Wang R, Wang HY, Song YF, Yu JH, Wang ZX, Duan YZ, Liu T, Han S. Coexistence of meningioma and other intracranial benign tumors in non-neurofibromatosis type 2 patients: A case report and review of literature. World J Clin Cases 2022; 10(13): 4249-4263
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4249.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4249
Meningioma is the most frequent intracranial benign tumor, accounting for 37.1% of tumors overall[1]. However, the concomitant occurrence of meningioma and other intracranial benign tumors is extremely rare[2]. Only a few coexisting meningioma and other intracranial benign tumor cases have been reported in previous publications, such as pituitary adenoma[3,4], craniopharyngioma[5] and vestibular schwannoma[6-9]. It is well-recognized that the occurrence of multiple nervous system tumors in the same patient is a characteristic of neurofibromatosis type 2 (NF2)[8]. Patients are diagnosed with NF2 when they meet the Manchester criteria (Supplementary Table 1)[10,11]. However, clinical and pathological features of non-NF2 patients with collision-tumors remain unclear. Here we report the clinical presentation, radiological features, surgical management and outcomes in our series of 5 non-NF2 patients with concomitant meningioma and other intracranial benign tumors, including one subependymoma, which was reported for the first time, and four pituitary adenomas. In addition, we also reviewed the available literature.
Case 1: A 45-year-old female patient presented with a sudden night epileptic seizure 10 d prior.
Case 2: A 40-year-old female patient presented with dizziness and headache for 1 mo.
Case 3: A 48-year-old male patient presented with frontal and bilateral temporal headache for 1 mo.
Case 4: A 52-year-old female patient presented with left progressive blurred vision for 6 mo and headaches for 2 mo.
Case 5: A 29-year-old female patient presented with menstrual disorder for 2 years and intermittent headache for 3 mo.
Case 1: The patient suffered a sudden epileptic seizure 10 d ago at night, which lasted for a few minutes.
Case 2: The patient had dizziness and headaches for 1 mo.
Case 3: The patient had a headache for 1 mo. The headache took place at night and affected his sleep.
Case 4: The patient had progressive vision loss in the left eye for 6 mo. She suffered from headache from 2 mo.
Case 5: The patient had menstrual disorder for 2 years. She developed amenorrhea and intermittent headaches 3 mo ago.
All patients had no history of specific illnesses.
All patients had no special personal and family history.
Case 1, 2, 3 and 5: Neurological examination of the patient found no positive signs.
Case 4: There was only slight sensation in her left eye when she was admitted.
Case 1, 3 and 4: Preoperative endocrine examination showed no abnormal changes.
Case 2: Endocrine examination indicated that prolactin levels were moderately elevated (61.19 ng/mL).
Case 5: Preoperative endocrine examination showed that prolactin levels were slightly elevated (59.17 ng/mL).
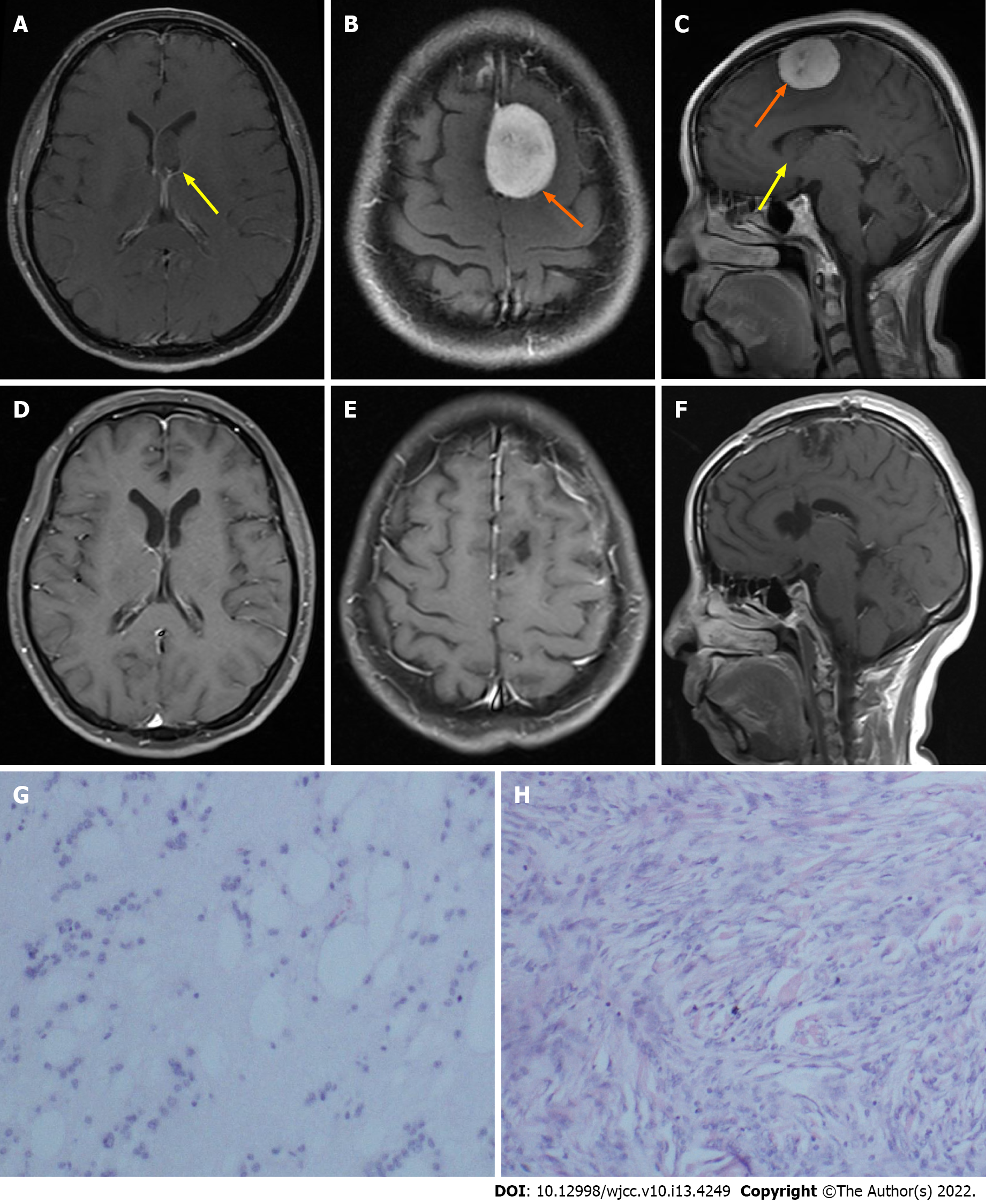
Case 1: Brain computed tomography at the local hospital suggested an intracranial space-occupying lesion in the left parietal lobe. Brain contrast-enhanced magnetic resonance imaging (MRI) showed a well-circumscribed mass (3.6 cm × 2.7 cm × 2.7 cm) in the left parietal parafalcine and a mass (1.9 cm × 1.2 cm × 1.1 cm) in the left lateral ventricle.
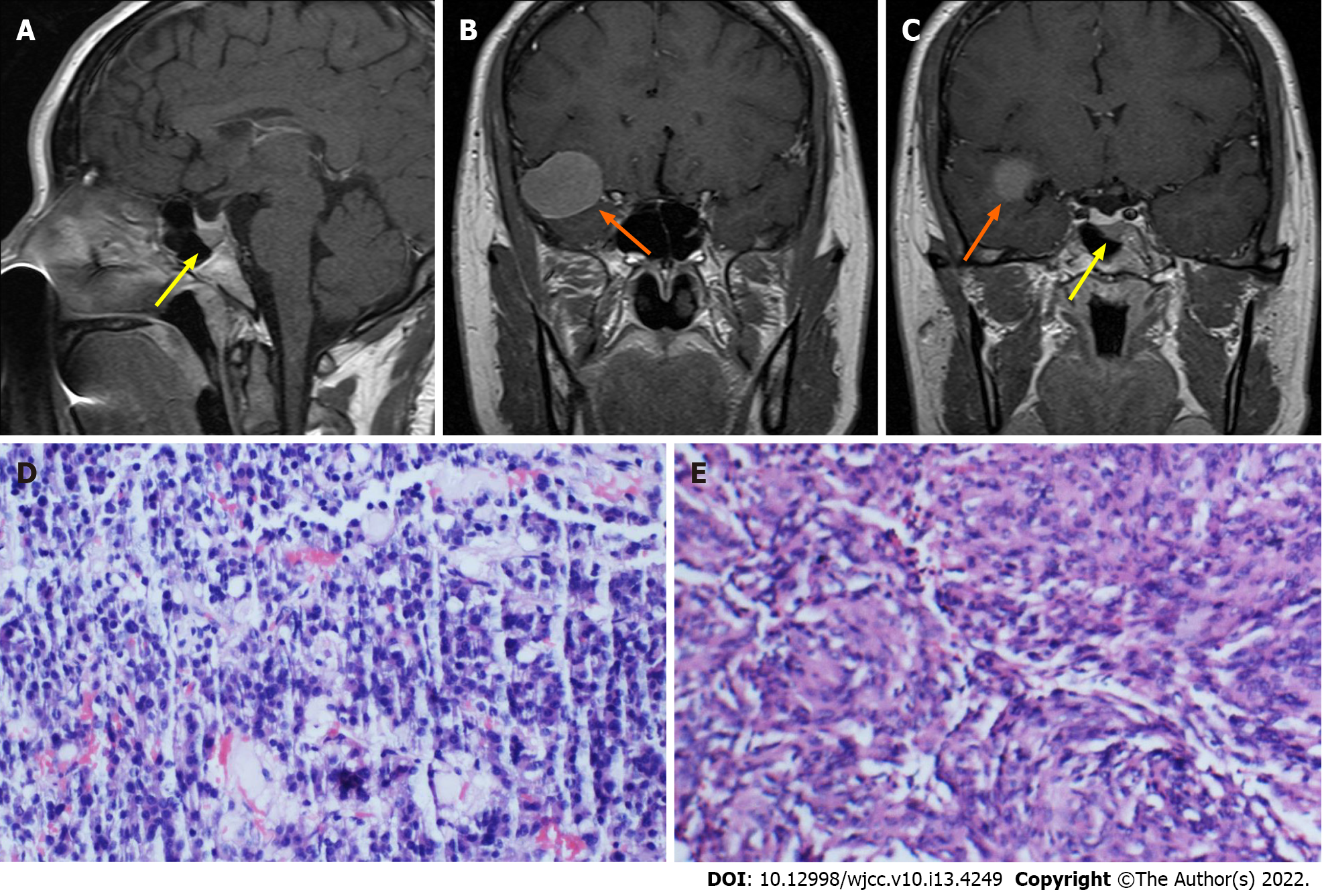
Case 2: MRI showed a mass (3.0 cm × 2.3 cm × 2.5 cm) in the right middle cranial fossa and a mass (0.5 cm × 0.5 cm × 0.5 cm) in the sellar region.
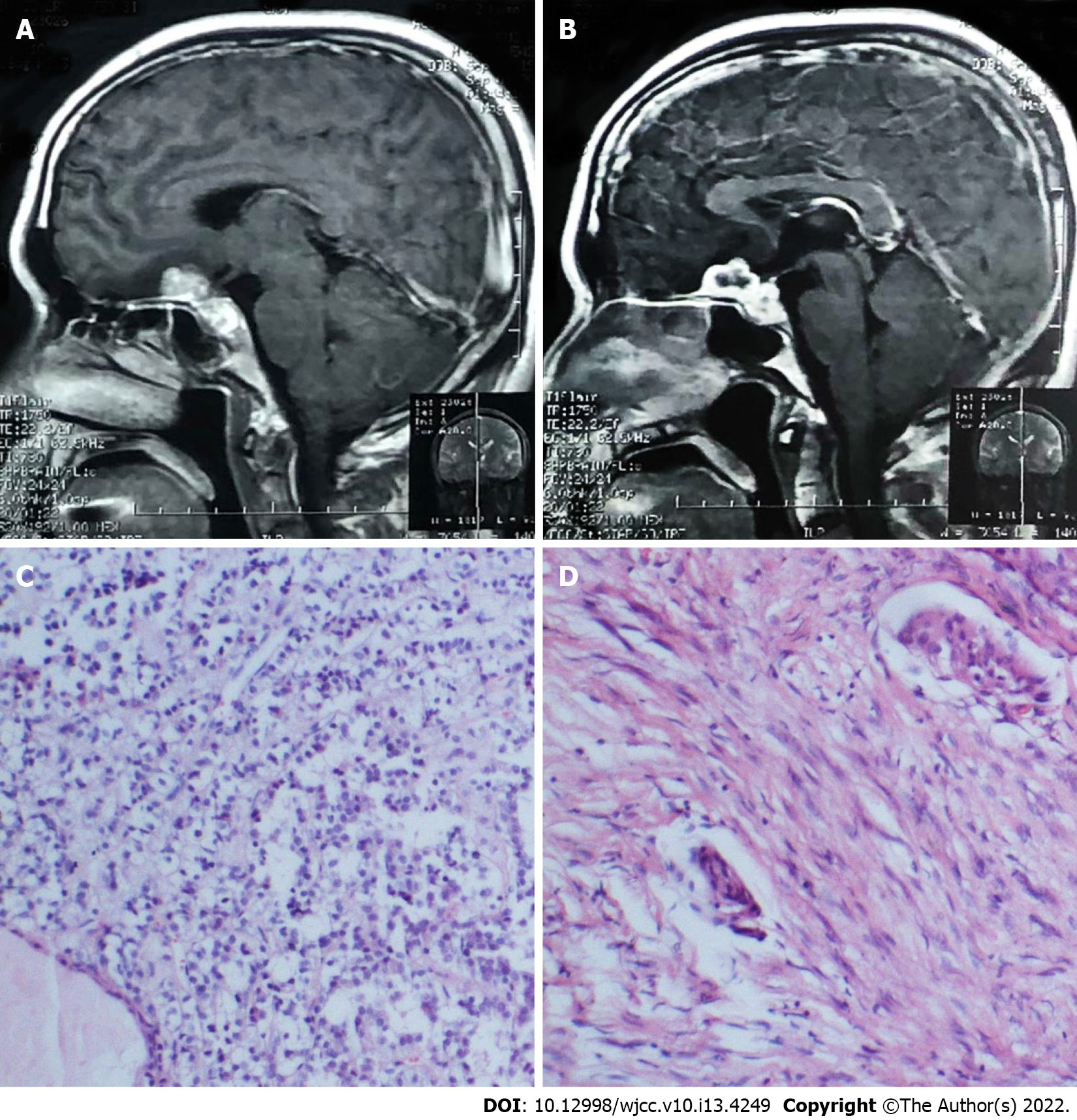
Case 3: Brain MRI showed lesions located in the planum sphenoidale and sellar regions.
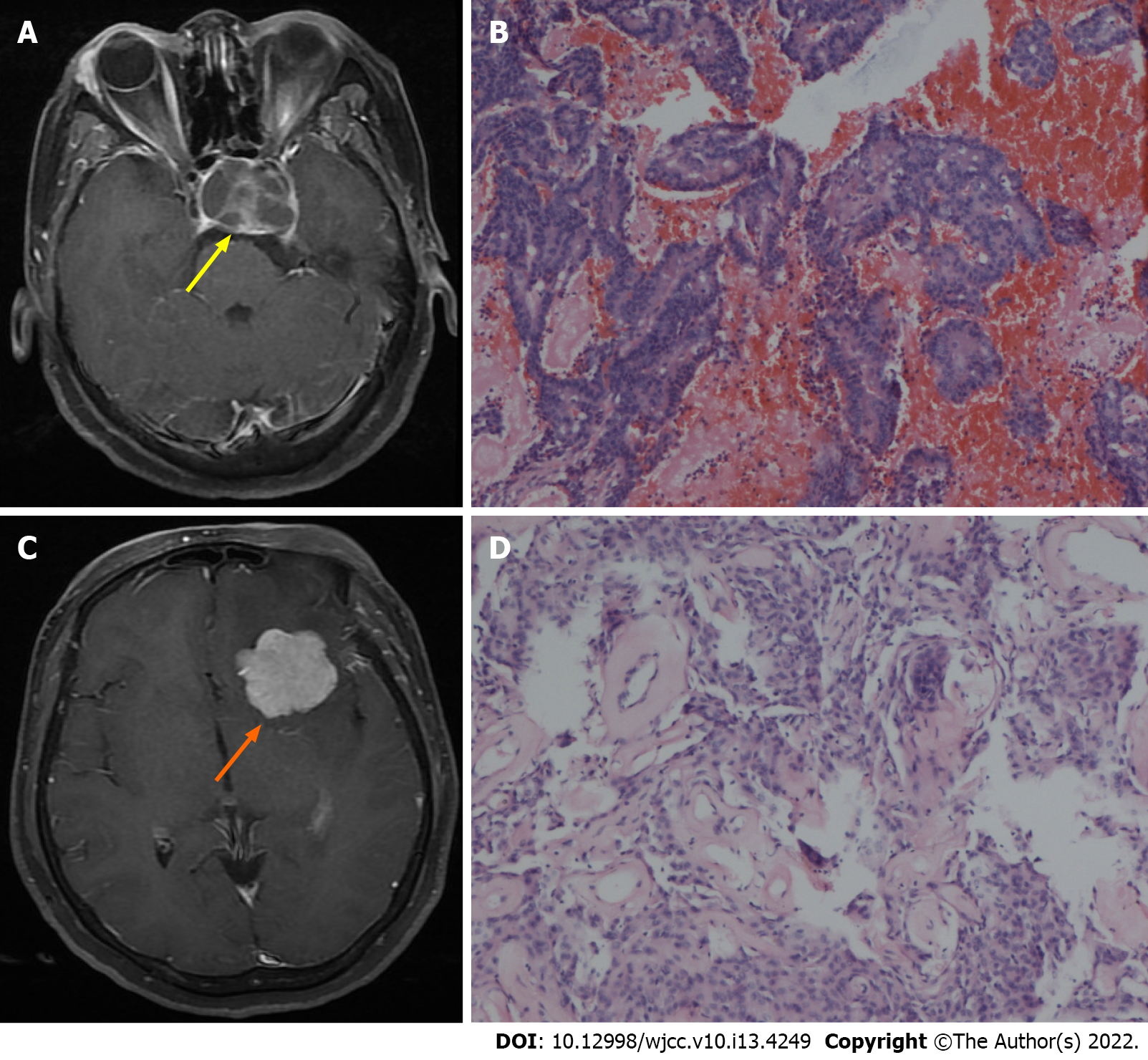
Case 4: There was a well-circumscribed mass (3.5 cm × 3.2 cm × 2.7 cm) surrounded with brain edema in the left sphenoid ridge and a mass (2.8 cm × 2.5 cm × 2.5 cm) encasing the internal carotid artery in the sellar and suprasellar regions, as determined by radiological examination.
Case 5: MRI showed a mass (5.7 cm × 3.3 cm) encasing the internal carotid artery located in the left petroclival region and a mass (maximum diameter 1.0 cm) located in the sellar region.
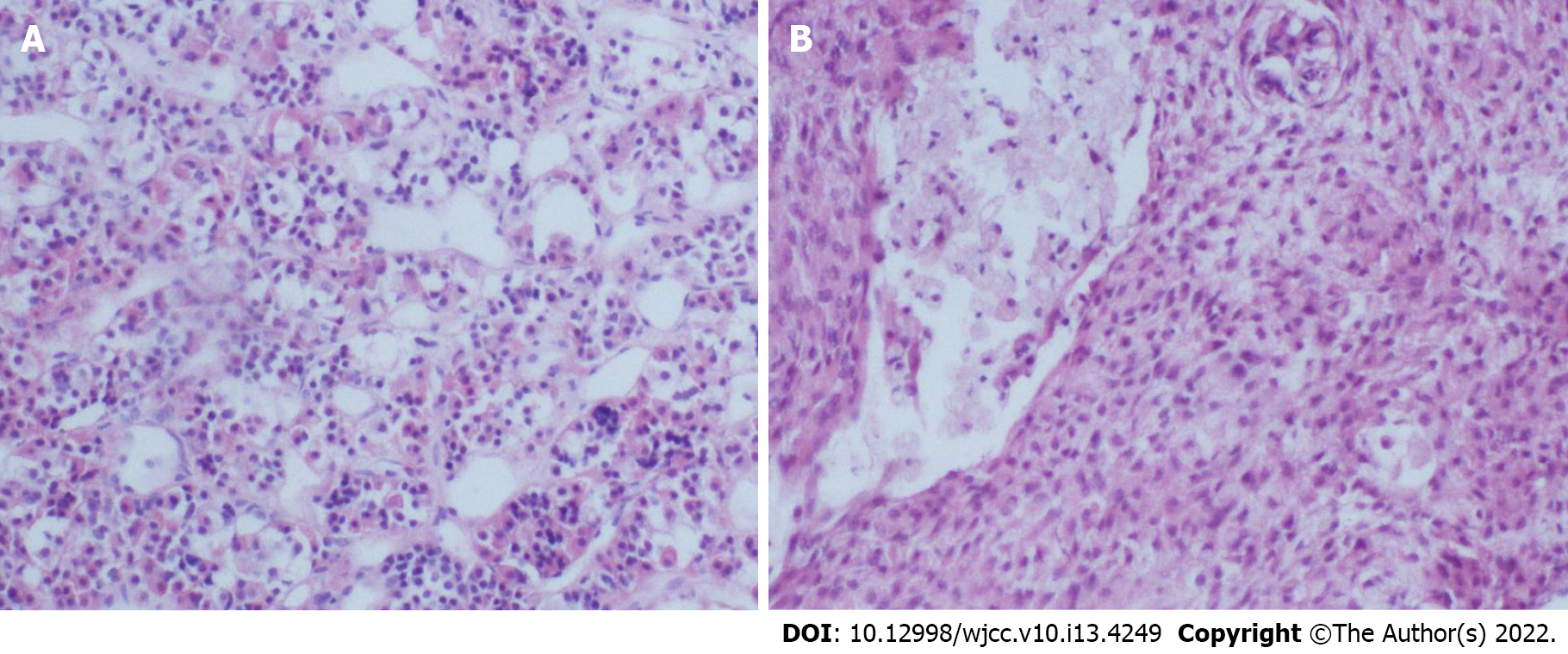
Case 1: Postoperative histopathological examination showed meningioma and subependymoma (Figure 1).
Case 2: Histopathological examination showed a meningioma and non-functioning pituitary adenoma (Figure 2).
Case 3: Histopathological examination showed a meningioma and non-functioning pituitary adenoma (Figure 3).
Case 4: Histopathological examination showed a meningioma and non-functioning pituitary adenoma (Figure 4).
Case 5: Postoperative histopathological examination showed a meningioma and non-functioning pituitary adenoma (Figure 5).
Case 1: After this discovery, she underwent craniotomy, and the two neoplasms were removed in one session.
Case 2: First, endonasal transsphenoidal surgery was performed for resection of the intrasellar mass. Then, the right middle cranial fossa mass was removed (Simpson grade II) by transcranial surgery.
Case 3: The patient underwent transcranial resection for the two tumors through the right transpterional approach.
Case 4: A single transcranial procedure was performed for removal of the sphenoid ridge mass (Simpson grade II) and sellar region mass. The pituitary tumor underwent subtotal resection.
Case 5: The patient underwent craniotomy for the two tumors. The petroclival tumor was hard in consistency and rich in blood supply with internal carotid artery encasement. The tumor was also close to cranial nerves II-VI and compressed the brain stem. A subtotal resection was performed (Simpson grade IV) for the petroclival tumor. Then the intrasellar tumor was removed.
Between January 2011 and January 2019, 2922 consecutive patients were diagnosed with meningioma in our institution. There were 5 meningioma patients (0.17%) with different intracranial benign tumors, and none of them were diagnosed with NF2. They had no history of previous irradiation. Clinical data were obtained and analyzed through retrospective medical history reviews, neuroimaging information, histopathological examination and follow-up. This retrospective study was approved by the institutional review board. Written consent was obtained from each patient for the use of their clinical data for research.
There were 4 female patients (80%) and 1 male patient (20%) with concomitant meningioma and other intracranial benign tumors. The mean age was 42.8 years (range: 29-79 years). The coexisting tumors included subependymoma in 1 case (20%) and pituitary adenoma in 4 cases (80%, four non-functional pituitary adenomas). The mean follow-up time was 38.8 mo (range: 23-96 mo). The clinical information of these cases is summarized in Table 1.
| Case No. | Sex/age | Symptom | Meningioma location | WHO grade of meningioma | Second primary tumor | Operation | Surgical approach | Simpson grade | Recurrence | Postoperative treatment | Follow-up, mo |
| 1 | F/45 | Seizure | Left frontal parafalcine | I | Subependymoma | Synchronous | TC | II | None | None | 27 |
| 2 | F/40 | Headache | Right middle cranial fossa | I | Pituitary adenoma | Synchronous | TS + TC | II | None | None | 24 |
| 3 | M/48 | Headache | Tuberculum sellae | I | Pituitary adenoma | Synchronous | TC | II | None | None | 23 |
| 4 | F/52 | Blurred vision, headache | Left sphenoid ridge | I | Pituitary adenoma | Synchronous | TC | II | Growth of residual pituitary tumor after 4 mo | Radiotherapy | 24 |
| 5 | F/29 | Amenorrhea and headache | Left petroclival | I | Pituitary adenoma | Synchronous | TC | IV | None | None | 96 |
Case 1: The patient was followed up for 27 mo with no evidence of recurrent disease.
Case 2: The patient was followed up for 24 mo, and she was in good health.
Case 3: He was treated by hormone replacement therapy because of postoperative hypopituitarism. The patient was followed up for 23 mo. He took LT4 regularly and is currently in good health.
Case 4: Four months after the surgery, MRI reexamination revealed the growth of residual pituitary tumor. Subsequently, she underwent radiotherapy. The patient was in good health at the 24-mo follow-up.
Case 5: As of this writing, the patient had been in stable condition for 96 mo.
The coexistence of meningioma and other intracranial benign tumors is a rare phenomenon that nevertheless deserves our attention. The most frequent coexistence of simultaneous benign tumors is pituitary adenoma with meningioma and schwannoma with meningioma[12]. The co-occurrence of meningioma and schwannoma is more likely to occur in patients with NF2, which has been well described previously. However, the clinical characteristics of non-NF2 patients with coexisting tumors are largely unknown.
To the best of our knowledge, we present the first case of concomitant meningioma and intracranial subependymoma. Subependymomas are rare, benign, slow-growing tumors and represent only 0.2% to 0.7% of intracranial tumors[13-16]. These tumors most often arise in the fourth ventricle (50%-60%) and the lateral ventricles (30%-40%)[14,15]. Most patients present with hydrocephalus as a consequence of ventricular obstruction or less commonly focal neurological dysfunction and seizures caused by mass effects[14,17]. The main purpose of surgery is to maximize the removal of the tumor[18]. In the published case series, there were satisfactory postoperative mortality and morbidity rates from supratentorial subependymomas[13-18]. In our case 1, resection of the left parietal meningioma and supratentorial subependymoma was accomplished via a single procedure. In a recent long-term outcome study of subependymoma, no patients exhibited a deterioration of performance status or tumor recurrence at medium to long-term follow-up[16]. As with the single subependymoma, the patient in our case also had a good prognosis after operation, and there was no evidence of recurrence at the last follow-up. Although the extremely rare coexistence of meningioma and subependymoma in our case may be an incidental event, the intrinsic relationship of these two tumors might require future investigation.
Until now, our understanding of the coexistence of meningioma and pituitary adenoma is based on occasional case reports[2-4,12,19-54]. We reviewed all the reported cases that were available to us, and the information was summarized in Table 2. The mean age of patients was 54.6 years (range: 26-82 years), and there were 39 women and 14 men among the published cases (female:male = 2.79:1). Our 4 cases also showed a female tendency (female:male = 3:1), and the mean age was 42.8 years (range: 29-52 years). A preference for parasellar, suprasellar and sphenoid ridge localization was found with 27 reported cases (50.94%)[2-4,20-23,25,26,30,32-37,42,47-49,51,54]. Consistently, the meningioma of our cases was in the petroclival, sellar and sphenoid wing regions.
| No. | Ref. | Age | Sex | Symptom | Size of meningioma, cm | Type of meningioma | Location of meningioma | Type of pituitary adenoma | History of radiation | Treatment | Postoperative therapy |
| 1 | Love et al[19], 1955 | 65 | F | NA | NA | Meningothelial | Sylvian fissure | Non-secreting | NA | NA | NA |
| 2 | O’Connell[20], 1961 | 47 | F | Failing vision in right eye | NA | Meningothelial | Tuberculum sellae | Non-secreting | No | TC (MN + PA) | No |
| 3 | Kitamura et al[21], 1965 | 66 | F | Headache and impaired vision | NA | Meningothelial | Sphenoid wing | Non-secreting | No | TC (MN + PA) | No |
| 4 | Probst[22], 1971 | 48 | F | NA | NA | Meningothelial | Suprasellar | ACTH-producing | NA | NA | NA |
| 5 | Brennan et al[23], 1977 | 36 | M | Blurred vision of right eye | NA | Transitional | Sphenoid wing | Non-secreting | No | TC (MN + PA) | Radiotherapy |
| 6 | Bunick et al[24], 1978 | 57 | M | Intermittent right-sided headache, acromegaly | 6 × 6 | Fibrous | Right frontal lobe | GH-producing | No | TC (MN + PA) | No |
| 7 | Hainer et al[25], 1978 | 72 | M | NA | NA | NA | Suprasellar | GH-producing | NA | NA | NA |
| 8 | Deen et al[26], 1981 | 75 | F | Chronic dementia, only had postmortem examination | NA | NA | Right sphenoid ridge | NA | NA | Autopsy | No |
| 9 | Hyodo et al[27], 1982 | 52 | F | Acromegaly, diabetes mellitus, headache, right hemiparesis and right impaired visual acuity | NA | Fibrous and meningothelial | Left parietal region | GH-producing | No | TC (MN) + TS (PA, 4 mo later) | No |
| 10 | Irsy et al[28], 1985 | 59 | F | NA | NA | NA | Centro-parietal | GH-producing | NA | NA | NA |
| 11 | Ohata[29], 1985 | 50 | F | Acromegaly, visual disturbance, headache, vomiting and a floating sensation | 4 × 4 × 4 | Transitional | Falcotentorial junction | GH-producing | No | TS (PA) + TC (MN, 2 mo later) | Bromocriptine, acetylcortisone, desiccated thyroid |
| 12 | Yamada et al[30], 1986 | 52 | F | Headache, disturbance of visual acuity and galactorrhea | NA | Meningothelial | Sphenoid ridge (parasellar) | Non-secreting | No | TC (PA + MN) | Bromocriptine |
| 13 | Honegger et al[31], 1989 | 37 | F | Marked alopecia | 2.5 | Meningothelial | Right temporal pole | PRL-producing | No | TC (MN) | Bromocriptine |
| 14 | Honegger et al[31], 1989 | 49 | F | Acromegaly persisted after radiotherapy | 3 | Meningothelial | Left parasagittal | GH-producing | Yes | TS (PA) + TC (MN, 2 mo later) | No |
| 15 | Honegger et al[31], 1989 | 74 | M | Recurrence of pituitary adenoma | 1.5 | NA | Left parietal | Non-secreting | Yes | TS (PA) + TC (PA, 5 yr later) | No |
| 16 | Zentner et al[32], 1989 | 46 | M | CT demonstration of a large intrasellar and suprasellar space-occupying lesion | 1.5 | Transitional | Planum sphenoidale | PRL-producing | No | TC (PA + MN) | No |
| 17 | Zentner et al[32], 1989 | 63 | F | Ataxia | NA | Meningothelial | Sphenoid wing | Non-secreting | No | TS (PA) + TC (MN, 1 mo later) | No |
| 18 | Zentner et al[32], 1989 | 61 | F | Frontal headache | NA | Meningothelial | Infradiaphragmatic | Non-secreting | No | TS + TS (PA + MN) + TC (PA, 1 d later) | No |
| 19 | Partington et al[2], 1989 | 26 | M | Evaluation of persistent symptom of Cushing’s disease; left temporal hemianopsia (10 yr later) | NA | Meningothelial | Tuberculum sellae | ACTH-secreting | Yes | TS (PA) + TS (PA, 8 yr later) + TC (1 yr later) | No |
| 20 | Uno et al[33], 1991 | 70 | F | Headache and acromegaly | NA | Meningothelial | Sphenoid ridge | GH-producing | No | TC (MN + PA) | No |
| 21 | Cannavo et al[34], 1993 | 47 | F | Acromegaly, diminished visual acuity, weakness and headache | NA | Meningothelial | Right latero- and retrosellar | GH-producing | No | TC (PA + MN) | No |
| 22 | Abs et al[35], 1993 | 47 | F | Aphasia and temporary right-sided hemiparesis | NA | Meningothelial | Tuberculum sellae | PRL-producing | No | TC (MN) + TS (PA, 3 mo later) | No |
| 23 | Abs et al[35], 1993 | 61 | F | A toxic thyroid adenoma, Cushing’s disease | NA | Meningothelial; transitional | Frontal convexity; occipital convexity | ACTH-producing | No | TC (MN) | No |
| 24 | Abs et al[35], 1993 | 45 | F | Amenorrhea and galactorrhea | NA | NA | Temporal fossa | PRL-producing | No | No surgery | No |
| 25 | Abs et al[35], 1993 | 45 | F | A toxic multinodular goiter and suspicion of acromegaly | NA | NA | Parietal convexity | GH-producing | No | TC (Schwannoma) + TS (PA) | No |
| 26 | Abs et al[35], 1993 | 82 | F | Bitemporal hemianopsia | NA | NA | Sphenoid ridge; parasellar | Non-secreting | No | TS (PA) | No |
| 27 | Abs et al[35], 1993 | 61 | F | Headache | 2.6 | NA | Choroid plexus (right lateral ventricle) | PRL-producing | No | No surgery | No |
| 28 | Abs et al[35], 1993 | 51 | F | Unilateral palpebral edema and exophthalmos | NA | NA | Sphenoid wing | Non-secreting | No | Embolization (MN) | No |
| 29 | Gorge et al[36], 1993 | 53 | M | Progressive impotence, decrease of libido and left-sided defective vision | NA | NA | Para- and suprasellar | PRL-producing | NA | TC (PA + MN) | No |
| 30 | Laun et al[37], 1993 | 61 | F | Deteriorating vision of the left eye and bitemporal hemianopia | NA | Meningothelial | Tuberculum sellae | Non-secreting | NA | NA | No |
| 31 | Mathuriya et al[38], 2000 | 58 | F | Acromegaly | NA | NA | Parasagittal | GH-producing | No | TC (PA + MN) | Radiotherapy |
| 32 | Maiuri et al[39], 2005 | 49 | M | Acromegaly, right hemiparesis | 3.5 | NA | NA | GH-producing | No | TC (MN) + TS (PA, 3 mo later) | No |
| 33 | Maiuri et al[39], 2005 | 63 | F | Left hemiparesis, bitemporal visual field defect | 4 | NA | NA | Non-secreting | No | TC (MN) + TS (PA, 1 mo later) | No |
| 34 | Curto et al[40], 2007 | 61 | F | Acromegaly and visual field impairment | 3.5 | NA | Right frontal | GH-producing | No | NA | No |
| 35 | da Costa et al[41], 2007 | 45 | M | Generalized malaise and weight loss for a few months. Progressive headache, vomiting and gait ataxia for 2 wk | NA | NA | The fourth ventricle | PRL-producing | No | TC (MN) | Bromocriptine |
| 36 | Prevedello et al[42], 2007 | 52 | F | Unremitting headache and profound right temporal visual field loss | 1.0 × 0.6 | NA | Right planum sphenoidale | Non-secreting | No | TS (PA + MN) | No |
| 37 | Basu et al[43], 2010 | 39 | M | Frontal headaches and erectile dysfunction with loss of libido | NA | Meningothelial | Left cavernous sinus | PRL-secreting | No | TC (MN) | No |
| 38 | Furtado et al[12], 2010 | 53 | M | Headache for 2 yr and altered sensorium for 2 wk | 6.0 × 5.5 × 5.0 | Meningothelial | Parasagittal | Non-secreting | No | TS (PA) + TC (MN) | No |
| 39 | Guaraldi et al[44], 2012 | 46 | F | Acromegaly | 2.3 × 2.6 × 2.6 | NA | Left parasagittal | GH-producing | No | TS (PA) + TC (MN, 10 yr later) + TS (Recurrence PA, 8 mo later) | No |
| 40 | Ramirez et al[45], 2012 | 61 | F | Headache and acute hydrocephalus for 2 mo | 1.0 × 1.0 | Psammomatous | Left anterior clinoidal | Silent corticotroph adenoma subtype II | No | TC (MN) + TS (PA, 1 mo later) | No |
| 41 | Masoodi et al[46], 2013 | 65 | M | Acromegaly | NA | NA | Frontal parasagittal | GH-producing | No | TS (PA) + TC (MN) | No |
| 42 | Mahvash et al[47], 2014 | 36 | F | Frontal headache and extended right visual field loss | 2.0 × 2.0 × 2.5 | NA | Tuberculum sellae | Non-secreting | No | TS (PA + MN) | No |
| 43 | Karsy et al[48], 2014 | 70 | F | Altered mental status, mutism and incontinence | 3.6 × 4.1 × 4.5 | Fibroepithelial | Tuberculum sellae | Non-secreting | No | TS (PA + MN) | No |
| 44 | Ruiz-Juretschke et al[49], 2015 | 61 | M | Progressive visual loss and bitemporal hemianopia for 6 mo | 2.0 × 2.2 × 2.2 | Meningothelial | Tuberculum sellae and planum sphenoidale | Non-secreting | No | TS (PA + MN) + TS (residual tumor) | No |
| 45 | Ben Nsir et al[50], 2016 | 61 | F | Loss of vision for 3 yr and occasional episode headache | NA | NA | Foramen magnum | Non-secreting | No | TC (MN) + [TS + TC] (PA) | No |
| 46 | Lim et al[51], 2016 | 65 | F | Visual symptoms and an episode of self-resolving vertigo | 1.5 × 1.3 × 1.3; 0.6 × 0.5 | NA | Tuberculum sellae and olfactory groove | Non-secreting | No | TS (PA + MN) | No |
| 47 | Amirjamshidi et al[3], 2017 | 37 | F | Oligomenorrhea for 8 mo, headache, diplopia and progressive visual impairment | 3.0 × 2.5 × 2.0 | NA | Suprasellar | PRL-secreting | No | TC (MN) | No |
| 48 | Amirjamshidi et al[3], 2017 | 42 | M | Acromegaly, visual acuity decreased and bitemporal hemianopia | 3.0 × 3.0 × 2.0 | NA | Suprasellar | Non-secreting | No | TS + TC (coexisting tumors) | No |
| 49 | Herrero-Ruiz et al[52], 2017 | 35 | F | Chagas disease and acromegaly | 2.0 × 2.0 × 1.2 | NA | Left parietal parasagittal | GH-producing | No | TS (Only PA) | No |
| 50 | Kumaria et al[53], 2017 | 46 | F | Lethargy, sleep disturbance, personality change and mild daily right-sided headache for 18 mo | NA | Transitional; Atypical | Right frontal and left temporal regions | Mammosomatotroph cell adenoma (PRL- and GH-secreting) | Yes | TC (MN) + TC (MN) + TS (PA) | No |
| 51 | Zhao et al[54], 2017 | 58 | F | Acromegaly and headache | NA | NA | Sellar | GH-producing | No | TS (PA) + TC (MN, 3 mo later) | No |
| 52 | Zhao et al[54], 2017 | 58 | F | Acromegaly | NA | NA | Sellar | GH-producing | No | TS (PA) + TC (MN, 4 mo later) | No |
| 53 | de Vries et al[4], 2019 | 75 | F | Depression, fatigue and unintended weight loss | NA | Meningothelial | Suprasellar | Non-secreting | No | TS (PA + MN) | No |
The most common type of pituitary adenoma with coexisting meningioma among reported cases was a non-secreting tumor (21/53, 39.62%) followed by growth hormone-producing tumor (17/53, 32.08%). In this paper, all 4 cases were non-functioning pituitary adenoma, representing the most common type. Although prolactinomas are the most frequent pituitary adenomas in general, the higher prevalence of acromegaly in patients with coexisting meningioma has led some authors to propose an association between growth hormone-producing adenomas and meningioma[29,34,40,42,49]. They suggested that persistently elevated growth hormone might stimulate arachnoid cap cells to play a role in the development of meningioma[24,39,51]. A recent study suggested that patients with acromegaly were at increased risk of meningioma[55].
Some researchers have tried to explore the association between meningiomas and pituitary tumors. The early reports suggested that this phenomenon was related to history of irradiation for pituitary tumors[2]. However, many cases with no history of radiotherapy were reported as well[24,27,29,48,49], including our cases. Therefore, Curto et al[40] suggested that the coexistence of meningioma and pituitary adenoma was a coincidental phenomenon. However, there was a higher proportion of involvement of chromosome 14 and 22 in estrogen receptor positive de novo meningiomas[56]. Similar genetic changes shared by two unrelated tumors found on the same chromosome may explain their coexistence[12].
Moreover, due to the indolence of benign tumors, a significant portion of this coexisting tumor population may remain undiagnosed[12]. MRI is useful for the diagnosis of the coexistence of two intracranial tumors but has limited significance for adjacent pituitary adenoma and meningioma[4]. Histological results are necessary for diagnosis because other preoperative findings cannot support accurate diagnosis[54]. For example, some reported cases were coexisting sellar meningioma and pituitary adenoma[4,42,47-49,54]. Because of the close location of the two tumors, it is difficult to produce an accurate diagnosis by preoperative imaging, as in our case 3. It deserves special attention because the two different types of tumors were not definitely diagnosed before surgery but later when the pathologist’s results were obtained. The patient in case 3 developed hypopituitarism after the operation, and this complication was also reported in other similar cases[47,49].
Traditionally, the treatment of these two coexisting tumors required one craniotomy[30,31,33] or two separate operations using two different approaches[2,32,35,39]. Prevedello et al[42] performed a single endoscopic expanded endonasal approach in patients with coexisting tuberculum sellae meningiomas and pituitary adenoma. In our opinion, surgical strategies should be decided according to the characteristics of the coexisting tumors (e.g., location, size and adjacent neurovascular structures) and the clinical features of individual patients (e.g., symptoms and systemic conditions). Like the single pituitary adenoma, the patient with coexisting meningioma and pituitary adenoma had a favorable prognosis using the retrospective case reports. Postoperative endocrine reexamination should be periodically monitored at the endocrinology outpatient department, especially in patients with postoperative hormone imbalance after surgery. However, as this study is a retrospective analysis and the case numbers are limited, we cannot draw strong conclusions.
The simultaneous occurrence of meningioma and other intracranial benign tumors is a rare clinical event, and histological examination is necessary for their accurate diagnosis. Neurosurgeons should select the appropriate surgical strategy according to the clinical features of individual patients, which may provide the patient with a more favorable prognosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Neurosurgery Society of Chinese Medical Association, Youth committee.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nuño JSZ, Mexico; Yelamanchi R, India S-Editor: Liu JH L-Editor: Filipodia P-Editor: Liu JH
| 1. | Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro Oncol. 2018;20:iv1-iv86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1156] [Cited by in RCA: 1563] [Article Influence: 260.5] [Reference Citation Analysis (0)] |
| 2. | Partington MD, Davis DH. Radiation-induced meningioma after treatment for pituitary adenoma: case report and literature review. Neurosurgery. 1990;26:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Amirjamshidi A, Mortazavi SA, Shirani M, Saeedinia S, Hanif H. 'Coexisting pituitary adenoma and suprasellar meningioma-a coincidence or causation effect: report of two cases and review of the literature'. J Surg Case Rep. 2017;2017:rjx039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | de Vries F, Lobatto DJ, Zamanipoor Najafabadi AH, Kleijwegt MC, Verstegen MJT, Schutte PJ, Biermasz NR, van Furth WR. Unexpected concomitant pituitary adenoma and suprasellar meningioma: a case report and review of the literature. Br J Neurosurg. 2019;1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Della Puppa A, Del Moro G, Tosatto L, Manara R, Orvieto E, Gardiman MP, Scienza R. Co-localisation of meningioma and craniopharyngioma mimicking a single skull base tumour in an elderly patient. J Neurooncol. 2011;102:167-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Chen AF, Samy RN, Gantz BJ. Cerebellopontine angle tumor composed of Schwann and meningeal proliferations. Arch Otolaryngol Head Neck Surg. 2001;127:1385-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Grauvogel J, Grauvogel TD, Taschner C, Baumgartner S, Maier W, Kaminsky J. A Rare Case of Radiologically Not Distinguishable Coexistent Meningioma and Vestibular Schwannoma in the Cerebellopontine Angle - Case Report and Literature Review. Case Rep Neurol. 2010;2:111-117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Bachir S, Shah S, Shapiro S, Koehler A, Mahammedi A, Samy RN, Zuccarello M, Schorry E, Sengupta S. Neurofibromatosis Type 2 (NF2) and the Implications for Vestibular Schwannoma and Meningioma Pathogenesis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 9. | Matyja E, Kunert P, Grajkowska W, Marchel A. Coexistence of meningioma and schwannoma in the same cerebellopontine angle in a patients with NF2. Folia Neuropathol. 2012;50:166-172. [PubMed] |
| 10. | Evans DG, Huson SM, Donnai D, Neary W, Blair V, Newton V, Harris R. A clinical study of type 2 neurofibromatosis. Q J Med. 1992;84:603-618. [PubMed] |
| 11. | Evans DG, King AT, Bowers NL, Tobi S, Wallace AJ, Perry M, Anup R, Lloyd SKL, Rutherford SA, Hammerbeck-Ward C, Pathmanaban ON, Stapleton E, Freeman SR, Kellett M, Halliday D, Parry A, Gair JJ, Axon P, Laitt R, Thomas O, Afridi S, Ferner RE, Harkness EF, Smith MJ; English Specialist NF2 Research Group. Identifying the deficiencies of current diagnostic criteria for neurofibromatosis 2 using databases of 2777 individuals with molecular testing. Genet Med. 2019;21:1525-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 12. | Furtado SV, Venkatesh PK, Ghosal N, Hegde AS. Coexisting intracranial tumors with pituitary adenomas: genetic association or coincidence? J Cancer Res Ther. 2010;6:221-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Bi Z, Ren X, Zhang J, Jia W. Clinical, radiological, and pathological features in 43 cases of intracranial subependymoma. J Neurosurg. 2015;122:49-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Jain A, Amin AG, Jain P, Burger P, Jallo GI, Lim M, Bettegowda C. Subependymoma: clinical features and surgical outcomes. Neurol Res. 2012;34:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Laghaei Farimani P, Fatehi M, Chaharyn BM, Akagami R. Large Subependymoma Inferior to the Cerebellopontine Angle With Significant Obstructive Hydrocephalus: A Case Report on an Extremely Rare Tumor. Cureus. 2021;13:e18686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Rincon-Torroella J, Rakovec M, Khalafallah AM, Liu A, Bettegowda A, Kut C, Rodriguez FJ, Weingart J, Luciano M, Olivi A, Jallo GI, Brem H, Mukherjee D, Lim M, Bettegowda C. Clinical features and surgical outcomes of intracranial and spinal cord subependymomas. J Neurosurg. 2022;1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Rushing EJ, Cooper PB, Quezado M, Begnami M, Crespo A, Smirniotopoulos JG, Ecklund J, Olsen C, Santi M. Subependymoma revisited: clinicopathological evaluation of 83 cases. J Neurooncol. 2007;85:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Kandenwein JA, Bostroem A, Feuss M, Pietsch T, Simon M. Surgical management of intracranial subependymomas. Acta Neurochir (Wien). 2011;153:1469-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | LOVE JG, BLACKBURN CM. Association of intracranial meningioma with pituitary adenoma; report of successfully treated case. Minn Med. 1955;38:335-336. [PubMed] |
| 20. | O'CONNELL JE. Intracranial meningiomata associated with other tumours involving the central nervous system. Br J Surg. 1961;48:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Kitamura K, Terao H, Kamano S, Nakamura N, Hayakawa I, Ishijima T, Sano K. [Primary Multiple Brain Tumors]. No To Shinkei. 1965;17:109-117. [PubMed] |
| 22. | Probst A. [Combined occurrence of Cushing-syndrome, hypophyseal adenoma and suprasellar meningeoma. Case report]. Zentralbl Neurochir. 1971;32:75-82. [PubMed] |
| 23. | Brennan TG Jr, Rao CV, Robinson W, Itani A. Case report. Tandem lesions: chromophobe adenoma and meningioma. J Comput Assist Tomogr. 1977;1:517-520. [PubMed] |
| 24. | Bunick EM, Mills LC, Rose LI. Association of acromegaly and meningiomas. JAMA. 1978;240:1267-1268. [PubMed] |
| 25. | Hainer V, Krejcík L, Pelikán J, Tvaroh F, Urbánek J. [Meningioma in contact with eosinophilic adenoma in a patient with acromegaly (author's transl)]. Cas Lek Cesk. 1978;117:829-831. [PubMed] |
| 26. | Deen HG Jr, Laws ER Jr. Multiple primary brain tumors of different cell types. Neurosurgery. 1981;8:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Hyodo A, Nose T, Maki Y, Enomoto T. [Pituitary adenoma and meningioma in the same patient (author's transl)]. Neurochirurgia (Stuttg). 1982;25:66-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 28. | Irsy G, Góth M, Slovik F, Bálint K, Szabolcs I, Pásztor E, Szilágyi G. Growth hormone producing pituitary adenoma and meningioma. Zentralbl Neurochir. 1985;46:337-343. [PubMed] |
| 29. | Ohata K. [Simultaneous occurrence of a pituitary adenoma and a falcotentorial junction meningioma. Case report]. Neurol Med Chir (Tokyo). 1985;25:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Yamada K, Hatayama T, Ohta M, Sakoda K, Uozumi T. Coincidental pituitary adenoma and parasellar meningioma: case report. Neurosurgery. 1986;19:267-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Honegger J, Buchfelder M, Schrell U, Adams EF, Fahlbusch R. The coexistence of pituitary adenomas and meningiomas: three case reports and a review of the literature. Br J Neurosurg. 1989;3:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Zentner J, Gilsbach J. Pituitary adenoma and meningioma in the same patient. Report of three cases. Eur Arch Psychiatry Neurol Sci. 1989;238:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Uno M, Ohshima T, Matsumoto K, Sano T. [A case report of adjacent tumor of sphenoid ridge meningioma and GH producing pituitary adenoma]. No Shinkei Geka. 1991;19:583-587. [PubMed] |
| 34. | Cannavò S, Curtò L, Fazio R, Paterniti S, Blandino A, Marafioti T, Trimarchi F. Coexistence of growth hormone-secreting pituitary adenoma and intracranial meningioma: a case report and review of the literature. J Endocrinol Invest. 1993;16:703-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Abs R, Parizel PM, Willems PJ, Van de Kelft E, Verlooy J, Mahler C, Verhelst J, Van Marck E, Martin JJ. The association of meningioma and pituitary adenoma: report of seven cases and review of the literature. Eur Neurol. 1993;33:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Görge HH, Pöll W, Gers B. [Para- and suprasellar meningioma coincident with a hormonally active intrasellar hypophyseal adenoma--case report]. Zentralbl Neurochir. 1993;54:190-196. [PubMed] |
| 37. | Laun A, Lenzen J, Hildebrandt G, Schachenmayr W. [Tuberculum sellae meningioma and hypophyseal adenoma in a woman]. Zentralbl Neurochir. 1993;54:119-124. [PubMed] |
| 38. | Mathuriya SN, Vasishta RK, Dash RJ, Kak VK. Pituitary adenoma and parasagittal meningioma: an unusual association. Neurol India. 2000;48:72-74. [PubMed] |
| 39. | Maiuri F, Cappabianca P, Iaconetta G, Esposito F, Messina A. Simultaneous presentation of meningiomas with other intracranial tumours. Br J Neurosurg. 2005;19:368-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Curto L, Squadrito S, Almoto B, Longo M, Granata F, Salpietro F, Torre ML, Marini F, Trimarchi F, Cannavo S. MRI finding of simultaneous coexistence of growth hormone-secreting pituitary adenoma with intracranial meningioma and carotid artery aneurysms: report of a case. Pituitary. 2007;10:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | da Costa LB, Riva-Cambrin J, Tandon A, Tymianski M. Pituitary adenoma associated with intraventricular meningioma: case report. Skull Base. 2007;17:347-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Prevedello DM, Thomas A, Gardner P, Snyderman CH, Carrau RL, Kassam AB. Endoscopic endonasal resection of a synchronous pituitary adenoma and a tuberculum sellae meningioma: technical case report. Neurosurgery. 2007;60:E401; discussion E401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Basu A, Brabant G, Gnanalingham KK. More than a prolactinoma. Pituitary. 2010;13:87-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Guaraldi F, Corazzini V, Gallia GL, Grottoli S, Stals K, Dalantaeva N, Frohman LA, Korbonits M, Salvatori R. Genetic analysis in a patient presenting with meningioma and familial isolated pituitary adenoma (FIPA) reveals selective involvement of the R81X mutation of the AIP gene in the pathogenesis of the pituitary tumor. Pituitary. 2012;15 Suppl 1:S61-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 45. | Ramirez Mdel P, Restrepo JE, Syro LV, Rotondo F, Londoño FJ, Penagos LC, Uribe H, Horvath E, Kovacs K. Neurocysticercosis, meningioma, and silent corticotroph pituitary adenoma in a 61-year-old woman. Case Rep Pathol. 2012;2012:340840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 46. | Masoodi SR, Mir SA, Farooqui KJ, Bhat AR, Wani AI, Bhat MA. Growth hormone secreting pituitary macroadenoma and meningioma: An association or coincidence? Indian J Endocrinol Metab. 2013;17:770-771. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 47. | Mahvash M, Igressa A, Pechlivanis I, Weber F, Charalampaki P. Endoscopic endonasal transsphenoidal approach for resection of a coexistent pituitary macroadenoma and a tuberculum sellae meningioma. Asian J Neurosurg. 2014;9:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 48. | Karsy M, Sonnen J, Couldwell WT. Coincident pituitary adenoma and sellar meningioma. Acta Neurochir (Wien). 2015;157:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Ruiz-Juretschke F, Iza B, Scola-Pliego E, Poletti D, Salinero E. Coincidental pituitary adenoma and planum sphenoidale meningioma mimicking a single tumor. Endocrinol Nutr. 2015;62:292-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Ben Nsir A, Khalfaoui S, Hattab N. Simultaneous Occurrence of a Pituitary Adenoma and a Foramen Magnum Meningioma: Case Report. World Neurosurg. 2017;97:748.e1-748.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 51. | Lim KZ, Goldschlager T, Chandra RV, Hall J, Uren B, Pullar M. Co-occurrence of Pituitary Adenoma with Suprasellar and Olfactory Groove Meningiomas. Basic Clin Neurosci. 2016;7:361-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Herrero-Ruiz A, Villanueva-Alvarado HS, Corrales-Hernández JJ, Higueruela-Mínguez C, Feito-Pérez J, Recio-Cordova JM. Coexistence of GH-Producing Pituitary Macroadenoma and Meningioma in a Patient with Multiple Endocrine Neoplasia Type 1 with Hyperglycemia and Ketosis as First Clinical Sign. Case Rep Endocrinol. 2017;2017:2390797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Kumaria A, Scott IS, Robertson IJ. An unusual pituitary adenoma coexistent with bilateral meningiomas: case report. Br J Neurosurg. 2019;33:579-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Zhao Y, Zhang H, Lian W, Xing B, Feng M, Liu X, Wang R. Collision tumors composed of meningioma and growth hormone-secreting pituitary adenoma in the sellar region: Case reports and a literature review. Medicine (Baltimore). 2017;96:e9139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Engelhardt J, Nunes ML, Pouchieu C, Ferrière A, San-Galli F, Gimbert E, Vignes JR, Laurent F, Berge J, Baldi I, Tabarin A, Loiseau H. Increased Incidence of Intracranial Meningiomas in Patients With Acromegaly. Neurosurgery. 2020;87:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 56. | Pravdenkova S, Al-Mefty O, Sawyer J, Husain M. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. 2006;105:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 117] [Article Influence: 6.5] [Reference Citation Analysis (0)] |