Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4190
Peer-review started: September 8, 2021
First decision: October 27, 2021
Revised: November 5, 2021
Accepted: March 14, 2022
Article in press: March 14, 2022
Published online: May 6, 2022
Processing time: 234 Days and 2.2 Hours
Paraneoplastic neurological syndrome (PNS) is a rare complication in patients with cancer. PNS can affect the central, peripheral, autonomic nervous system, neuromuscular junction, or muscles and cause various neurological symptoms. Anti-Yo antibody-positive neurological paraneoplasms and anti-Hu antibody-positive neurological paraneoplasms are common, but coexistence of both types has not been described in the literature.
Here we present a rare case of paraneoplastic neuropathy occurring in both breast and lung cancers. A 55-year-old woman was admitted to our hospital with unsteadiness while walking. The patient had a history of breast cancer two years previously. Chest computed tomography revealed a 4.6 cm × 3.6 cm mass in the right lung, which was diagnosed as small-cell lung cancer (SCLC). Blood test was positive for anti-Yo antibodies, and the cerebrospinal fluid was positive for both anti-Yo and anti-Hu antibodies, and the neurological symptoms were considered to be related to the paraneoplasm. The patient was treated with a course of intravenous immunoglobulin, without noticeable improvement. After being discharged from hospital, the patient underwent regular chemotherapy for SCLC and periodic reviews. The patient’s neurological symptoms continued to deteriorate at the follow-up visit in April 2021.
This case suggests the possibility of two types of tumors appearing simultaneously with two paraneoplastic antibodies. The clinical appearance of two or more paraneoplastic tumors requires additional attention.
Core Tip: Neurological paraneoplastic syndrome is a common manifestation in patients with tumors, and in some cases, it appears earlier than the tumor. We report a rare case in which two different paraneoplastic antibodies were present. While most previous studies have reported this syndrome due to one tumor, this patient presented with two different tumors and two paraneoplastic antibodies at the same time, causing neurological symptoms such as ataxia, limb numbness, and weakness. This case highlights the specificity of paraneoplastic antibodies to their corresponding tumors, avoiding a missed diagnosis when multiple antibodies are present and providing a reference for future clinical diagnosis.
- Citation: Li ZC, Cai HB, Fan ZZ, Zhai XB, Ge ZM. Paraneoplastic neurological syndrome with positive anti-Hu and anti-Yo antibodies: A case report. World J Clin Cases 2022; 10(13): 4190-4195
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4190.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4190
Paraneoplastic neurological syndromes (PNS) are rare immune-mediated disorders that occur in 3.5%–5.0% of patients with small-cell lung cancer (SCLC). Breast cancer is also a disease characterized by immune-mediated syndromes occurring in less than 1.0% of patients[1,2]. With the continuous progress in cancer research, the paraneoplastic syndrome of the nervous system caused by cancer has attracted increasing attention. PNS is characterized by diverse neurological syndromes caused by cancer and always antedate its diagnosis. PNS is associated with various cancers, such as lung cancer, Hodgkin’s lymphoma, testicular cancer, breast cancer, and gynecologic malignancies.
PNS can affect the central, peripheral, autonomic nervous system, neuromuscular junction or muscles and cause various neurological symptoms. Because of the underlying neoplasm, PNS has often been misdiagnosed as other nervous system diseases such as viral encephalitis or myasthenia gravis, which may delay therapy and result in the deterioration of patients’ conditions, causing ataxia, rapid progressive dementia, status epileptics, and even coma. Antibodies and T cells share epitope expression responses to the nervous system and tumors, which may be the main cause of most PNS[3].
Although the coexistence of two or more different tumors in the same patient with PNS is rare, the tumors and multiple paraneoplastic antibodies can appear at the same time. Only a few such cases have been reported.
Here we describe a patient with PNS, involving both SCLC and breast cancer.
The patient was a 55-year-old Chinese woman who was admitted to hospital with numbness and weakness of limbs in July 2020.
She experienced numbness and discomfort in the extremities without any obvious cause, with limited movement and inability to walk on their own for ten days. In the following days, her gait gradually became unsteady, and she developed weakness of her lower limbs.
The patient presented to the local oncology hospital with a history of breast cancer 2 years ago and underwent radical mastectomy after 8 chemotherapy sessions at that time. The patient was reviewed regularly for two years after surgery, and no recurrence was observed.
There was no family history of neurological or autoimmune disorders.
Neurological examination revealed weakness of proximal muscles of lower limb at grade 3 on the Medical Research Council scale, and she had signs associated with cerebellar degeneration, such as mild dysarthria and clear ataxia, especially with prolonged speech. However, her cranial nerves and cognitive function were normal.
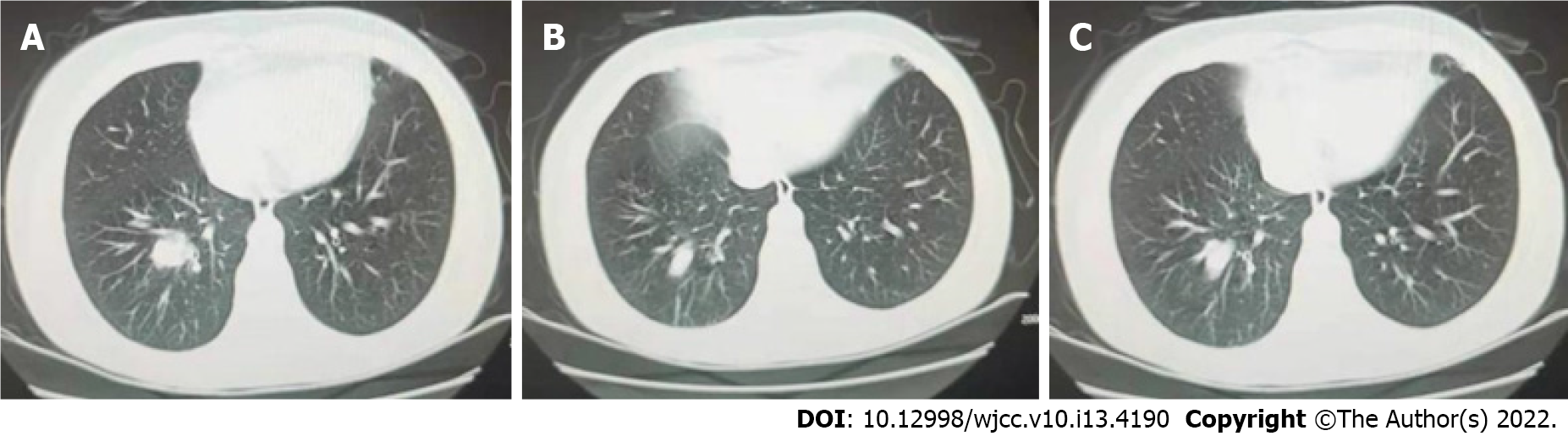
Cerebrospinal fluid examination and routine hematological, biochemical, and serological tests were normal. The neuron-specific enolase in blood was 45.90 ng/mL and the precursor of gastrin-releasing peptide was 976 pg/mL, suggesting the possibility of SCLC. Indirect immunofluorescence test (IIFT), for blood paraneoplastic anti-Hu antibody IgG was negative, and anti-Yo antibody IgG was positive (+ +, 1:10). Cerebrospinal fluid paraneoplastic anti-Hu antibody IgG was negative and anti-Yo antibody IgG was positive (+ +, 1:3.2). BLOT test for blood paraneoplastic anti-Hu antibody IgG and anti-Yo antibody IgG was positive (both + + +), and cerebrospinal fluid paraneoplastic anti-Hu antibody IgG was positive (+) and anti-Yo antibody IgG was strongly positive (+ + +). No tumor cells were detected in microscopic examination of cerebrospinal fluid (Figure 1A-D).
The patient had two paraneoplastic antibodies examined in the cerebrospinal fluid, but no associated tumor cells were detected, and no significant meningeal enhancement was seen on the cranial magnetic resonance imaging (MRI), so there was insufficient evidence for the diagnosis of leptomeningeal disease. However, considering the low positive rate of tumor cells in cerebrospinal fluid of patients with carcinomatous meningocele and related neurological paraneoplastic syndrome, the possibility of leptomeningeal disease was not excluded.
The MRI of cranial and cervical spine did not reveal any significant abnormalities, and electro
After completing the examinations, she was eventually diagnosed with PNS, involving SCLC and breast cancer.
The patient was treated with a course of intravenous immunoglobulin without noticeable improvement. During the month of onset at that time, the patient's limb numbness and cerebellar symptoms did not improve significantly. After being discharged from hospital, the patient underwent regular chemotherapy for SCLC and was reviewed periodically.
The size of her lung tumor has become significantly smaller over the past ten months and no recurrent breast cancer was seen. However, her numbness in the extremities and cerebellar symptoms did not resolve, and the neurological symptoms continued to deteriorate at the follow-up visit in April 2021.
Current research suggests that the main etiology of PNS is immune-related, with the presence of tumor-associated antigens in neurons, which causes a cross-immune reaction between antibodies produced by the body's immune response, consequently neural tissue-damaging symptoms appear. The clinical symptoms include mainly subacute cerebellar ataxia, abnormal mental behavior, and seizures. Anti-Hu and anti-Yo antibodies are the most common markers, with the former being seen in SCLC and the latter in patients with gynecological tumors or breast cancer[4].
SCLC can cause a variety of paraneoplastic syndromes. One of the most common paraneoplastic syndromes in patients with SCLC is anti-Hu peripheral neuropathy or encephalitis[5,6]. Anti-Hu is also known as type IIa neuronal antibodies and type I anti-European nuclear antibodies, originally named Hu by Graus et al[7]. Anti-Hu antigen is expressed in the nucleus and cytoplasm of neuronal cells and cancerous cells[8,9]. In patients with positive anti-Hu antibodies, the most common clinical features are sensorimotor neuropathy, and typical electrophysiological features are predominantly sensory nerve conduction block with normal motor nerve conduction. Anti-Hu antibodies have also been reported to cause damage to the enteric nervous system (ENS), but its pathogenic role is unclear and is presumed to be related to activation of the immune system and promotion of immune cell infiltration into the ENS microenvironment[10]. There have also been cases of pupillary tonicity, suggesting that the anti-Hu antibodies associated with neuropathy may be heterogeneous[11]. Small-cell lung cancers tend to have multiple autoantibodies and can be recognized several years after the onset of the disease in some patients.
PNS in breast cancer are rare (1%), and the spectrum of syndromes includes subacute cerebellar degeneration, myoclonic ataxia, limbic encephalitis, and subacute sensory and motor neuropathies, most of the syndromes have a subacute and progressive course. However, the primary tumor is usually insidious, and neurological diseases usually precede the diagnosis of the tumor[12,13]. Anti-Yo antibody is the most common antibody involved in breast cancer, and paraneoplastic cerebellar degeneration (PCD) is the most common clinical presentation of anti-Yo-positive PNS. The final outcomes are better for patients with paraneoplastic syndrome than in those without paraneoplastic syndrome. Compared to other paraneoplastic antibodies, PCD associated with anti-Yo antibodies has a poorer prognosis[14,15].
Both anti-Hu and anti-Yo antibodies were detected in blood and cerebrospinal fluid in the present case, suggesting the coexistence of the two antibodies. Small-cell lung cancer was revealed on chest CT, but there was no imaging evidence of breast cancer metastasis at this time. Small-cell lung cancer is a tumor that is most likely to cause neurological paraneoplastic syndrome, and patients may present with two or more antibodies, but the incidence is low[16-20]. Considering the patient’s history of breast cancer, and the IIFT and BLOT both suggested positive anti-Yo antibodies with high titers, the neurological paraneoplastic syndrome may occur earlier than solid tumors, the possibility of potential breast cancer in the patient is high and the prognosis is unknown.
Anti-Hu antibody is associated with SCLC. Anti-Yo antibody positivity is mostly observed in gynecological and breast tumors. As the neurological paraneoplastic syndrome often precedes the appearance of solid tumors, patients with a history of tumors might have recurrent tumors, and whole-body imaging and pathology should be completed to clarify whether the patient has recurrent tumors or new and different tumors. Therefore, clinicians should consider the possibility of two malignancies when these two paraneoplastic antibodies are both positive. Early diagnosis and treatment of PNS and the tumors can significantly improve the prognosis of patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical Neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Melo FF, Brazil; Valencia GA, Peru S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Kanaji N, Watanabe N, Kita N, Bandoh S, Tadokoro A, Ishii T, Dobashi H, Matsunaga T. Paraneoplastic syndromes associated with lung cancer. World J Clin Oncol. 2014;5:197-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 118] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (4)] |
| 2. | Gatti G, Simsek S, Kurne A, Zurrida S, Naninato P, Veronesi P, Frasson A, Millen E, Rososchansky J, Luini A. Paraneoplastic neurological disorders in breast cancer. Breast. 2003;12:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Bhat R, Steinman L. Innate and adaptive autoimmunity directed to the central nervous system. Neuron. 2009;64:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Seluk L, Taliansky A, Yonath H, Gilburd B, Amital H, Shoenfeld Y, Kivity S. A large screen for paraneoplastic neurological autoantibodies; diagnosis and predictive values. Clin Immunol. 2019;199:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000;123 (Pt 7):1481-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 662] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 6. | Senties-Madrid H, Vega-Boada F. Paraneoplastic syndromes associated with anti-Hu antibodies. Isr Med Assoc J. 2001;3:94-103. [PubMed] |
| 7. | Graus F, Cordon-Cardo C, Posner JB. Neuronal antinuclear antibody in sensory neuronopathy from lung cancer. Neurology. 1985;35:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 245] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Jean WC, Dalmau J, Ho A, Posner JB. Analysis of the IgG subclass distribution and inflammatory infiltrates in patients with anti-Hu-associated paraneoplastic encephalomyelitis. Neurology. 1994;44:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 106] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Dalmau J, Furneaux HM, Rosenblum MK, Graus F, Posner JB. Detection of the anti-Hu antibody in specific regions of the nervous system and tumor from patients with paraneoplastic encephalomyelitis/sensory neuronopathy. Neurology. 1991;41:1757-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 160] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Li Q, Michel K, Annahazi A, Demir IE, Ceyhan GO, Zeller F, Komorowski L, Stöcker W, Beyak MJ, Grundy D, Farrugia G, De Giorgio R, Schemann M. Anti-Hu antibodies activate enteric and sensory neurons. Sci Rep. 2016;6:38216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Zhang L, Luo S, Jin H, Lv X, Chen J. Anti-Hu Antibody-Associated Adie's Pupil and Paraneoplastic Sensorimotor Polyneuropathy Caused by Primary Mediastinal Small Cell Carcinoma. Front Neurol. 2019;10:1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543-1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 662] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 13. | Kawasoe T, Yamamoto Y, Okumura Y, Iwase H. A case report of paraneoplastic neurological syndrome associated with occult breast cancer. Breast Cancer. 2006;13:202-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Shams'ili S, Grefkens J, de Leeuw B, van den Bent M, Hooijkaas H, van der Holt B, Vecht C, Sillevis Smitt P. Paraneoplastic cerebellar degeneration associated with antineuronal antibodies: analysis of 50 patients. Brain. 2003;126:1409-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 345] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 15. | Hammack JE, Kimmel DW, O'Neill BP, Lennon VA. Paraneoplastic cerebellar degeneration: a clinical comparison of patients with and without Purkinje cell cytoplasmic antibodies. Mayo Clin Proc. 1990;65:1423-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Sun X, Tan J, Sun H, Liu Y, Guan W, Jia J, Wang Z. Anti-SOX1 Antibodies in Paraneoplastic Neurological Syndrome. J Clin Neurol. 2020;16:530-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Hardy-Werbin M, Arpí O, Taus A, Rocha P, Joseph-Pietras D, Nolan L, Danson S, Griffiths R, Lopez-Botet M, Rovira A, Albanell J, Ottensmeier CH, Arriola E. Assessment of neuronal autoantibodies in patients with small cell lung cancer treated with chemotherapy with or without ipilimumab. Oncoimmunology. 2018;7:e1395125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Altaha R, Abraham J. Paraneoplastic neurologic syndrome associated with occult breast cancer: a case report and review of literature. Breast J. 2003;9:417-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Saraya AW, Worachotsueptrakun K, Vutipongsatorn K, Sonpee C, Hemachudha T. Differences and diversity of autoimmune encephalitis in 77 cases from a single tertiary care center. BMC Neurol. 2019;19:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Dik A, Strippel C, Mönig C, Golombeck KS, Schulte-Mecklenbeck A, Wiendl H, Meuth SG, Johnen A, Gross CC, Melzer N. Onconeural antigen spreading in paraneoplastic neurological disease due to small cell lung cancer. Oxf Med Case Reports. 2018;2018:omy034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |