Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4145
Peer-review started: July 30, 2021
First decision: October 25, 2021
Revised: November 2, 2021
Accepted: March 14, 2022
Article in press: March 14, 2022
Published online: May 6, 2022
Processing time: 273 Days and 15.2 Hours
Primitive neuroectodermal tumor (PNET), especially located in the prostate, is a rare tumor that mainly occurs in young men. Bladder or rectum invasion and distant metastasis are strongly associated with a poor prognosis. Combination therapy, including radical surgery, adjuvant chemotherapy, and radiotherapy, is available. We present a case of prostatic PNET and a review of 17 cases identified in the literature.
A 58-year-old man was admitted complaining of dysuria for 2 years. Computed tomography and magnetic resonance imaging showed a large cystic-solid mass in the pelvic cavity compressing the surrounding bladder and rectum. The mass was iso- to hyperintense on T1-weighted imaging (WI) and heterogeneously hyperintense on T2WI. Cystic degeneration and necrosis were seen in the tumor, and solid tissues within the mass enhanced on contrast-enhanced scan. The patient underwent robot-assisted laparoscopic pelvic tumor resection. Histologically, the presence of many small round cells that were positive for expression of CD99, vimentin, and synaptophysin established the diagnosis of PNET in the prostate after surgery. The patient underwent adjuvant chemotherapy. During 34 mo of follow-up, the patient had no signs or symptoms of recurrence or residual disease.
We present the case of the oldest prostatic PNET patient, who has a good prognosis. This illustrates how older men with prostatic PNET may also benefit from the combination therapy, like younger adults, and achieve a long-term survival. As always, PNET should be considered in the differential diagnosis of aggressive prostatic tumors in young men.
Core Tip: Prostatic primitive neuroectodermal tumors (PNETs) are usually malignant and occur in young men (median age: 29 years; range: 20 to 58 years) with predominant complaints of dysuria, often with normal prostate specific antigen levels. Prostatic PNETs may invade adjacent organs, including the bladder, rectum, and seminal vesicles, and are prone to distant metastases. Forty-four percent of patients develop metastases, most commonly (75%) in the lung. CD99 is the most accepted immunohistochemical marker for prostatic PNETs. Almost all patients receive chemotherapy. Despite combination therapy, including surgery, chemotherapy, and radiotherapy, the median survival of the patients remains unsatisfactory at 13 mo.
- Citation: Tian DW, Wang XC, Zhang H, Tan Y. Primitive neuroectodermal tumor of the prostate in a 58-year-old man: A case report. World J Clin Cases 2022; 10(13): 4145-4152
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4145.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4145
Primitive neuroectodermal tumor (PNET) is an extremely rare neural crest tumor with a poor prognosis that mainly affects children and adolescents. The clinical, morphological, and immunophenotypic characteristics of PNET are similar to Ewing’s sarcoma, and the two are thought to be related. Histologically, PNET is characterized by small round and oval cells. CD99, an antigen encoded by the Mic-2 gene, is present on the surface of most PNET cells, and therefore represents a useful diagnostic marker for PNET[1].
PNET is divided into central PNET and peripheral PNET according to its location. Peripheral PNETs have occurred in the kidney, bladder, prostate, and adrenal gland, often revealing an infiltrative mass with an ill-defined and necrotic region on imaging[2]. PNET of the prostate is extremely rare, with significant malignant potential. We here present the oldest prostatic PNET patient reported to date. Clinicopathological features of 18 cases reported since 2003, including ours, are reviewed.
A 58-year-old man presented with a 2-year history of dysuria without obvious inducement.
The patient had dysuria without obvious cause accompanied by urinary hesitancy, which was progressively worsening. The pelvic ultrasound showed a cystic-solid mass. During 3 mo before admission, the patient had also presented with constipation and occasional pain.
The patient had no prior urologic history or significant medical history.
There was no personal or family history.
The examination revealed a softly distended tympanitic abdomen with tenderness near the pubic symphysis.
The serum prostate specific antigen (PSA) level was 0.82 ng/mL, the cytokeratin-19 fragment level was 6.79 ng/mL, and the other tumor markers including neuron specific enolase (NSE), alpha fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 199 were all within normal ranges.
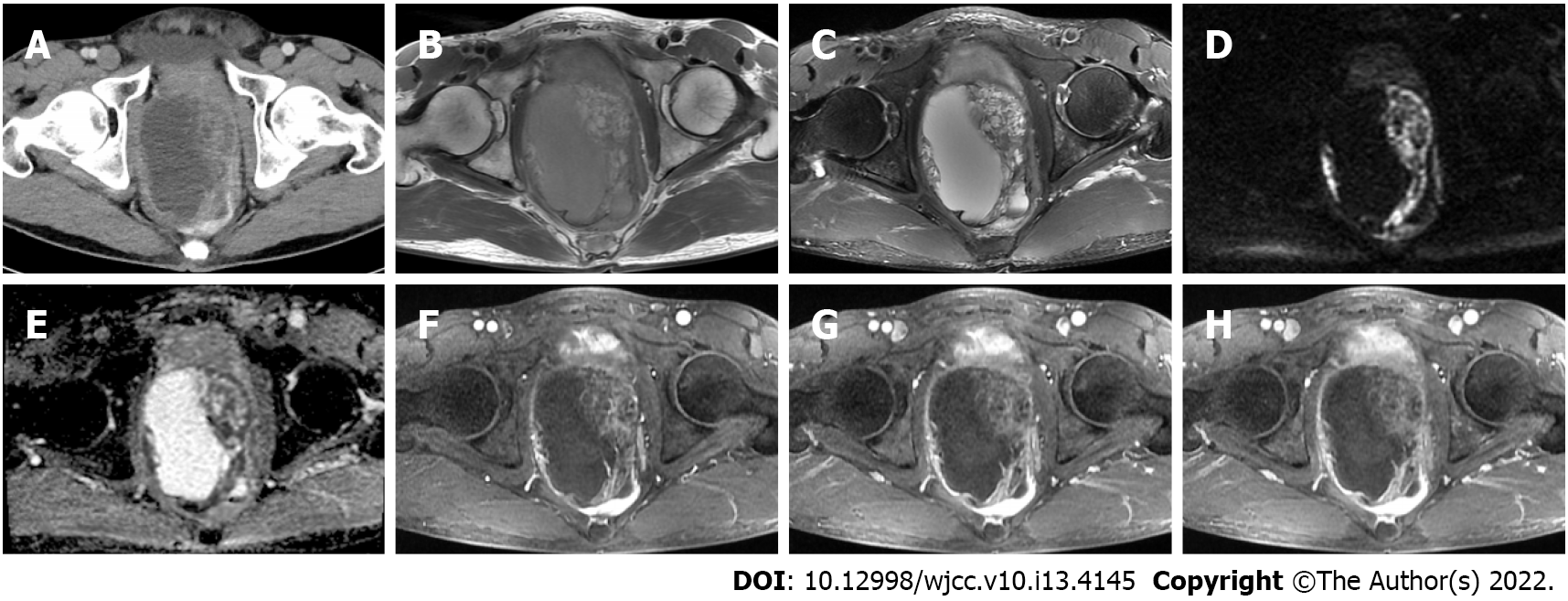
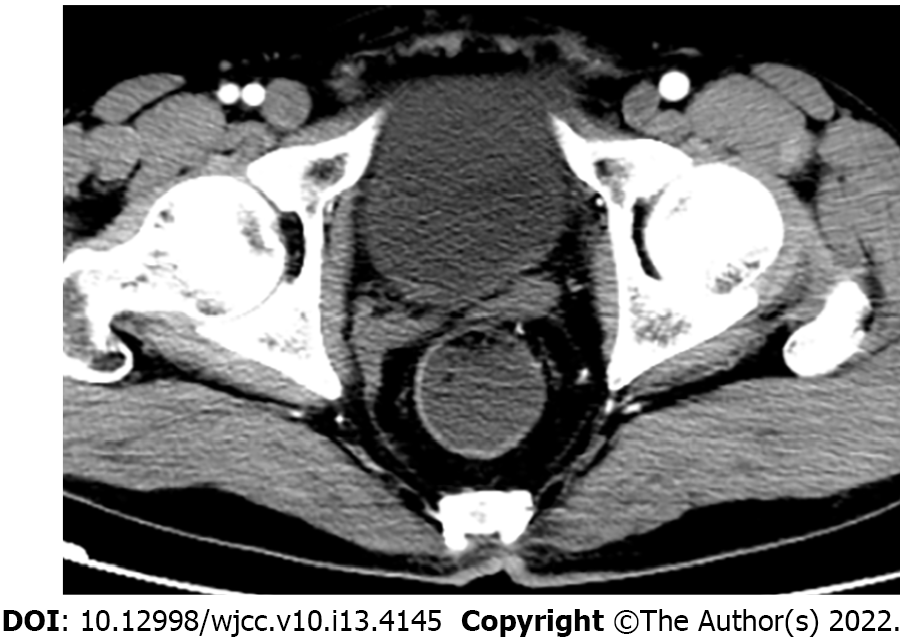
Contrast-enhanced computed tomography (CT) showed a mass between the bladder and rectum with cystic and necrotic components and heterogeneous enhancement (Figure 1A). Magnetic resonance imaging (MRI) of the pelvis confirmed a large cystic-solid mass measuring 10.7 cm × 10.8 cm × 8.1 cm near the prostate and compressing the rectum and bladder (Figure 1B-H). The lesion appeared isointense to slightly hyperintense on T1-weighted imaging (WI) and was heterogeneously hyperintense on T2WI. The solid portion of the tumor was hyperintense on diffusion-WI and correspondingly hypointense on the apparent diffusion coefficient maps. The mass showed prominent heterogeneous enhancement in the arterial phase and continuous enhancement in the venous and delayed phases. These findings initially suggested prostatic cystadenoma. However, prostate cancer could not be excluded considering the patient's age. At repeat CT examination 2 mo after surgery and the first cycle of chemotherapy, there was no evidence of residual or recurrent tumor (Figure 2).
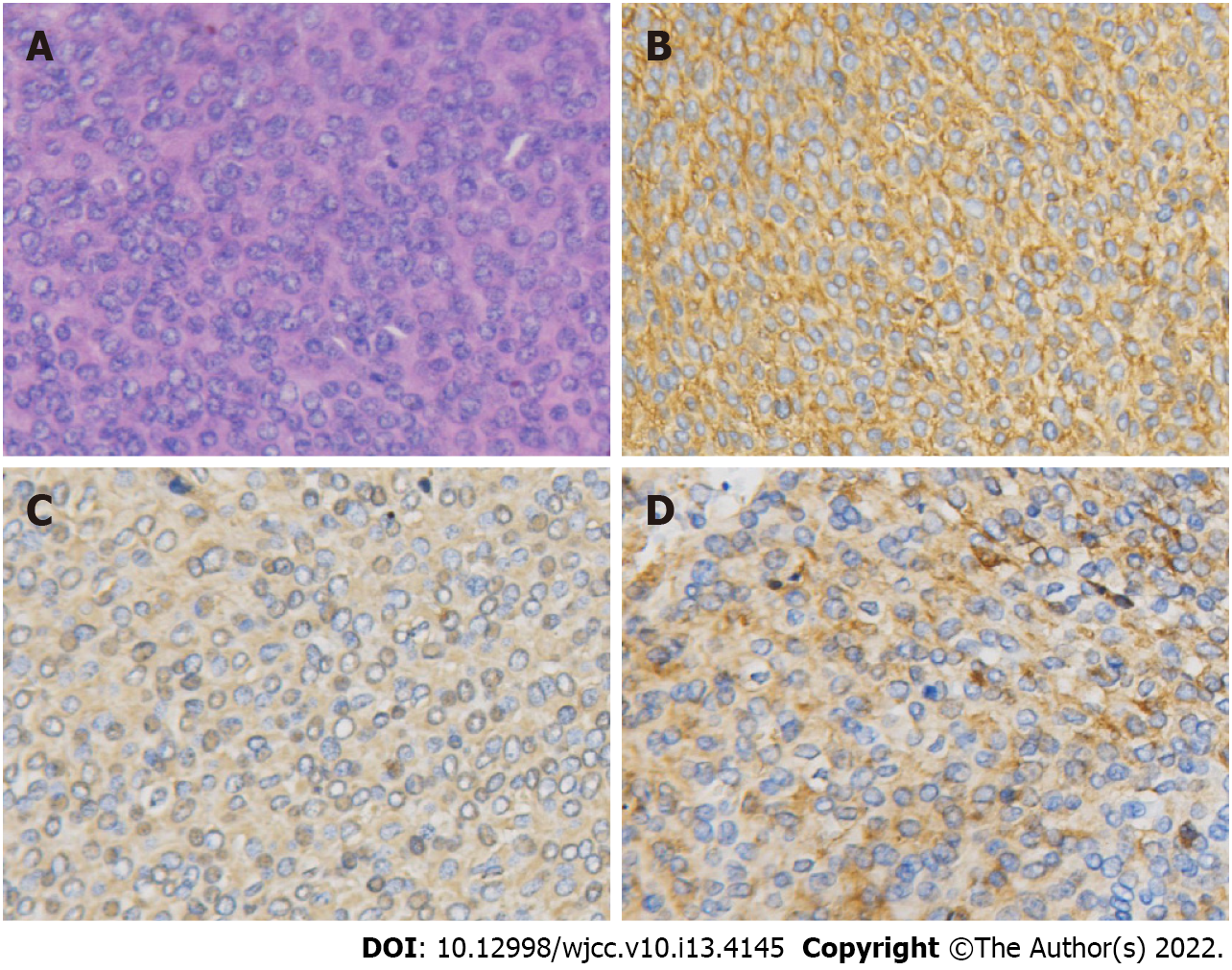
The final diagnosis was prostatic PNET with cystic degeneration. Histopathology of the surgical specimens showed strongly staining small round cells (Figure 3A). Immunohistochemistry analysis showed strong positivity for CD99 and positivity for vimentin and synaptophysin (Figure 3B-D).
The patient underwent robot-assisted laparoscopic resection. An insufflation needle was inserted from the edge of the umbilicus and a longitudinal incision of about 1 cm was made at 2 cm from the upper edge of the umbilicus. A large cystic-solid mass was observed in the rectum and bladder space which adhesions to the surrounding prostate and rectum. The neoplasm had a vascularized appearance. The cystic fluid was extracted with an aspirator and the tumor was removed gradually and completely. The resected cystic-solid tumor measured about 8 cm × 7 cm. Histological examination and immunohistochemical staining ultimately confirmed the PNET. After surgery, the patient received adjuvant chemotherapy based on an alternating VEC (vincristine, etoposide, carboplatin) and IE (ifosfamide, etoposide) regimen. Chemotherapy was repeated every 3 wk for up to six cycles as tolerated.
There was complete remission of the tumor after radical surgery and chemotherapy. At the most recent follow-up visit (34 mo), the patient was alive and well, and there was no recurrence.
PNET is an extremely rare malignancy that is aggressive and has a poor prognosis[3]. In 2003, Colecchia et al[1] were the first to report PNET of the prostate, and besides the present case, only 17 cases of prostatic PNET have been reported to date. We review 18 cases including ours and summarize the clinicopathological features in Table 1.
| No. | Ref. | Age | Symptoms | PSA (ng/mL) | Size (cm) | Immunohistochemistry (positive) | Molecular studies | Treatment | Follow-up (mo) | Metastases |
| 1 | Colecchia et al[1], 2003 | 31 | NA | 2.06 | 7 | CD99, vimentin, NSE, synaptophysin | EWS/FLI1 fusion | CT, RT, SR | NA | NA |
| 2 | Peyromaure et al[21], 2003 | 27 | Dysuria and pelvic discomfort | NA | NA | CD99 | t (11; 22) (q24; q12) | CT, SR, RT | 2+ | NA |
| 3 | Thete et al[10], 2007 | 26 | Dysuria and pelvic discomfort | 0.3 | NA | CD99(Mic-2) | NA | NA | NA | NA |
| 4 | Kumar et al[16], 2008 | 25 | Burning micturition and dysuria | 0.88 | 6.7 | CD99, vimentin, S100, NSE, synaptophysin | NA | CT | NA | NA |
| 5 | Funahashi et al[13], 2009 | 20 | Gross hematuria and miction pain | 0.7 | 10 | CD99, NSE, CD56, MIB-1, p53 | t (11; 22) (q24; q12) | SR | 10+ | Lung |
| 6 | Mohsin et al[14], 2011 | 29 | Burning micturition and urinary retention | 1.3 | NA | CD99 | NA | CT | 4 | Lung |
| 7 | Al Haddabi et al[4], 2012 | 24 | Dysuria, constipation, back pain, and pelvic discomfort | 0.7 | 10 | CD99, Bcl2, CK19, AE1/AE3, CK, vimentin | Rearrangement of chromosome 22 | CT | NA | No |
| 8 | Wu et al[5], 2013 | 29 | Difficult defecation and anus distention | 1.19 | 7.4 | CD99, Bcl-2 | NA | SR, CT | 12+ | Lung |
| 9 | Liao et al[17], 2015 | 49 | Frequent urination, dysuria, and pelvic discomfort | NA | 7.1 | CD99, CD56, NSE, Ki-67 | NA | CT, RT | 24+ | No |
| 10 | Shibuya et al[3], 2015 | 23 | Dysuria and anal pain | NA | NA | MIC-2, cytokeratin, vimentin, N-CAM | Translocation at chromosome 22q12 | CT | 4 | Bone, lung, meninge |
| 11 | Esch et al[11], 2016 | 33 | Pelvic pain, dysuria, and urgency | NA | 6 | Cytokeratin | EWSR1-gene | SR | 12+ | No |
| 12 | Kord et al[8], 2018 | 38 | Abdominal pain, constipation, pain with defecation, and hematuria | NA | 14.4 | CD99 | EWSR1/FLI1 fusion | CT, SR | 14 | Lung, liver, peritoneum |
| 13 | Du et al[9], 2019 | 39 | Notalgia and paraplegia | 1.07 | 2.6 | CD99, CD56, P63, vimentin | NA | RT, CT | 17 | Thoracic spine |
| 14 | Javanmard et al[22], 2019 | 37 | Painless gross hematuria | 1.07 | 8.6 | CD99, vimentin, BCL2, Ki67 | NA | SR, CT | 16 | Yes |
| 15 | Liu et al[23], 2020 | 40 | Dysuria | NA | 11.2 | CD99, synaptophysin, CD56, vimentin | FLI1 | SR, CT | 14+ | No |
| 16 | Teng et al[15], 2020 | 27 | Dysuria and dyschezia | 1.52 | 8.4 | CD99, CD56, desmin, vimentin | NA | SR | 5 | Lung and peritoneum |
| 17 | da Ponte et al[12], 2021 | 29 | Pelvic discomfort and dysuria | 0.4 | 8.8 | CD99/MIC-2, synaptophysin | EWS gene rearrangement | CT, SR | 84+ | No |
| 18 | Present case | 58 | Dysuria, constipation, and pain with defecation | 0.82 | 10.8 | CD99, vimentin, synaptophysin | NA | SR, CT | 34+ | No |
In the published cases, the patients were mainly young adults (median age: 29 years; range: 20 to 58 years). Our 58-year-old patient is the oldest patient described thus far. Patients with prostatic PNET may present with dysuria, hematuria, pelvic discomfort, constipation, and hematochezia[3-5]. In these 18 cases, ten (56%) had dysuria, six (33%) were accompanied with pelvic discomfort or pain, and three (17%) presented with hematuria. Prostatic PNET should be suspected when young men present with dysuria. Although PSA is an essential serum marker for the diagnosis of prostate cancer, with a positive detection rate reaching 82%[6], the PSA values in all 18 patients with prostatic PNET were within normal limits (0 to 4 ng/mL). Approximately 44% of patients had distant metastases, with the lung as the most common site, accounting for 75% of all metastases, followed by the bone (25%), liver (12.5%), and meninges (12.5%). Distant metastasis is known as the most unfavorable prognostic factor for Ewing's sarcoma[7]. Therefore, the search for metastasis must be emphasized in patients with prostatic PNET as the early detection of metastasis is crucial.
Imaging examination is beneficial in diagnosis, clarifying the internal structure, and assessing local invasion and distant metastasis. In 14 cases, including ours, the average size of the tumor was 8.5 cm (range: 2.6 to 14.4 cm). PNET of the prostate has been described as an ill-defined aggressive soft tissue mass with hemorrhage, necrosis, and cystic degeneration; as a multilobulated mass with heterogeneous enhancement; and as a mass replacing the prostate on CT and MRI[8,9]. MRI generally shows the lesion to be hypointense on T1WI and iso- to hyperintense on T2WI, and contrast-enhanced T1WI shows heterogeneous enhancement[8,10-12]. MRI is sensitive to evaluate local tumor invasion. In nine reported cases, there was compression or involvement of the bladder; five of these had distant metastases[5,8,13-15]. There was compression or involvement of the rectum in six cases, two of which had metastases to distant sites[8,15]. Four tumors were in close association with seminal vesicles but without metastases[5,8,16,17]. Additionally, there was one lesion that invaded the left ureter and bladder with bilateral hydroureteronephrosis[16]. In general, the imaging examination is useful to identify the relationship with adjacent tissues and distant metastasis, but preoperative diagnosis based on imaging alone is challenging.
The final diagnosis of a PNET involving the prostate relies on histopathological features. Under the light microscope, PNET is a mass of undifferentiated small round cells, which are arranged closely in a flaky, lobulated, or nest-like pattern. The characteristic small round cells of PNET are reactive to anti-CD99 antibody (Mic-2), and more than 90% of PNETs have demonstrated a translocation between the long arms of chromosomes 11 and 22, and are positive for the EWS-FLI1 fusion gene[18]. The translocation can be confirmed by molecular techniques such as fluorescence in situ hybridization and reverse transcriptase-polymerase chain reaction[4]. At present, the diagnosis scheme proposed by Schmidt et al[19] has been extensively adopted, including the presence of Homer-Wright rosettes and/or the expression of at least two neural markers. Among 18 cases of prostatic PNET, 89% were immunohistochemically positive for CD99, 44% for vimentin, 28% for synaptophysin, 28% for CD56, and 22% for NSE. Molecular analyses in eight cases showed translocations of the chromosomes or EWSR1/FLI1 fusion. However, molecular techniques were not used to detect chromosomal translocations in our case. Identification of translocation may be crucial, as some translocation types are associated with a poor prognosis.
Combinations of surgery, chemotherapy, and radiotherapy can form an effective treatment strategy for prostatic PNET. The commonly recommended chemotherapy drugs include vincristine, doxorubicin, cyclophosphamide, etoposide, and ifosfamide[18,20]. In the 18 cases that we summarize, adjuvant or neoadjuvant chemotherapy was administered in all cases of prostatic PNET except for one case without detailed treatment strategy. In two cases in the literature, the patients underwent radical surgery combined with chemotherapy and radiotherapy[1,21]. Our patient and five others were treated by radical surgery combined with chemotherapy[5,8,12,22,23], four patients received chemotherapy alone[3,4,14,16], and in other cases, the patients adopted single radical resection or chemoradiotherapy. Although standard treatment has not been established, a multimodal approach is recommended. Follow-up information was available for 13 patients and our case. In general, during an average follow-up period of approximately 18 mo (median period: 13 mo; range: 2 to 84 mo), patients with combination therapy had longer survival than patients with monotherapy.
PNET of the prostate shows aggressive biological behavior and is often overlooked in the differential diagnosis due to its rare occurrence. It should be considered in young men with the complaint of dysuria to contribute to early diagnosis. The appropriate therapeutic schedule is radical surgery as early as possible, and combined chemotherapy or radiotherapy, which could be helpful to improve prognosis. Further studies and longer-term follow-up await.
We thank the pathology department of The First Hospital of Shanxi Medical University for providing pathological information and the assistance of the patient.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gokce E, Turkey S-Editor: Zhang H L-Editor: Wang TQ P-Editor: Zhang H
| 1. | Colecchia M, Dagrada G, Poliani PL, Messina A, Pilotti S. Primary primitive peripheral neuroectodermal tumor of the prostate. Immunophenotypic and molecular study of a case. Arch Pathol Lab Med. 2003;127:e190-e193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Gupta P, Hari S, Thulkar S. Imaging spectrum of peripheral primitive neuroectodermal tumours. Singapore Med J. 2013;54:463-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Shibuya T, Mori K, Sumino Y, Sato F, Mimata H. Rapidly progressive primitive neuroectodermal tumor of the prostate: A case report and review of the literature. Oncol Lett. 2015;9:634-636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Al Haddabi I, Al Bahri M, Burney I. Cytokeratin-positive primitive neuroectodermal tumor of the prostate: case report and review of literature. Indian J Pathol Microbiol. 2012;55:569-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Wu T, Jin T, Luo D, Chen L, Li X. Ewing's sarcoma/primitive neuroectodermal tumour of the prostate: A case report and literature review. Can Urol Assoc J. 2013;7:E458-E459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, DeKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL, Waters WB, MacFarlane MT, Southwick PC. Comparison of Digital Rectal Examination and Serum Prostate Specific Antigen in the Early Detection of Prostate Cancer: Results of a Multicenter Clinical Trial of 6,630 Men. J Urol. 2017;197:S200-S207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Subbiah V, Anderson P, Lazar AJ, Burdett E, Raymond K, Ludwig JA. Ewing's sarcoma: standard and experimental treatment options. Curr Treat Options Oncol. 2009;10:126-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Kord Valeshabad A, Choi P, Dababo N, Shamim E, Alsadi A, Xie KL. Metastatic Primitive Neuroectodermal Tumor of the Prostate: A Case Report and Review of the Literature. Clin Genitourin Cancer. 2018;16:e343-e347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Du TQ, Li X, Diao HR, Chen YZ, Yan Y, Zhao YX. Primitive Neuroectodermal Tumor of the Prostate with Notalgia and Paraplegia as the Initial Symptoms: A Case Report and Literature Review. J Adolesc Young Adult Oncol. 2019;8:697-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Thete N, Rastogi D, Arya S, Singh A, Rao P, Chandge A, Ramadwar M. Primitive neuroectodermal tumour of the prostate gland: ultrasound and MRI findings. Br J Radiol. 2007;80:e180-e183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Esch L, Barski D, Bug R, Otto T. Prostatic sarcoma of the Ewing family in a 33-year-old male - A case report and review of the literature. Asian J Urol. 2016;3:103-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Borges da Ponte C, Leitão TP, Miranda M, Polido J, Alvim C, Fernandes I, Braga T, Pena B, de Almeida JM, Costa L, Dos Reis JP. Prostate Ewing Sarcoma/PNET: A case of long survival in a highly aggressive malignancy. Urology. 2021;154:e11-e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Funahashi Y, Yoshino Y, Hattori R. Ewing's sarcoma/primitive neuroectodermal tumor of the prostate. Int J Urol. 2009;16:769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Mohsin R, Hashmi A, Mubarak M, Sultan G, Shehzad A, Qayum A, Naqvi SA, Rizvi SA. Primitive neuroectodermal tumor/Ewing's sarcoma in adult uro-oncology: A case series from a developing country. Urol Ann. 2011;3:103-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Teng L, Wei L, Li L, Xu Y, Chen Y, Cao Y, Wang W, Li C. Total pelvic exenteration and a new model of diversion for giant primitive neuroectodermal tumor of prostate: A case report and review of the literature. Asian J Urol. 2020;7:181-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Kumar V, Khurana N, Rathi AK, Malhotra A, Sharma K, Abhishek A, Bahadur AK. Primitive neuroectodermal tumor of prostate. Indian J Pathol Microbiol. 2008;51:386-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Liao C, Wu X, Wang X, Li H. Primitive neuroectodermal tumor of the prostate: Case report from China. J Cancer Res Ther. 2015;11:668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Bernstein M, Kovar H, Paulussen M, Randall RL, Schuck A, Teot LA, Juergens H. Ewing's sarcoma family of tumors: current management. Oncologist. 2006;11:503-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 336] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 19. | Schmidt D, Herrmann C, Jürgens H, Harms D. Malignant peripheral neuroectodermal tumor and its necessary distinction from Ewing's sarcoma. A report from the Kiel Pediatric Tumor Registry. Cancer. 1991;68:2251-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 20. | Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore S, Rausen AR, Vietti TJ, Miser JS. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 942] [Cited by in RCA: 915] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 21. | Peyromaure M, Vieillefond A, Boucher E, De Pinieux G, Beuzeboc P, Debré B, Flam TA. Primitive neuroectodermal tumor of the prostate. J Urol. 2003;170:182-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Javanmard B, Fallah Karkan M, Yousefi MR, Ahadi M. Painless Gross Hematuria: A New Presentation of Primitive Neuroectodermal Tumor of the Prostate. Int J Cancer Manag. 2019;12:e86352. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Liu Y, Xu B. Primary Peripheral Primitive Neuroectodermal Tumor of the Prostate on 18F-DCFPyL PET/CT. Clin Nucl Med. 2020;45:e249-e251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |