Published online May 6, 2022. doi: 10.12998/wjcc.v10.i13.4020
Peer-review started: November 2, 2021
First decision: February 14, 2022
Revised: February 25, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 6, 2022
Processing time: 178 Days and 18.7 Hours
Superior mesenteric artery embolism (SMAE) has acute onset and fast progression, which seriously threatens the life of patients. Multidetector computed tomography (MDCT) is one of the most important diagnostic methods for SMAE, which plays an important role in the diagnosis and prognosis of SMAE.
To evaluate the value of combined clinical data and MDCT findings in the diagnosis of acute SMAE and predict the risk factors for SMAE-related death.
Data from 53 SMAE patients who received abdominal MDCT multi-phase enhancement and superior mesenteric artery digital subtraction angiography examinations were collected. Univariate cox regression and multivariate cox model were used to analyze the correlation between death risk and clinical and computed tomography features in SMAE patients.
Univariate Cox regression model showed that intestinal wall thinning, intestinal wall pneumatosis, blood lactate > 2.1 mmol/L and blood pH < 7.35 increased the risk of death in patients with SMAE. After adjusting for age, sex, embolic involvement length and embolic distribution region, multivariate Cox regression model I showed that blood lactate > 2.1 mmol/L (HR = 5.26, 95%CI: 1.04-26.69, P = 0.045) and intestinal wall thinning (HR = 9.40, 95%CI: 1.05-83.46, P = 0.044) were significantly increases the risk of death in patients with SMAE.
For patients with SAME, increased blood lactate and intestinal wall thinning are the risk factors for death; hence, close monitoring may reduce the mortality rate. Clinical observation combined with MDCT signs can significantly improve SMAE diagnosis.
Core Tip: Acute superior mesenteric artery embolism (SAME) has a rapid onset and progression and lacks specific clinical and biochemical diagnostic indicators, resulting in delayed diagnosis and poor prognosis. Currently, multidetector computed tomography (MDCT) is recommended as the first-line examination method for diagnosing mesenteric vascular diseases. However, when there is no clinical doubt, radiologists can easily miss SMAE with length ≤ 20 mm and in regions III and IV, affecting early treatment of patients. We found that a serum lactate level > 2.1 mmol/L and intestinal wall thinning on MDCT could independently predict the risk factors of death in patients with SMAE.
- Citation: Yang JS, Xu ZY, Chen FX, Wang MR, Cong RC, Fan XL, He BS, Xing W. Role of clinical data and multidetector computed tomography findings in acute superior mesenteric artery embolism. World J Clin Cases 2022; 10(13): 4020-4032
- URL: https://www.wjgnet.com/2307-8960/full/v10/i13/4020.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i13.4020
Superior mesenteric artery embolism (SMAE) has a rapid onset and progression and lacks specific clinical and biochemical diagnostic indicators, which leads to delayed diagnosis and increased mortality[1,2]. Early and accurate diagnosis can shorten duration of intestinal ischemia, reduce the occurrence of intestinal ischemic necrosis, and improve outcomes of clinical treatment and prognosis.
Multidetector computed tomography (MDCT) is a fast, accurate, and noninvasive method that has been recommended as a first-line imaging in diagnosing mesenteric vascular disease[3,4], with the sensitivity of 93% and specificity of 100%[5-7]. In addition, MDCT can assess extravascular conditions, including the intestine, mesentery, and other abdominal organs, which can help distinguish non-SMAE abdominal pain and evaluate complications.
Irreversible intestinal necrosis in patients with SMAE often indicates poor prognosis. To date, few studies have integrated clinical data, biochemical indicators and imaging signs to predict the occurrence of irreversible intestinal necrosis and patient death in the early stage of SMAE[8-10]. This study aimed to evaluate the value of combining clinical data and MDCT findings in the diagnosis of SMAE and to predict the risk factors for patient death. The results can provide a reference for clinical decision-making and improve the survival rate of patients.
This was a retrospective, observational, single-center study. The study was approved by the ethics committee of the Second Affiliated Hospital of Nantong University (No. 2021KT154). Since this was a retrospective study, informed consent was waived.
The data of 84 patients who were suspected of having SMAE and treated at the 2nd Affiliated Hospital of Nantong University from January 2012 to December 2020 were collected. The exclusion criteria were as follows: no computed tomography (CT) examination (n = 2), no enhanced CT examination (n = 7), no digital subtraction angiography (DSA) examination (n = 12), superior mesenteric artery dissection (n = 3), pancreatic cancer invading superior mesenteric artery (SMA) (n = 2), pancreatitis (n = 1), portal vein thrombosis (n = 2), mesenteric torsion (n = 1), and lung cancer (n = 1). Finally, 53 patients with SMAE were enrolled. The patients were then divided into a survival group (n = 44) and a death group (n = 9). General clinical data (including sex, age, history of heart disease, smoking, alcohol, hypertension, diabetes, surgery, time of abdominal pain and body temperature at admission) and biochemical indicators at admission (including blood cell count, D-dimers, blood lactic acid, blood pH) were collected from the patients.
Siemens Somatom Sensation 64-slice spiral CT, Siemens Definition Flash CT, or Siemens Somatom FORCE CT were used to perform three-phase scans (unenhanced phase, arterial phase and venous phase). The scan parameters are listed in Table 1.
| Group | Scan protocol/parameters |
| Scan area | From diaphragm top to lower edge of symphysis pubis |
| Body position | Supine position |
| Tube voltage (kV)/tube current (mAs) | 120/care dose 4D |
| Linear fusion coefficient | 0.5 |
| Pitch | 1.0 |
| Rotate speed | 0.5 s |
| Collimation (mm) | 2 × 192 × 0.6 |
| Thickness of collection layer (mm) | 1 mm |
| Interlayer spacing | 0.6 mm |
| Convolution kernel | Br40 |
| Enhancement protocol | Arterial phase (automatic tracking technology: when the aortic monitoring threshold reached 100 HU, the arterial phase scan was triggered); intravenous phase (scan started 75 s after the contrast agent was injected) |
| Contrast agent (concentration) | Iopromide (370 mgI/mL) |
| Dose and rate of contrast agent administration | 1.5 mL/kg, 3.5 mL/s |
All collected raw images were sent to a Synovia post-processing workstation. Multiplanar reconstruction was performed on the three-phase image, with slice thickness of 2 mm and 2 mm increments. The maximum intensity projection (MIP) was reconstructed in the arterial phase, and the conventional coronary thin-MIP image (parallel to the SMA trunk, with a slice thickness 10 mm, and 5 mm increments), oblique coronal thin-MIP image (parallel to the long axis of the ileocolonic and jejunal arteries, with a slice thickness 10 mm, and 5 mm increments) and sagittal thin-MIP image (slice thickness 10 mm, 5 mm increments) were reconstructed as needed. The images in the arterial phase were used to reconstruct the volume rendered technique (VRT) images of the blood vessels.
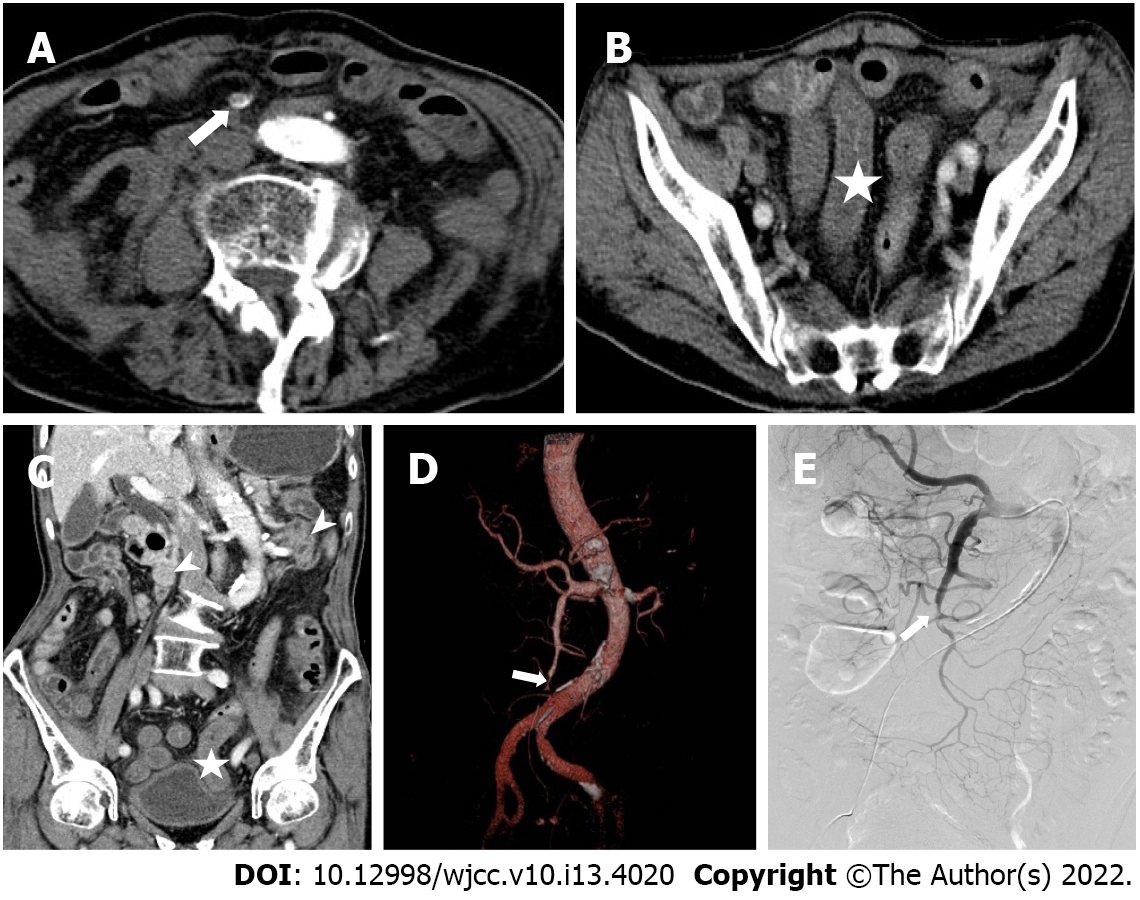
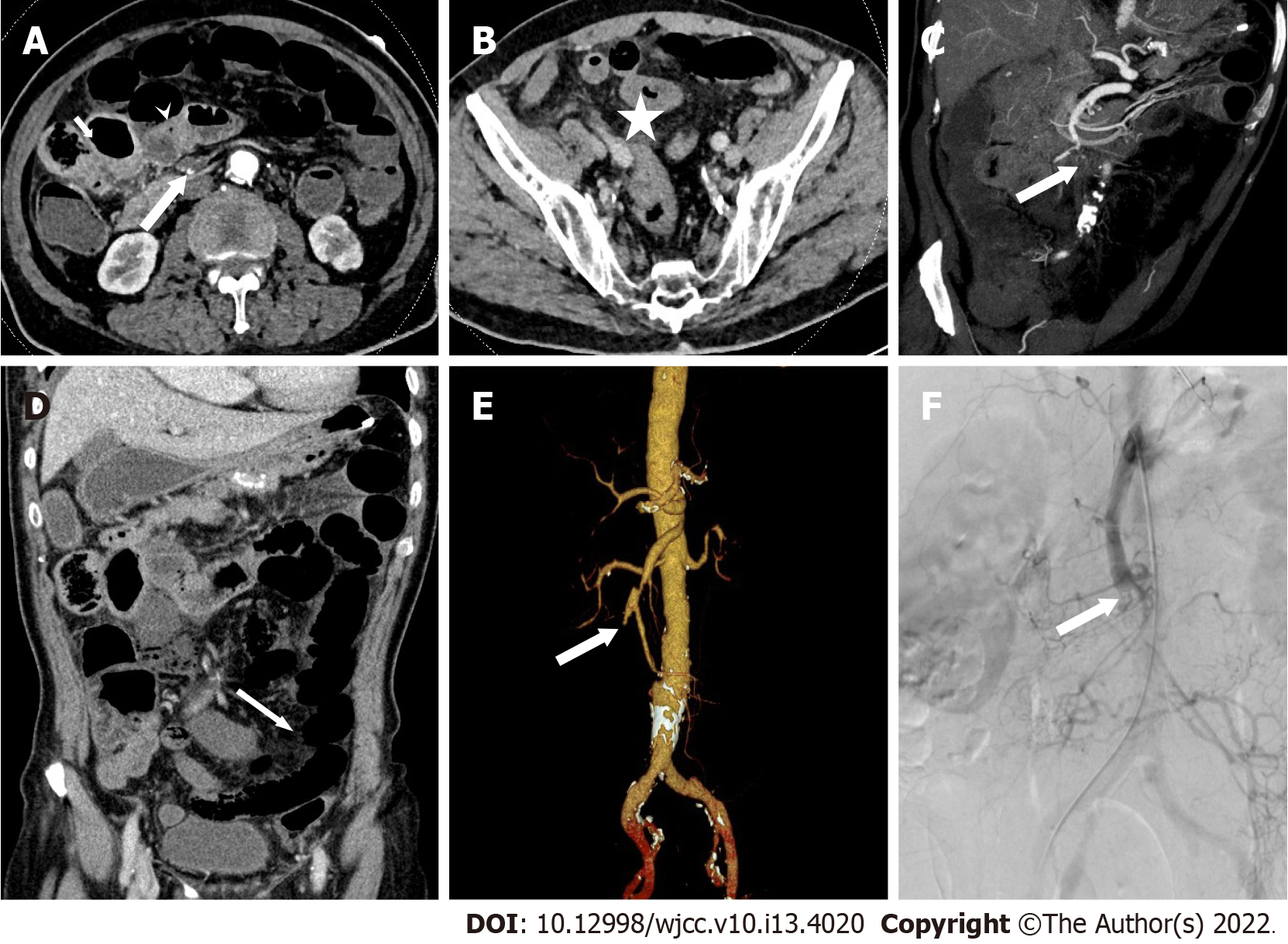
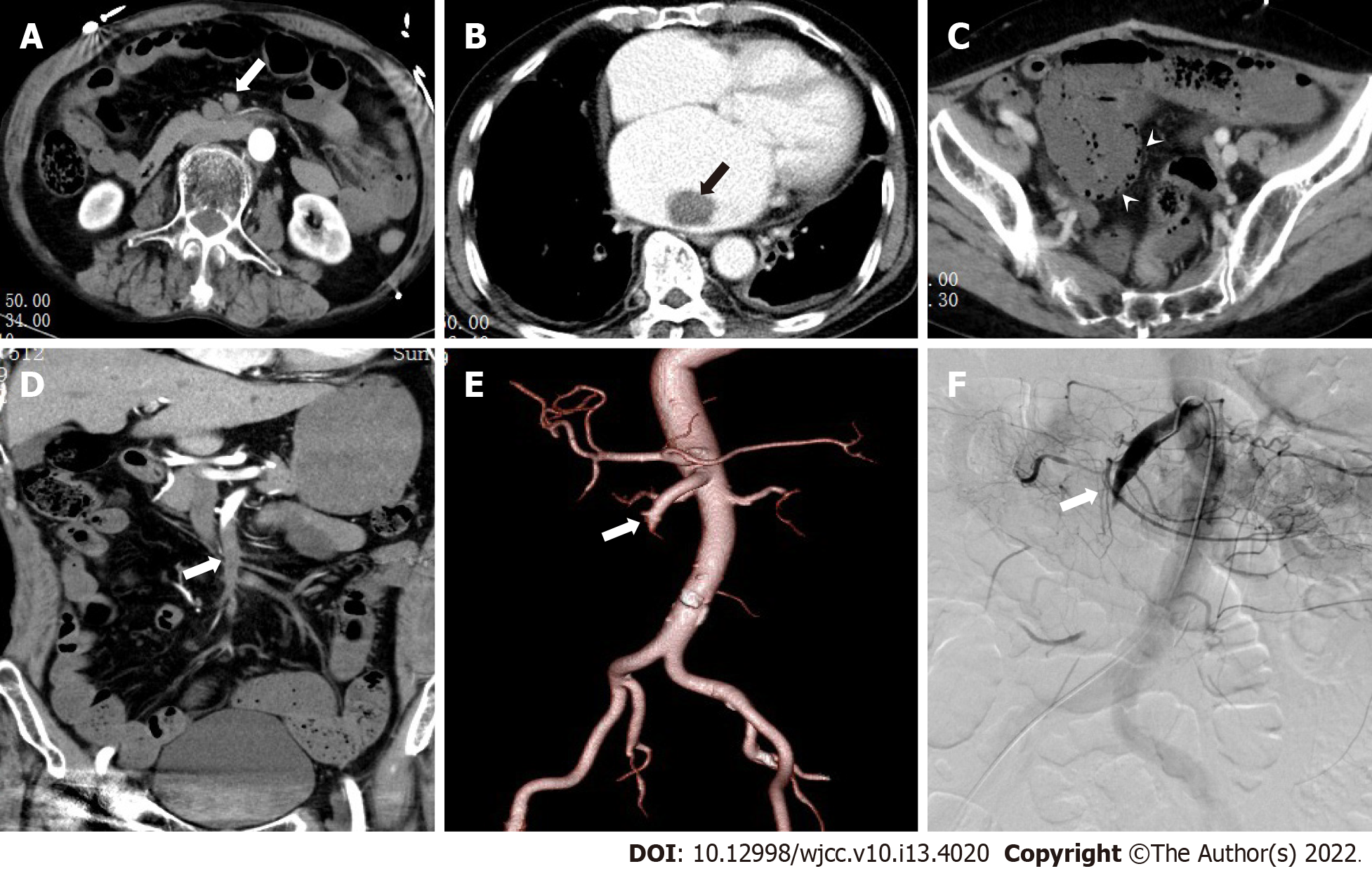
MDCT images were independently evaluated by two radiologists, A and B, with experience in abdominal CT diagnosis. Only doctor B was informed that the patients had been initially diagnosed with or suspected of having mesenteric vascular disease. The main CT findings included SMAE length (divided into three groups: ≤ 20 mm, 20-50 mm, and ≥ 50 mm), distribution area (SMA was divided into four regions: I, II, III, and IV[11]), thickening of intestinal wall (≥ 3 mm), intestinal wall thinning (same thickness as paper), decreased or no enhancement of intestinal wall (compared to the CT value of the adjacent normal intestinal wall), pneumatosis intestinalis, mesenteric fat stranding, and infarction of other abdominal organs. Blood vessel conditions were evaluated using DSA as the reference standard, and inconsistent CT evaluations were discussed between the two doctors. The imaging data of typical patients are shown in Figures 1-3.
The prognoses of these patients were followed up after admission. The follow-up period was 15 (3, 24) mo.
Quantitative data were expressed as median (range). The normal range was used to classify some continuous variables into two categories: D-dimer, blood lactic acid level, blood pH, blood amylase, and blood creatinine. Qualitative data were recorded as patient numbers (percentages). Pearson χ2 test or Fisher's exact test was used to compare categorical variables, and the independent sample t-test was used for normally distributed continuous variables.
Univariate Cox regression and multivariate Cox models with adjusted confounding factors were used to analyze the correlation and effect size between the death risk of SMAE patients and general clinical data, biochemical indicators, and MDCT findings. The risk ratio and 95%CI for each influencing factor were analyzed and calculated.
The stata16 software was used for statistical analysis, with a two-sided α of 0.05. Statistical significance was set at P < 0.05.
Among the 53 SMAE patients diagnosed by DSA, 41 patients received endovascular or surgical treatment after admission and subsequent anticoagulation, and two died. One patient died of intracerebral hemorrhage after surgery; another patient progressed to extensive small intestinal necrosis after conservative treatment, followed by laparotomy for ischemic and necrotic parts of the jejunum, ileum and ileocecal area, and finally died during hospitalization. Twelve patients chose medical anticoagulant therapy, and seven died, of which four died during hospitalization, two within 30 d of follow-up, and one after 9 mo of follow-up. Causes of death included multiple organ failure and extensive intestinal avascular necrosis.
The median age of nine died patients who died was 75 (73, 82) years, including five men and four women; the duration of abdominal pain at admission was 1 d (0.75, 2). Among the 44 surviving patients, the median age was 72 (range, 62.25-80) years, including 28 males and 16 females; and the duration of abdominal pain at admission was 2 (range, 1-5) d. There were no significant difference in sex (P = 0.938), age (P = 0.231), duration of abdominal pain at admission (P = 0.298), or basic clinical history between the two groups, as shown in Table 2.
| Total number (n = 53) | Survival group (n = 44) | Death group (n = 9) | P value | |
| Sex (%) | 0.938 | |||
| Male | 33 (62.3) | 28 (52.8) | 5 (9.4) | |
| Female | 20 (37.7) | 16 (30.2) | 4 (7.5) | |
| Smoking (%) | 1.000 | |||
| No | 35 (66.0) | 29 (54.7) | 6 (11.3) | |
| Yes | 18 (34.0) | 15 (28.3) | 3 (5.7) | |
| Alcohol (%) | 0.667 | |||
| No | 35 (66.0) | 28 (52.8) | 7 (13.2) | |
| Yes | 18 (34.0) | 16 (30.2) | 2 (3.8) | |
| Diabetes (%) | 0.978 | |||
| No | 44 (83.0) | 36 (67.9) | 8 (15.1) | |
| Yes | 9 (17.0) | 8 (15.1) | 1 (1.9) | |
| Hypertension (%) | 1.000 | |||
| No | 27 (50.9) | 22 (41.5) | 5 (9.4) | |
| Yes | 26 (49.1) | 22 (41.5) | 4 (7.5) | |
| Heart disease (%) | 1.000 | |||
| No | 26 (49.1) | 22 (41.5) | 4 (7.5) | |
| Yes | 27 (50.9) | 22 (41.5) | 5 (9.4) | |
| Surgery (%) | 0.395 | |||
| No | 38 (71.7) | 30 (56.6) | 8 (15.1) | |
| Yes | 15 (28.3) | 14 (26.4) | 1 (1.9) | |
| Vomitus (%) | 0.938 | |||
| No | 20 (37.7) | 16 (30.2) | 4 (7.5) | |
| Yes | 33 (62.3) | 28 (52.8) | 5 (9.4) | |
| Age (yr) | 74 (63.50, 80.50) | 72 (62.25, 80.00) | 75 (73.00, 82.00) | 0.231 |
| Abdominal pain duration (d) | 1.00 (1.00, 5.00) | 2.00 (1.00, 5.00) | 1.00 (0.75, 2.00) | 0.298 |
| Body temperature at admission (℃) | 36.7 (36.5, 37.7) | 36.6 (36.4, 37.3) | 37.7 (36.6, 38.4) | 0.055 |
A comparison of the biochemical indicators between the death and survival groups is shown in Table 3. Among the nine patients in the death group, five had blood lactate > 2.1 mmol/L, four had blood pH < 7.35, while the corresponding numbers in the survival group were four and two, respectively; the difference was significant (P = 0.004). Blood amylase indices were collected from 38 cases, including six cases in the death group (three with increased blood amylase) and 32 in the survival group (six with increased blood amylase), and the difference was significant (P = 0.027). There was no statistical difference in the other biochemical indicators between the two groups.
| Total number (n = 53) | Survival group (n = 44) | Death group (n = 9) | P value | |
| D-dimer > 1000 μg/mL (%) | 0.499 | |||
| No | 20 (37.7) | 18 (34.0) | 2 (3.8) | |
| Yes | 33 (62.3) | 26 (49.1) | 7 (13.2) | |
| Blood lactate > 2.1 mmol/L (%) | 0.004 | |||
| No | 44 (83.0) | 40 (75.5) | 4 (7.5) | |
| Yes | 9 (17.0) | 4 (7.5) | 5 (9.4) | |
| Blood pH < 7.35 (%) | 0.004 | |||
| No | 47 (88.7) | 42 (79.2) | 5 (9.4) | |
| Yes | 6 (11.3) | 2 (3.8) | 4 (7.5) | |
| Blood amylase > 110 U/L (%) | 0.027 | |||
| No | 32 (84.2) | 29 (76.3) | 3 (7.9) | |
| Yes | 6 (15.8) | 3 (7.9) | 3 (7.9) | |
| Serum creatinine > 120 μmol/L (%) | 0.097 | |||
| No | 36 (78.3) | 32 (69.6) | 4 (8.7) | |
| Yes | 10 (21.7) | 6 (13.0) | 4 (8.7) | |
| White blood cell | 10.6 (6.95, 15.45) | 9.1 (6.73, 16.48) | 10.7 (9.2, 14.6) | 0.362 |
| Red blood cell | 4.19 (3.89, 4.64) | 4.18 (3.97, 4.63) | 4.48 (3.60, 4.94) | 0.887 |
| Neutrophil | 8.5 (5.42, 13.66) | 7.55 (5.36, 14.46) | 9.42 (7.75, 12.90) | 0.236 |
| Lymphocyte | 0.9 (0.6, 1.2) | 0.90 (0.60, 1.20) | 0.70 (0.55, 1.20) | 0.392 |
| Neutrophil/lymphocyte | 9.3 (6.00, 16.22) | 8.47 (5.48, 16.42) | 13.46 (8.52, 17.33) | 0.169 |
| Total protein | 64.4 (56.55, 70.85) | 64.2 (56.73, 69.45) | 67.0 (51.3, 76.05) | 0.713 |
| Albumin to total protein ratio | 1.20 (1.0, 1.3) | 1.20 (1.00, 1.38) | 1.10 (1.00, 1.30) | 0.516 |
| Blood glucose | 6.90 (5.65, 8.44) | 6.8 (4.93, 8.44) | 7.70 (6.90, 16.28) | 0.107 |
The 53 patients with SMAE were confirmed to have SMAE using DSA. The length and distribution of the SMAE were (Table 4): ≤ 20 mm (14/53), 20-50 mm (11/53), ≥ 50 mm (28/53); I (5/53), II (1/53), III (2/53), IV (8/53), I + II (2/53), III + IV (19/53), I + II + III (1/53), II + III + IV (7/53) and I + II + III + IV (8/53). Doctor B was significantly better than doctor A in the diagnosis of embolism with a length ≤ 20 mm (P = 0.014) and in regions III and IV (P = 0.024). The length and distribution of SMAE were not statistically different between the death and survival groups.
| Total number | A | B | P value | |
| Embolus involvement length (%) | ||||
| ≤ 20 mm | 14 | 6 | 13 | 0.014 |
| 20-50 mm | 11 | 10 | 11 | 1 |
| ≥ 50 mm | 28 | 25 | 28 | 0.236 |
| Embolus region (%) | ||||
| I | 5 | 4 | 5 | 1 |
| II | 1 | 1 | 1 | 1 |
| III | 2 | 0 | 2 | 0.024 |
| IV | 8 | 3 | 7 | |
| I + II | 2 | 2 | 2 | 1 |
| III + IV | 19 | 18 | 19 | 1 |
| I + II + III | 1 | 1 | 1 | 1 |
| II + III + IV | 7 | 6 | 7 | 1 |
| I + II + III + IV | 8 | 8 | 8 | 1 |
| Extravascular signs (%) | ||||
| Decreased intestinal wall enhancement | 30 | 24 | 28 | 0.255 |
| Intestinal wall thinning | 22 | 9 | 14 | 0.131 |
| Intestinal wall thickening | 14 | 14 | 14 | 1 |
| Pneumatosis intestinalis | 5 | 3 | 3 | 1 |
| Infarction of the organs | 22 | 18 | 22 | 0.108 |
| Mesenteric fat stranding | 29 | 25 | 27 | 0.67 |
Among the nine patients in the death group, four (44%) had varying degrees of intestinal wall thickening, eight (89%) had intestinal wall thinning, nine (100%) had decreased intestinal wall enhancement, three (33%) had pneumatosis intestinalis, five (56%) had infarction of other organs, seven (78%) had mesenteric fat stranding. Among these indicators, the proportions of patients with decreased intestinal wall enhancement (P = 0.004), intestinal wall thinning (P = 0.002), and pneumatosis intestinalis (P = 0.007) were higher in the death group than in the survival group. There was no difference between doctor A and B in the diagnosis of extravascular CT signs (Table 5).
| Survival group (n = 44) | Death group (n = 9) | P value | |
| Embolus involvement length (%) | 0.311 | ||
| ≤ 20 mm | 12 (27.27) | 1 (11.11) | |
| 20-50 mm | 10 (22.73) | 1 (11.11) | |
| ≥ 50 mm | 22 (50.00) | 7 (77.78) | |
| Embolus region (%) | 0.212 | ||
| I | 4 (9.09) | 1 (11.11) | |
| II | 1 (2.27) | 0 (0) | |
| III | 2 (4.55) | 0 (0) | |
| IV | 8 (18.18) | 0 (0) | |
| I + II | 1 (2.27) | 1 (11.11) | |
| III + IV | 17 (38.64) | 2 (22.22) | |
| I + II + III | 1 (2.27) | 0 (0) | |
| II + III + IV | 6 (13.64) | 1 (11.11) | |
| I + II + III + IV | 4 (9.09) | 4 (44.44) | |
| Decreased intestinal wall enhancement (%) | 0.004 | ||
| No | 23 (52.27) | 0 (0) | |
| Yes | 21 (47.73) | 9 (100) | |
| Intestinal wall thinning (%) | 0.002 | ||
| No | 30 (68.18) | 1 (11.11) | |
| Yes | 14 (31.82) | 8 (88.89) | |
| Intestinal wall thickening (%) | 0.178 | ||
| No | 34 (77.27) | 5 (55.56) | |
| Yes | 10 (22.73) | 4 (44.44) | |
| Pneumatosis intestinalis (%) | 0.007 | ||
| No | 42 (95.45) | 6 (66.67) | |
| Yes | 2 (4.55) | 3 (33.33) | |
| Infarction of other organs (%) | 0.348 | ||
| No | 27 (61.36) | 4 (44.44) | |
| Yes | 17 (38.64) | 5 (55.56) | |
| Mesenteric fat stranding (%) | 0.127 | ||
| No | 22 (50) | 2 (22.22) | |
| Yes | 22 (50) | 7 (77.78) |
As shown in Table 6, the results of the univariate Cox regression analysis showed that intestinal wall thinning (HR, 13.35, 95%CI: 1.54-99.01) and pneumatosis intestinalis (HR, 5.73, 95%CI: 1.41-23.35) increased the risk of death in patients with SMAE. Blood lactate levels > 2.1 mmol/L and blood pH < 7.35 caused a 6.82-fold (HR, 6.82; 95%CI: 1.82-25.60) and 7.36-fold (HR, 7.36; 95%CI: 1.94-27.86) increase in risk of death, respectively.
| HR | 95%CI | P value | |
| Intestinal wall thinking | 2.27 | (0.61-8.45) | 0.223 |
| Intestinal wall thinning | 13.35 | (1.54-99.01) | 0.018 |
| Pneumatosis intestinalis | 5.73 | (1.41-23.35) | 0.015 |
| Other organ infarction | 1.79 | (0.48-6.67) | 0.386 |
| mesenteric fat stranding | 2.96 | (0.62-14.27) | 0.176 |
| Blood lactate > 2.1 mmol/L | 6.82 | (1.82-25.60) | 0.004 |
| Blood pH < 7.35 | 7.36 | (1.94-27.86) | 0.003 |
| Embolus distribution region (multi-classification variable) | 1.42 | (0.92-1.74) | 0.156 |
| Embolus involvement length | |||
| 20-50 mm (vs ≤ 20 mm) | 1.09 | (0.07-17.50) | 0.951 |
| ≥ 50 mm (vs ≤ 20 mm) | 3.14 | (0.39-25.54) | 0.284 |
As shown in Table 7, the multivariate Cox regression model I, adjusted for age, sex, embolic involvement range, and embolic distribution area, showed that intestinal wall thinning (HR, 9.40, 95%CI: 1.05-83.46) and blood lactate > 2.1 mmol/L (HR, 5.26, 95%CI: 1.04-26.69) increased the risk of death in SMAE patients. Multivariate Cox regression model II, adjusted for age, sex and embolic distribution showed that intestinal wall thinning (HR, 9.66, 95%CI: 1.15-81.03), intestinal wall pneumatosis (HR, 6.09, 95%CI: 1.25-29.69), blood lactic acid > 2.1 mmol/L (HR, 5.26, 95%CI: 1.04-26.69) and pH < 7.35 (HR, 5.59, 95%CI: 1.26-24.88) increased the risk of death in SMAE patients. Multivariate Cox regression model III, adjusted for sex, embolic involvement range and embolic distribution area, showed that intestinal wall thinning (HR, 11.01, 95%CI: 1.32-92.22), intestinal wall pneumatosis (HR, 6.01, 95%CI: 1.25-28.97), blood lactic acid > 2.1 mmol/L (HR, 6.34, 95%CI: 1.56-25.73) and pH < 7.35 (HR, 6.26, 95%CI: 1.47-26.59) increased the risk of death in SMAE patients.
| Model1 | Model2 | Model3 | ||||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | ||
| Intestinal wall thinning | 9.40 | (1.05-83.46) | 0.044 | 9.66 | (1.15-81.03) | 0.037 | 11.01 | (1.32-92.22) | 0.027 | |
| Pneumatosis intestinalis | 4.58 | (0.86-24.36) | 0.074 | 6.09 | (1.25-29.69) | 0.025 | 6.01 | (1.25-28.97) | 0.025 | |
| Blood lactate > 2.1 mmol/L | 5.26 | (1.04-26.69) | 0.045 | 5.21 | (1.12-24.22) | 0.035 | 6.34 | (1.56-25.73) | 0.010 | |
| Blood pH < 7.35 | 4.84 | (0.94-25.09) | 0.060 | 5.59 | (1.26-24.88) | 0.024 | 6.26 | (1.47-26.59) | 0.013 | |
Early diagnosis and treatment is key to reduce the mortality of patients with SMAE, but it is also a clinical challenge. Intestinal ischemia during the early stage of SMAE is often reversible. At this stage, the physical examination and laboratory findings are non-specific. As the disease progresses, intestinal ischemia becomes irreversible, and patients may experience multiple organ failure[8,10,12], increased blood lactate levels[8,12], increased blood amylase[13], increased white blood cells[14] and other abnormal clinical and laboratory findings. Even with clinical intervention, the patient's prognosis is poor. In this study, we also observed that the increase in blood lactate level was an important factor in the death of patients with SMAE, because it usually represents the late stage of intestinal ischemia. In this study, the mortality rate of patients with SMAE was approximately 17%, which was lower than that reported in the literature[15]. It is possible that most of the enrolled patients were in a state of reversible intestinal ischemia. After vascular recanalization through intravascular thrombectomy or thrombolysis, intestinal blood supply can be restored.
The difficulty in the early diagnosis of SMAE also lies in the interference from other common acute abdominal pain lesions, such as acute appendicitis, peptic ulcer, acute pancreatitis, digestive tract inflammation, cholecystitis, especially when the two are concurrent. Therefore, clinicians should consider SMAE from the above-mentioned common diseases as critical to the final diagnosis. According to previous reports[16], SMAE is not uncommon in patients aged > 75 years, with a higher incidence than that of appendicitis. Cardiovascular and cerebrovascular diseases, such as cerebral infarction, hypertension, heart disease, are risk factors for SMAE[17]. In this study, 49.1% and 50.9% of patients with SMAE had hypertension and heart-related diseases, respectively. Therefore, SMAE should be considered in elderly patients with abdominal pain and a history of cardiovascular and cerebrovascular diseases.
Clinically suspected SMAE is helpful for accurate MDCT diagnosis. Multi-phase MDCT enhanced scanning has become an important means of diagnosing SMAE[3,4,18], which can not only accurately assess the embolism of mesenteric vessels, but also assess the conditions of the extravascular digestive tract and solid organs. Wadman et al[19] found that SMAE patients with multi-phase enhanced CT scan had lower hospital mortality rates than those with unenhanced CT scans. Nuzzo et al[20] proposed that if SMAE is suspected clinically, an unenhanced CT scan may delay its diagnosis and treatment, and a CT multi-phase enhanced scan should be performed immediately. In addition, if SMAE is clinically suspected before a MDCT scan, the accuracy of the radiologist's diagnosis can be significantly increased[19,21]. There are differences in the length and region of involvement of the SMAE. For SMA peripheral blood vessels or embolism with a small length, MDCT diagnosis may not be optimal; if SMAE is clinically suspected before a CT scan, purposeful inspection of the image by a radiologist will help detect concealed embolism. This study found that a radiologist (doctor B) who was informed of clinical suspicion of SAME before the CT scan was significantly better in diagnosing SMAE ≤ 20 mm (P = 0.014) and in regions III and IV (P = 0.024) than an uninformed radiologist (doctor A). Therefore, if SMAE is highly suspected, and the CT scan is negative, SMAE cannot be excluded with certainty, and the SMA peripheral blood vessels or small embolism should be paid more attention.
SMAE usually co-occurs with extravascular CT signs such as intestinal wall thickening, intestinal wall thinning, decreased enhancement of intestinal wall, pneumatosis intestinalis, mesenteric fat stranding, and infarction of other solid organs. Careful search for the above CT signs can help in the diagnosis of SMAE[22,23] and the prediction of prognosis[8,24-26]. In our study, three Cox regression models were established by adjusting for confounding factors, such as age, sex and embolism. Each model showed that intestinal wall thinning in CT signs significantly increased the risk of death in SMAE patients. Early intestinal ischemia caused by SMAE usually shows intestinal wall thickening due to intestinal mucosal edema, which is the most common CT sign. With the continuation of the ischemic state, the capillary blood volume in the intestinal wall mucosa decreases, the nerves and muscle layers in the mucosa are destroyed, the intestinal tension is lost and expanded, the intestinal wall becomes as thin as paper, and the degree of enhancement is further reduced, and even the intestinal wall necrosis and gas accumulation. It is usually accompanied by an increase in blood lactate levels, suggesting irreversible intestinal ischemia and an increased risk of death. In this study, the signs of intestinal wall thinning, decreased intestinal wall enhancement, and intestinal wall pneumatosis in the SMAE death group were significantly higher than those in the survival group, Similar to the findings of previous studies[8,12].
This study had some limitations. First, because this was a retrospective study, patient selection bias might have occurred, and some clinical or biochemical data were incomplete. Second, the clinical treatment schemes of patients with SMAE were different, and their impact on prognosis was not considered in this study.
In summary, for patients with abdominal pain and previous cardiovascular or cerebrovascular events, clinicians should consider the possibility of SMAE and perform MDCT multi-phase enhanced scanning. Active and effective communication between clinicians and radiologists before the examination is particularly useful in rapidly and accurately detecting SMAE ≤ 20 mm and in regions III and IV. Simultaneously, the combination of patients' MSCT imaging signs and clinical information can provide important information for the prognosis of patients with SMAE and a basis for reasonable treatment of the disease.
Superior mesenteric artery embolism (SMAE) has acute onset and fast progression, which seriously threatens the life of patients. The early diagnosis of SMAE is related to the patients’ recovery. However, to date, there are serious challenges in the early diagnosis of SMAE, and clinical suspicion is the key to diagnosis. MDCT is one of the most important diagnostic methods for SMAE, which plays an important role in the diagnosis and prognosis of SMAE.
Superior mesenteric artery (SMA) in peripheral vessels or small-scale emboli is easy to be missed, leading to irreversible intestinal necrosis, which affects the prognosis of patients. High clinical suspicion and some extra-vascular computed tomography (CT) signs are helpful for the diagnosis of SMAE. Currently, few studies have combined clinical, biochemical and MDCT to predict the risk of irreversible intestinal necrosis and death in SMAE at early stages.
The purpose of this study is to evaluate the value of combining clinical data and MDCT in the diagnosis of SMAE and to predict the risk factors for death.
We retrospectively analyzed the clinical and MDCT data of 53 patients with SMAE confirmed by digital subtraction angiography. We analyzed the impact of a high clinical suspicion on the radiologist's diagnosis of SAME on MDCT. The patients were divided into two groups: the death and survival groups. Univariate cox regression and multivariate cox model adjusted for confounding factors were used to analyze the association trend of mortality risk with clinical and CT signs in SMAE patients.
Under the premise of high clinical suspicion of SMAE, the radiologist was able to more accurately diagnose emboli with lengths ≤ 20 mm(P = 0.014) and in areas III and IV (P = 0.024). Univariate cox regression and multivariate cox model analysis adjusted for confounding factors determined that blood lactate > 2.1 mmol/L (HR, 5.26, 95%CI: 1.04-26.69, P = 0.045) and intestinal wall thinning (HR, 9.40, 95%CI: 1.05-83.46, P = 0.044) were consistently significantly associated with mortality in SAME patients.
Increased blood lactate and intestinal wall thinning are risk factors for death in patients with SMAE. Meaningful clinical cues combined with MDCT can significantly improve the accuracy of radiologists in diagnosing SMAE with the length ≤ 20 mm and embolism in regions III and IV.
With clinical suspicion of SMAE, a multiphase enhanced CT should be performed immediately to observe the SMA trunk and peripheral vessels, as well as extravascular MDCT. Intestinal wall thinning and increased blood lactate levels might be effective predictors for death in patients with SMAE, although further validation in large sample, prospective and multicenter studies is needed. With the advent of dual-energy CT, new post-processing techniques (Iodine mapping, virtual monoenergetic imaging) may provide important information on SMA peripheral small vessel embolization and whether intestinal wall is enhanced.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gumustas OG, Turkey; Yadav P, India S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Tang W, Jin B, Kuang LQ, Zhang J, Li CX, Wang Y. Risk factors of geriatrics index of comorbidity and MDCT findings for predicting mortality in patients with acute mesenteric ischemia due to superior mesenteric artery thromboembolism. Br J Radiol. 2020;93:20190605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Zhang Z, Wang D, Li G, Wang X, Wang Y, Jiang T. Endovascular Treatment for Acute Thromboembolic Occlusion of the Superior Mesenteric Artery and the Outcome Comparison between Endovascular and Open Surgical Treatments: A Retrospective Study. Biomed Res Int. 2017;2017:1964765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Expert Panels on Vascular Imaging and Gastrointestinal Imaging; Ginsburg M, Obara P, Lambert DL, Hanley M, Steigner ML, Camacho MA, Chandra A, Chang KJ, Gage KL, Peterson CM, Ptak T, Verma N, Kim DH, Carucci LR, Dill KE. ACR Appropriateness Criteria® Imaging of Mesenteric Ischemia. J Am Coll Radiol. 2018;15:S332-S340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Bala M, Kashuk J, Moore EE, Kluger Y, Biffl W, Gomes CA, Ben-Ishay O, Rubinstein C, Balogh ZJ, Civil I, Coccolini F, Leppaniemi A, Peitzman A, Ansaloni L, Sugrue M, Sartelli M, Di Saverio S, Fraga GP, Catena F. Acute mesenteric ischemia: guidelines of the World Society of Emergency Surgery. World J Emerg Surg. 2017;12:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 5. | Cudnik MT, Darbha S, Jones J, Macedo J, Stockton SW, Hiestand BC. The diagnosis of acute mesenteric ischemia: A systematic review and meta-analysis. Acad Emerg Med. 2013;20:1087-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 6. | Menke J. Diagnostic accuracy of multidetector CT in acute mesenteric ischemia: systematic review and meta-analysis. Radiology. 2010;256:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 182] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Yikilmaz A, Karahan OI, Senol S, Tuna IS, Akyildiz HY. Value of multislice computed tomography in the diagnosis of acute mesenteric ischemia. Eur J Radiol. 2011;80:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 8. | Nuzzo A, Maggiori L, Ronot M, Becq A, Plessier A, Gault N, Joly F, Castier Y, Vilgrain V, Paugam C, Panis Y, Bouhnik Y, Cazals-Hatem D, Corcos O. Predictive Factors of Intestinal Necrosis in Acute Mesenteric Ischemia: Prospective Study from an Intestinal Stroke Center. Am J Gastroenterol. 2017;112:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 9. | Zogheib E, Cosse C, Sabbagh C, Marx S, Caus T, Henry M, Nader J, Fumery M, Bernasinski M, Besserve P, Trojette F, Renard C, Duhaut P, Kamel S, Regimbeau JM, Dupont H. Biological scoring system for early prediction of acute bowel ischemia after cardiac surgery: the PALM score. Ann Intensive Care. 2018;8:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Grotelüschen R, Bergmann W, Welte MN, Reeh M, Izbicki JR, Bachmann K. What predicts the outcome in patients with intestinal ischemia? J Visc Surg. 2019;156:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Ottinger LW. The surgical management of acute occlusion of the superior mesenteric artery. Ann Surg. 1978;188:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 75] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Canfora A, Ferronetti A, Marte G, Maio VD, Mauriello C, Maida P, Bottino V, Aprea G, Amato B. Predictive Factors of Intestinal Necrosis in Acute Mesenteric Ischemia. Open Med (Wars). 2019;14:883-889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Acosta-Mérida MA, Marchena-Gómez J, Cruz-Benavides F, Hernández-Navarro J, Roque-Castellano C, Rodríguez-Méndez A, Alonso-Alvarado A, Hernández-Romero J. [Predictive factors of massive intestinal necrosis in acute mesenteric ischemia]. Cir Esp. 2007;81:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Emile SH. Predictive Factors for Intestinal Transmural Necrosis in Patients with Acute Mesenteric Ischemia. World J Surg. 2018;42:2364-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Acosta S, Ogren M, Sternby NH, Bergqvist D, Björck M. Clinical implications for the management of acute thromboembolic occlusion of the superior mesenteric artery: autopsy findings in 213 patients. Ann Surg. 2005;241:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Kärkkäinen JM, Lehtimäki TT, Manninen H, Paajanen H. Acute Mesenteric Ischemia Is a More Common Cause than Expected of Acute Abdomen in the Elderly. J Gastrointest Surg. 2015;19:1407-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Wyers MC. Acute mesenteric ischemia: diagnostic approach and surgical treatment. Semin Vasc Surg. 2010;23:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Chen YC, Huang TY, Chen RC, Tsai SH, Chang WC, Fan HL, Huang GS, Ko KH, Chou YC, Hsu HH. Comparison of Ischemic and Nonischemic Bowel Segments in Patients With Mesenteric Ischemia: Multidetector Row Computed Tomography Findings and Measurement of Bowel Wall Attenuation Changes. Mayo Clin Proc. 2016;91:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Wadman M, Block T, Ekberg O, Syk I, Elmståhl S, Acosta S. Impact of MDCT with intravenous contrast on the survival in patients with acute superior mesenteric artery occlusion. Emerg Radiol. 2010;17:171-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Nuzzo A, Joly F, Ronot M, Castier Y, Huguet A, Paugam-Burtz C, Cazals-Hatem D, Tran-Dinh A, Becq A, Panis Y, Bouhnik Y, Maggiori L, Corcos O; SURVI group. Normal Lactate and Unenhanced CT-Scan Result in Delayed Diagnosis of Acute Mesenteric Ischemia. Am J Gastroenterol. 2020;115:1902-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Lehtimäki TT, Kärkkäinen JM, Saari P, Manninen H, Paajanen H, Vanninen R. Detecting acute mesenteric ischemia in CT of the acute abdomen is dependent on clinical suspicion: Review of 95 consecutive patients. Eur J Radiol. 2015;84:2444-2453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 22. | Olson MC, Fletcher JG, Nagpal P, Froemming AT, Khandelwal A. Mesenteric ischemia: what the radiologist needs to know. Cardiovasc Diagn Ther. 2019;9:S74-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 23. | Barmase M, Kang M, Wig J, Kochhar R, Gupta R, Khandelwal N. Role of multidetector CT angiography in the evaluation of suspected mesenteric ischemia. Eur J Radiol. 2011;80:e582-e587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Atre ID, Eurboonyanun K, O'Shea A, Lahoud RM, Shih A, Kalva S, Harisinghani MG, Hedgire S. Predictors of transmural intestinal necrosis in patients presenting with acute mesenteric ischemia on computed tomography. Abdom Radiol (NY). 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Wang X, Chu C, Sun S, Xie T, Duan Z, Wang K, Liu B, Fan X, Wu X, Ding W. Outcomes and clinical characteristics of transmural intestinal necrosis in acute mesenteric ischemia. Scand J Gastroenterol. 2019;54:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Emile SH, Khan SM, Barsoum SH. Predictors of bowel necrosis in patients with acute mesenteric ischemia: systematic review and meta-analysis. Updates Surg. 2021;73:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |