Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3916
Peer-review started: September 16, 2021
First decision: October 25, 2021
Revised: November 18, 2021
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 216 Days and 15.7 Hours
Anti-glomerular basement membrane (GBM) disease is a rare autoimmune disease manifesting as acute progressive nephritis syndrome with or without varying degrees of pulmonary hemorrhage. Anti-GBM disease coexisting with Immunoglobulin A (IgA) nephropathy is rarer and has different clinical manifestations and prognoses than simple anti-GBM disease. We describe a case of coexistence of these two diseases.
A 49-year-old man with hematuria and proteinuria accompanied by a slight elevation of serum creatinine was admitted to our hospital. The pathological results of renal biopsy and the elevated serum anti-GBM antibody titer supported a diagnosis of anti-GBM disease combined with IgA nephropathy. After treatment with corticosteroids and cyclophosphamide, the patient's serum creatinine was relatively stable, and the hematuria and proteinuria moderately improved in the subsequent six months.
Anti-GBM disease coexisting with IgA nephropathy is rare. The clinical manifestations and prognosis are better than those of simple anti-GBM disease. In this case, the patient's condition was improved and his renal function remained relatively stable with corticosteroid and cyclophosphamide treatment. New detection methods to identify whether the crescents in this case were derived from anti-GBM disease or IgA nephropathy are worthy of further exploration.
Core Tip: This case reported a rare disease with both anti-glomerular basement membrane (GBM) disease and Immunoglobulin A (IgA) nephropathy. Its clinical manifestations and prognosis are better than those of simple anti-GBM disease. The patient's condition was improved and the renal function was relatively stable with the treatment of corticosteroids and cyclophosphamide. About 70% of the glomeruli contained crescents, and multiple crescents formed in this patient's renal biopsy pathology. New detection methods to identify whether the crescents in this case were derived from anti-GBM disease or IgA nephropathy are worthy of further exploration.
- Citation: Guo C, Ye M, Li S, Zhu TT, Rao XR. Anti-glomerular basement membrane disease with IgA nephropathy: A case report. World J Clin Cases 2022; 10(12): 3916-3922
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3916.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3916
Anti-glomerular basement membrane (GBM) disease is an autoimmune disease with anti-GBM antibody deposition, and the incidence rate of anti-GBM disease in the population is 1 to 2 cases per million population per year in European populations, accounting for 10%-15% of crescentic glomerulonephritis cases[1,2]. The main clinical manifestation is acute progressive nephritis syndrome with or without varying degrees of pulmonary hemorrhage. The disease progresses rapidly, and the prognosis is poor. The main renal pathology is crescentic glomerulonephritis, with Immunoglobulin G (IgG) deposited in a linear pattern along the capillary loop on direct immunofluorescence examination. The coexistence of anti-GBM disease and Immunoglobulin A (IgA) nephropathy is rare. Last year, our hospital diagnosed and treated a patient with concurrent anti-GBM disease and IgA nephropathy. A report of the characteristics of the patient and the disease follows.
A greater than 9-year history of hematuria was present, as was a greater than 1-year history of proteinuria.
A 49-year-old man was admitted to the nephrology department of Guang'anmen Hospital in November 2020. Nine years before this hospitalization, when the patient underwent a physical examination in the local hospital, the routine urine examination revealed 1+ blood, while urinary protein was negative and serum creatinine was normal. At that time, the patient had no obvious symptoms of discomfort, such as arthralgias, oral ulceration, or photosensitivity. Subsequently, the patient’s annual physical examination showed urinary occult blood fluctuating from 1+ to 3+, but the urinary protein was always negative, and the serum creatinine remained normal. Nearly 2 mo before admission, a routine urine examination showed 3+ blood and 3+ protein, and the patient was administered irbesartan 150 mg once a day. One day before admission, the patient’s urinalysis results revealed 3+ blood and 2+ protein, and the 24-h urine protein result was 5.158 g. In addition, the serum creatinine level was 133 μmol per liter.
The patient had a history of hypertension, hyperuricemia, and gout. He had hepatitis A as a child and was later cured.
The patient had a history of inhaling gasoline and diesel in his working environment and had a long history of smoking and drinking. He had no known allergic reactions to drugs or food. He denied a family history of kidney disease.
The patient’s vital signs were stable, and his blood pressure was 120/78 mmHg. His weight was 67 kg, and his body mass index was 23.2 kg/m2. The patient had mildly depressed edema in both lower limbs.
The novel coronavirus nucleic acid test was negative, and the novel coronavirus IgM antibody and IgG antibody tests were negative. The hepatitis B virus antigen, hepatitis C antibody, syphilis, and HIV tests were also negative. The other laboratory examination results are shown in Table 1. Subsequently, a positive anti-GBM antibody result was obtained at a titer of 68 RU/mL, though anti-nuclear antibodies and anti-neutrophil cytoplasmic antibodies were absent.
| Variable | Reference range | Initial value on admission | Value after intervention for half a year |
| Routine blood examination | |||
| White cell count (× 109/L) | 3.5−9.5 | 8.08 | 12.43 |
| Red cell count (× 1012/L) | 4.3−5.8 | 3.55 | 4.04 |
| Hemoglobin (g/L) | 130−175 | 108 | 126 |
| Biochemical blood examination | |||
| Urea nitrogen (mmol/L) | 2.9−8.2 | 6.04 | 8.85 |
| Creatinine (μmol/L) | 59−104 | 133 | 109 |
| Glucose (mmol/L) | 3.9−6.1 | 4.99 | 4.99 |
| Sodium (mmol/L) | 137−147 | 139.8 | 139 |
| Potassium (mmol/L) | 3.5−5.3 | 3.81 | 3.90 |
| Chloride (mmol/L) | 99−101 | 104.7 | 103.90 |
| Calcium (mg/dL) | 2.2−2.65 | 2.15 | 2.37 |
| Phosphorus (mg/dL) | 0.81−1.45 | 1.25 | 0.96 |
| Albumin (g/L) | 40−55 | 32.51 | 41.75 |
| Immunological indexes | |||
| ANA | Negative | − | |
| ASO (U/mL) | 0−116 | Less than 25 | − |
| C3 (g/L) | 0.79−1.52 | 1.15 | − |
| C4 (g/L) | 0.16−0.38 | 0.339 | − |
| IgG (g/L ) | 7.51−15.6 | 9.87 | − |
| IgA (g/L) | 0.82−4.53 | 2.27 | − |
| IgM (g/L) | 0.46−3.04 | 0.685 | − |
| Anti-PR3-ANCA (RU/mL) | 0−20 | 0 | − |
| Anti-MPO-ANCA (RU/mL) | 0−20 | 0 | − |
| Kappa light chain (mg/dL) | Negative | − | |
| Lambda light chain (mg/dL) | Negative | − | |
| Anti-PLA2R antibody (RU/mL) | Less than 5 | − | |
| Anti-THSD7A antibody | Negative | − | |
| Anti-GBM antibody (RU/mL) | 0−20 | 68 | − |
| Urinalysis | |||
| Occult blood | 3+ | 2+ | |
| RBCs (per high-power field) | 0−3 | 45.09 | 10.6 |
| Protein | 2+ | 2+ | |
| 24 h urinary total protein (g/24 h) | 5.16 | 1.68 |
Renal ultrasonography revealed that the size of the kidneys was normal and that the echo pattern from the renal cortex was normal.
Chest high-resolution computed tomography (CT) showed small solid nodules in the lower lobe of the left lung.
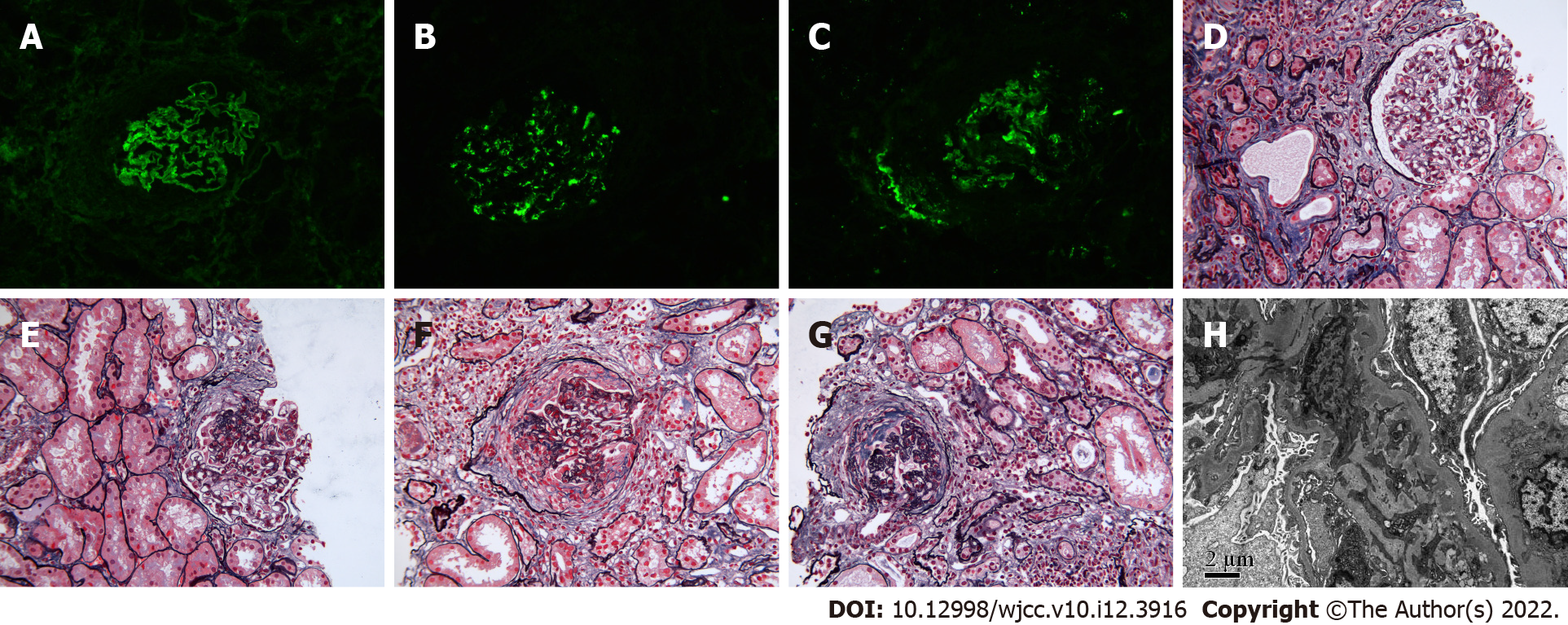
Immunofluorescence staining showed linear deposition of IgG, kappa, and lambda along the capillary wall. IgG1 and IgG4 were the main subtypes of IgG, and granular and bolus-type deposits of IgA and C3 were seen in the mesangial area.
Light microscopy demonstrated glomerulosclerosis and ischemic sclerosis, and approximately 70% of glomeruli contained crescents, including small-cell crescents, small cellular fibrous crescents, cellular fibrous crescents, and fibrous crescents with sclerosis, accompanied by seriously damaged glomerular capillary loops and partial destruction of Bowman's capsules. In addition, the glomerular mesangial cells and matrix were slightly proliferative. Renal interstitial inflammatory cell infiltration was accompanied by fibrosis. In addition, there were vacuoles and granular degeneration of renal tubular epithelial cells, the hair margin of the small focus brush had deteriorated, and multiple lesions showed atrophy. Immunohistochemistry revealed granular deposition of C4d in the capillary wall, mesangial area, and arteriole wall.
Electron microscopy showed massive electron-dense deposits in the mesangial area. The pathological results were consistent with a diagnosis of type I crescentic nephritis with IgA nephropathy (Figure 1).
A diagnosis of anti-GBM glomerulonephritis and IgA nephropathy was confirmed.
Methylprednisolone (500 mg) was given intravenously once a day for 3 consecutive days, and then 50 mg prednisolone acetate was given orally once a day. After 2 mo of continuous oral administration, the daily dosage of hormone was decreased by 5 mg per month. In addition, 0.8 g cyclophosphamide was given intravenously once a month.
After two months of treatment, the anti-GBM antibody result turned negative. On May 31, 2021, the dosage of corticosteroids was adjusted to 20 mg every day, and the cumulative amount of cyclophosphamide was 4.8 g. Subsequently, the patient’s serum creatinine fluctuated between 105 µmol/L and 123 µmol/L after discharge, the 24-hour urine protein quantity decreased from 5.16 g to 1.68 g, and the urine red blood cell count decreased from 45.09/HPF to 10.6/HPF under a high-power microscope (Table 1).
Anti-GBM disease is a rare systemic vasculitis mediated by autoantibodies produced against antigens in the glomerular and alveolar basement membrane that is often characterized by a rapid decline in renal function and alveolar hemorrhage[3,4]. Although the patient in this case did not show obvious pulmonary-renal syndrome symptoms, the anti-GBM antibody titer in the serum increased, and immunofluorescence analysis showed linear deposition of IgG along the capillary wall. Therefore, the diagnosis of anti-GBM disease was clear. In addition, IgA nephropathy was diagnosed in this patient because immunofluorescence analysis demonstrated IgA deposited along the mesangial area.
Based on a literature analysis, anti-GBM disease is closely related to genetic and environmental factors[5]. In terms of genetic susceptibility, anti-GBM disease is closely related to the HLA-DRB1 × 1501 allele and is also associated with genes of the KLK and FCGR families[6]. The patient in this case had no family history of kidney disease. In terms of environmental factors, anti-GBM disease is often associated with cigarette smoking or hydrocarbon inhalation, such as inhalation of gasoline, diesel, or paint, which may trigger exposure of cryptic collagen epitopes in the alveolar basement membrane, inducing the formation of anti-basement membrane antibodies and leading to nephritis and hemorrhage through cross-reaction with the GBM[5,7,8]. In contrast to the hemoptysis of most patients with a history of hydrocarbon inhalation, only small nodules were found on lung CT in this case[9].
Approximately 80% of cases of anti-GBM disease show crescents of similar age and activity in renal biopsy findings, reflecting the sudden onset of disease and distinguishing it from ANCA-associated vasculitis, in which a mixture of cellular, fibrocellular and fibrous crescents are often observed[3,10,11]. In addition, the proportion of glomeruli containing crescents in IgA nephropathy is usually low[12]. However, in this case, approximately 70% of the glomeruli contained crescents, and the multiple crescents seen on this patient’s renal biopsy pathology results were negative for ANCAs. Therefore, it is difficult to determine whether the crescents in this patient resulted from anti-GBM disease or IgA nephropathy. Kojima et al[13] previously reported a patient diagnosed with anti-GBM disease during IgA nephropathy progression; in this case, the researchers were also unable to determine whether the anti-GBM disease was primary or secondary because the source of the crescents was unknown.
In regard to treatment, plasma exchange (PE) is often the first choice to eliminate existing anti-GBM antibodies, followed by administration of corticosteroids and cyclophosphamide to suppress inflammation and reduce renal damage[11]. After analyzing some similar case reports in recent 5 years searching from PubMed (https://pubmed.ncbi.nlm.nih.gov/) (Table 2), we found that although the patients had rapidly decreasing renal function and positive anti-GBM antibody, the disease could be improved without PE. Even if PE was used, the patients might not get rid of dialysis[13-16]. For this patient, considering that there was no rapid and progressive increase in serum creatinine and that the serum anti-GBM antibody titer was not high, PE was not used. After 2 mo of treatment with corticosteroids and cyclophosphamide, the patient's serum anti-GBM antibody result turned negative. After half a year of follow-up, the patient's serum creatinine remained relatively stable, and the hematuria and proteinuria were improved.
| Ref. | Sex (Male/Female) | Age (yr) | Initial serum creatinine (μmol/L) | Pulmonary hemorrhage | Gross hematuria | Anti-GBM antibody | Crescent ratio | Treatment methods | Treatment outcome |
| Annamalai et al[14], 2021 | Female | 22 | 168.53 increase to 282.88 in one week | Negative | Positive | 96 U/mL | 7/10 | IV Methyl-PD + oral-PD | Improved, nondialysis-dependent renal dysfunction |
| Suh et al[15], 2019 | Female | 38 | 57.46 increase to 481.78 in three months | Negative | Positive | 187.2 U/mL | 11/16 | IV Methyl-PD + oral-PD + IV cyclophsphamide | Improved, nondialysis-dependent renal dysfunction |
| Kojima et al[13], 2019 | Female | 66 | 91.62 increase to 400.45 in one month | Negative | Negative | 116 IU/mL | 18/25 | HD + PE + IV Methyl-PD + oral-PD | HD |
| Xu et al[16], 2016 | Female | 50 | 157 increase to 220 in ten days | Negative | Positive | 258.3 EU/mL | 16/18 | IV Methyl-PD + Oral-PD + Mycophenolate mofetil | Improved, nondialysis-dependent renal dysfunction |
| This case | Male | 49 | 133 without rapid progress | Negative | Negative | 68 RU/mL | 21/29 | IV Methyl-PD + oral-PD + IV cyclophsphamide | Improved, nondialysis-dependent renal dysfunction |
A relevant literature review showed that the prognosis of concurrent anti-GBM disease and IgA nephropathy seems to be better than that of simple anti-GBM disease; This improved prognosis with concomitant disease may be due to the deposition of immune complexes associated with IgA nephropathy, which can result in changes in the composition of the GBM[15-17]. In the case reports summarized (Table 2), four of five cases showed rapidly decreasing kidney function at the beginning, but four of five cases showed improvement after treatment and did not rely on dialysis[13-16]. However, more clinical and laboratory data are needed to further confirm the prognosis of this coexisting disease.
This is a case report of anti-GBM disease coexisting with IgA nephropathy. The patient had a history of hydrocarbon inhalation and smoking, which are environmental factors associated with anti-GBM disease. Fortunately, there was no pulmonary hemorrhage or rapidly progressive glomerulonephritis. After combined administration of corticosteroids and cyclophosphamide, the patient's anti-GBM antibody result turned negative, and serum creatinine remained relatively stable. The origin of the crescents and the relationship between IgA nephropathy and anti-GBM nephropathy in this patient are worthy of further study and analysis.
We would like to thank the patient and the medical staff of the Nephrology Department of Guang'anmen Hospital, China Academy of Chinese Medical Sciences. We thank the Institute of Nephrology of Peking University First Hospital for providing renal pathology pictures and pathological diagnosis. We thank Dr. Yang XF for his constructive comments.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Urology and nephrology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Le PH, Taiwan; Tsilivigkos C, Greece S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | McAdoo SP, Pusey CD. Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. 2017;12:1162-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 262] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 2. | Canney M, O'Hara PV, McEvoy CM, Medani S, Connaughton DM, Abdalla AA, Doyle R, Stack AG, O'Seaghdha CM, Clarkson MR, Griffin MD, Holian J, Dorman AM, Niland A, Keogan M, Wallace EM, Conlon NP, Walsh C, Kelly A, Little MA. Spatial and Temporal Clustering of Anti-Glomerular Basement Membrane Disease. Clin J Am Soc Nephrol. 2016;11:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | Jennette JC. Rapidly progressive crescentic glomerulonephritis. Kidney Int. 2003;63:1164-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 273] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | McAdoo SP, Pusey CD. Antiglomerular Basement Membrane Disease. Semin Respir Crit Care Med. 2018;39:494-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Cui Z, Zhao MH. Advances in human antiglomerular basement membrane disease. Nat Rev Nephrol. 2011;7:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Zhou XJ, Lv JC, Zhao MH, Zhang H. Advances in the genetics of anti-glomerular basement membrane disease. Am J Nephrol. 2010;32:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Povey J, Rutherford E, Levy J, Muniraju T. Relapse of treated anti-GBM disease following hair dye use. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Troxell ML, Houghton DC. Atypical anti-glomerular basement membrane disease. Clin Kidney J. 2016;9:211-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Cui Z, Zhao MH, Singh AK, Wang HY. Antiglomerular basement membrane disease with normal renal function. Kidney Int. 2007;72:1403-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | L'Imperio V, Ajello E, Pieruzzi F, Nebuloni M, Tosoni A, Ferrario F, Pagni F. Clinicopathological characteristics of typical and atypical anti-glomerular basement membrane nephritis. J Nephrol. 2017;30:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Gulati K, McAdoo SP. Anti-Glomerular Basement Membrane Disease. Rheum Dis Clin North Am. 2018;44:651-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Roberts IS. Pathology of IgA nephropathy. Nat Rev Nephrol. 2014;10:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 161] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 13. | Kojima T, Hirose G, Komatsu S, Oshima T, Sugisaki K, Tomiyasu T, Yoshikawa N, Yamada M, Oda T. Development of anti-glomerular basement membrane glomerulonephritis during the course of IgA nephropathy: a case report. BMC Nephrol. 2019;20:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Annamalai I, Chandramohan G, Srinivasa Prasad ND, Fernando E, Sujith S. Rapidly progressive glomerulonephritis due to anti-glomerular basement membrane disease accompanied by IgA nephropathy: An unusual association. Saudi J Kidney Dis Transpl. 2017;28:1404-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Suh KS, Choi SY, Bae GE, Choi DE, Yeo MK. Concurrent Anti-glomerular Basement Membrane Nephritis and IgA Nephropathy. J Pathol Transl Med. 2019;53:399-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Xu D, Wu J, Xu C, Zhang Y, Mei C, Gao X. Novel therapy for anti-glomerular basement membrane disease with IgA nephropathy: A case report. Exp Ther Med. 2016;11:1889-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Cui Z, Zhao MH, Wang SX, Liu G, Zou WZ, Wang HY. Concurrent antiglomerular basement membrane disease and immune complex glomerulonephritis. Ren Fail. 2006;28:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |