Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3808
Peer-review started: December 7, 2021
First decision: January 25, 2022
Revised: February 15, 2022
Accepted: March 7, 2022
Article in press: March 7, 2022
Published online: April 26, 2022
Processing time: 135 Days and 3.5 Hours
Thrombotic thrombocytopenic purpura (TTP) is a life-threatening but treatable disorder. Acute pancreatitis is a well-described consequence of TTP, but TTP as a consequence of acute pancreatitis is rare.
A 32-year-old male developed acute pancreatitis due to a fatty diet and suffered splenectomy 3 years ago due to trauma. From day 4 of his onset of pain the blood examination showed the platelet extremely reduced, bilirubin elevated and creatinine increased. High clinical suspicion of TTP was made and prompt initiation of plasma exchange was given followed intravenous drip methylprednisolone. After 7 sessions of plasm exchange and the laboratory parameters were back to normal and the patient was discharged from the hospital on the 13th day of admission.
Patients develop acute pancreatitis with no apparent causes for hemolytic anemia and thrombocytopenia, the possibility of TTP should be considered. Treatments for TTP including plasm exchange should be evaluated as soon as a diagnosis is made.
Core Tip: This subject is a rare case report encountered in clinical work. Acute pancreatitis accompanied thrombotic thrombocytopenic purpura is fatal but treatable. Highly recognized this disease could save lives. When acute pancreatitis accompanied hemolytic anemia, thrombocytopenia, renal impairment, fever, and neurological disorders, a high index of clinical suspicion of thrombotic thrombocytopenic purpura is required for prompt diagnosis and early treatment, which is associated with good outcome.
- Citation: Wang CH, Jin HF, Liu WG, Guo Y, Liu Z. Acute pancreatitis-induced thrombotic thrombocytopenic purpura: A case report. World J Clin Cases 2022; 10(12): 3808-3813
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3808.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3808
Thrombotic thrombocytopenic purpura (TTP) is a rare but fatal disease characterized by fever, thrombocytopenia, microangiopathic hemolytic anemia, renal failure, and neurological manifestations[1]. The underlying pathophysiological mechanism of TTP is deficiency or the production of antibodies against a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13). ADAMTS13 splits the ultra-large von Willebrand factor (ULVWF) polymers into smaller particles. In the absence of ADAMTS13, excess polymeric ULVWF leads to diffuse platelet-rich microthrombosis, leading to ischemia in different organs[2]. TTP-induced acute pancreatitis this mechanism is well known[3,4]. However, only a few cases of TTP as a complication of acute pancreatitis have been reported[5,6]. Herein we report a case of TTP secondary to acute pancreatitis caused by a high-fat diet.
A 32-year-old male presented with an 8 d history of upper abdominal pain and thrombocytopenia was observed for 1 d.
A previously healthy 32-year-old Asian male presented to a local hospital with a 4 d history of upper abdominal pain. He had eaten some fatty food a day prior to the onset of the symptom. After 4 d of treatment at local hospital for acute pancreatitis, his abdominal pain was markedly relieved, but the blood routine showed extremely low platelets.
The patient underwent splenectomy after an abdominal trauma three years ago, and did not undergo any follow-up.
There was no other relevant past medical and family histories.
There was tenderness in the upper abdomen. No petechiae found on the skin. Heart and lung examination showed no abnormality. No masses or hepatosplenomegaly. There were no abnormal neurological signs.
On the day of admission at the local hospital, his initial laboratory studies showed a white blood cell (WBC) count of 17.14 × 109/L (normal 4-10 × 109/L); neutrophils, 63.04%; lymphocytes, 31.64%; hemoglobin (HB), 152 g/L (normal 100-160 g/L); platelet count, 426 × 109/L (normal 150-350 × 109/L). The serum creatinine was 65 μmol/L (normal 62-106 μmol/L); blood urea nitrogen (BUN), 5.46 mmol/L (normal 1.7-8.3 mmol/L); serum amylase, 364 U/L (normal 0-100 U/L); total bilirubin, 11.5 μmol/L (normal 3.4-20.3 μmol/L), indirect bilirubin, 7.5 μmol/L (normal 0.3-16 μmol/L). After four days of treatment, the abdominal pain subsided. However, a repeat blood routine test revealed a WBC count of 13.71 × 109/L; neutrophils, 79.21%; lymphocytes, 12.32%; HB, 104 g/L; platelet count 30 × 109/L. Serum amylase was at 230 U/L, total bilirubin, 43.8 μmol/L, and indirect bilirubin, 21.7 μmol/L. The patient was transferred to our hospital for severe thrombocytopenia. laboratory data (day four of symptom onset) showed WBC, 12.75 × 109/L; neutrophils, 76%; lymphocytes, 15%; HB, 102 g/L; platelets, 7 × 109/L; urinalysis showed protein 2+, red blood cell 45/μL (normal, 0-25/μL); and stool occult blood tests were weakly positive. Coagulation studies revealed normal prothrombin time of 13.3 s (normal 9.4-12.5 s); fibrinogen, 4.76 g/L (2.38-4.98 g/L); D-dimer, 0.978 (normal 0-0.243 mg/L). Serum creatinine was 241 μmol/L; BUN, 18.5 mmol/L; serum amylase, 195 U/L; total bilirubin, 64.2 μmol/L, direct bilirubin, 8.6 μmol/L (normal 0-4 μmol/L). Lactate dehydrogenase was at 1559 U/L. There were schistocytes in his peripheral blood smear (3+; 0.5%). Both direct and indirect Coombs tests were negative (laboratory parameters showed in Table 1).
| Day from illness | Reference range | Day 1 | Day 4 | Day4 (transfer) | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 |
| WBC (× 109/L) | 4-10 | 17.14 | 13.71 | 12.75 | 13.55 | 18.67 | 22.91 | 23.56 | 24.04 | 22.35 |
| n (%) | 40-75 | 63.04 | 79.21 | 76 | 87.5 | 81.6 | 75.3 | 73.8 | 71.3 | 71.3 |
| RBC (× 1012/L) | 4.3-5.8 | 4.52 | 4.01 | 3.38 | 3.07 | 2.74 | 2.56 | 2.71 | 2.76 | 2.76 |
| HB (g/L) | 130-175 | 152 | 104 | 102 | 92 | 82 | 76 | 82 | 84 | 84 |
| PLT (× 109/L) | 150-350 | 426 | 30 | 7 | 42 | 24 | 56 | 74 | 148 | 319 |
| Creatinine (μmol/L) | 62-106 | 65 | - | 241 | 198 | - | 146 | 112 | 107 | 96 |
| BUN (mmol/L) | 1.7-8.3 | 5.46 | - | 18.5 | 15.2 | - | 13.8 | 9.9 | 7.8 | 3.7 |
| TBil (μmol/L) | 3.4-20.3 | 11.5 | 43.8 | 64.2 | 58.8 | - | 21.2 | 16.7 | 14.4 | 11.8 |
| IBil (μmol/L) | 0.3-16 | 7.5 | 21.7 | 55.6 | 47.3 | - | 15.8 | 12.8 | 10.7 | 9 |
| LDH (U/L) | 120-250 | - | - | 1559 | - | - | 630 | - | 359 | 285 |
| PT (s) | 9.4-12.5 | - | - | 13.3 | - | 21.4 | 13.3 | - | - | 12.8 |
| D-dimer (mg/L) | 0-0.243 | - | - | 0.978 | - | - | - | - | - | 0.623 |
| Fibrinogen (g/L) | 2.38-4.98 | - | - | 4.76 | - | - | - | - | - | 2.12 |
| Amylase (U/L) | 0-100 | 364 | 230 | 195 | 105 | - | - | - | - | - |
| Plasma exchange | - | - | + | + | + | + | + | + | + |
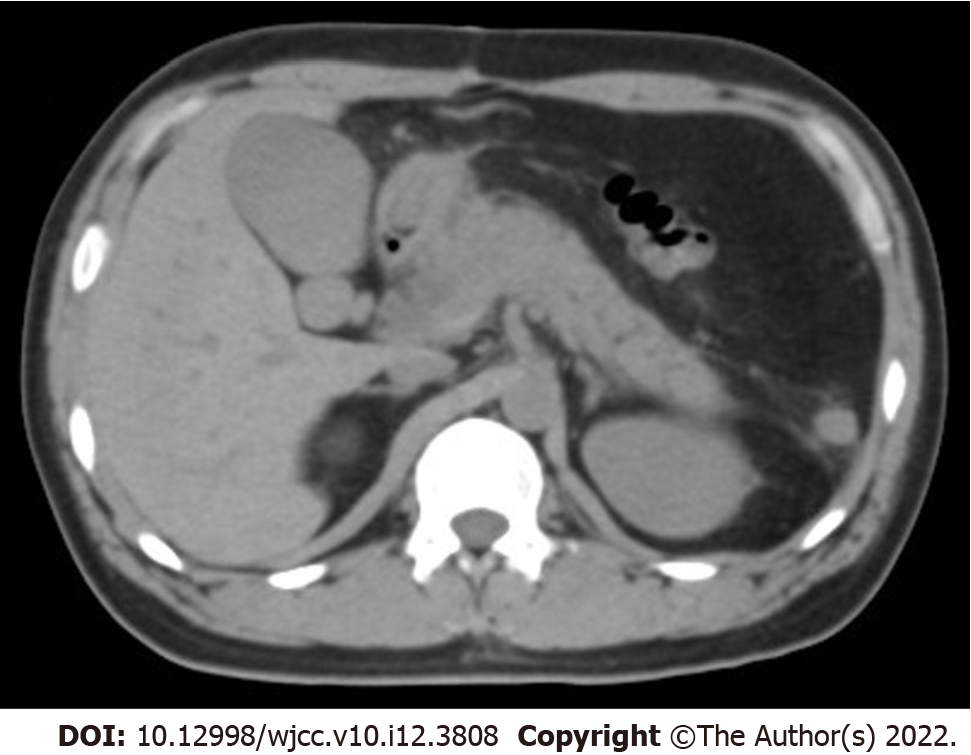
Abdominal computed tomography revealed signs of pancreatitis (Figure 1).
The patient's symptoms of acute pancreatitis and abdominal pain improved. Combined with laboratory examination and abdominal computed tomography review, the thrombocytopenia caused by infection associated to acute pancreatitis was considered to be excluded. Our hospital does not have the technical platform to test for ADAMTS13. The patient’s typical laboratory data of severe thrombocytopenia with mild renal impairment were in favor of TTP over hemolytic uremic syndrome.
Based on the severity of TTP, plasma exchange and intravenous methylprednisolone (40 mg/d) was initiated. After 7 sequences of plasmapheresis, oral methylprednisolone (28 mg/d) was continued.
After 7 sequences of plasmapheresis, the patient’s platelet count increased to 147 × 109/L, and urea, creatinine, and HB returned to normal (laboratory parameters showed in Table 1). Plasma exchange was stopped and oral methylprednisolone (28 mg/d) was continued. The patient was discharged 13 d after admission. Discharge follow-up showed recurrent pancreatitis 8 mo later without TTP. Due to coronavirus disease 2019 and his condition improved, the patient was not able to complete the test for ADAMTS13.
TTP is a rare disease with an annual incidence of approximately 6 per 1 million[7]. It can be congenital or acquired by any cause of ADAMTS13 deficiency or dysfunction of the ADAMTS13 enzyme. Acquired TTP is usually due to autoantibodies that inhibit ADAMTS13 activity and impair ULVWF function. TTP usually manifests as an acute and fulminant, sometimes fatal process. However, acute inflammatory disease has been known to reduce the activity of ADAMTS13[8].
Pancreatic injury caused by TTP is common, the mechanism of TTP-induced pancreatic injury is believed to be the pancreatic circulation disturbance caused by thrombus occlusion of small vessels[9]. Only a few cases of TTP as a complication of acute pancreatitis have been reported. Acute pancreatitis is an inflammatory disease characterized by tissue damage in situ. Increased levels of cytokines including interleukins (IL-8, IL-1, and IL-6) and tumor necrosis factor (TNF-α) may stimulate the release of ULVWF by endothelial cells. This may account for the relative deficiency of the ADAMTS13 protease in acute pancreatitis, which is rapidly consumed. Acute pancreatitis mediated TTP typically occurs within 1 to 13 d (median 3 d) of the diagnosis of acute pancreatitis, possibly due to the peak levels of inflammatory cytokines IL-6 and IL-8 on the 3 d after the onset of pancreatitis[10]. Nitric oxide may also be involved in the development of TTP after acute pancreatitis[11]. In vitro studies have shown that inflammatory factors stimulate the release of ULVWF from endothelial cells and inhibit the cleavage of ULVWF by ADAMTS13[12].
Due to the high fatality rate of TTP, diagnostic treatment is initiated before more definitive test results such as ADAMTS13 Levels can be obtained. Before the 1980s, prior to the era of therapeutic plasmapheresis for TTP, the fatality rate was greater than 90%[13]. Treatment includes immediate and daily therapeutic plasmapheresis with oral or intravenous glucocorticoids, depending on the patient’s neurological status. Because the platelet count reflects the disease’s response to treatment, it should be monitored daily. Once the platelet count exceeds 150000/mL for more than 2 d, therapeutic plasma
About 40% of patients with TTP are likely to experience a relapse[15]. One study found a recurrence rate of about 36% during a 30-mo follow-up period, with about 76% occurring within the first 24 mo[16]. Splenectomy remains a viable, but non-curative, treatment option for patients with recurrent or refractory disease. In a case series by Dubois and Gray, patients who underwent splenectomy for recurrent TTP had better outcomes than patients who underwent splenectomy for refractory disease. They noted that in the recurrent TTP group and the refractory group that underwent splenectomy, the overall complication rate was 6% and 10%, respectively, and the mortality rate was 1.2% and 5%, respectively. In addition, they found that the recurrence rate of TTP after splenectomy was about 17%[17]. It has been suggested that splenectomy may benefit patients with TTP because it removes a large number of B lymphocytes that produce pathogenic autoantibodies. By eliminating the source of pathogenic autoantibody production, splenectomy can be a successful treatment option for patients with recurrent or plasma refractory acquired TTP due to autoantibody mediated defects in ADAMTS13[18]. However, in the present case, the patient underwent splenectomy three years previously, and in the absence of a spleen, the patient still had a recurrence of acute pancreatitis at eight months follow-up albeit without being complicated with TTP. However, more follow-up in the future is required to examine whether TTP will relapse.
TTP, a life-threatening disorder, is sometimes caused by acute pancreatitis. TTP is a rare and serious complication of acute pancreatitis. Therefore, when TTP is highly suspected clinically but cannot be diagnosed early, prompt plasmapheresis and glucocorticoid therapy are necessary and may lead to a favorable outcome.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hakim GD, Turkey; Sikiric P, Croatia S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | George JN. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116:4060-4069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 306] [Article Influence: 20.4] [Reference Citation Analysis (2)] |
| 2. | Joly BS, Coppo P, Veyradier A. Thrombotic thrombocytopenic purpura. Blood. 2017;129:2836-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 467] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 3. | Ridolfi RL, Bell WR. Thrombotic thrombocytopenic purpura. Report of 25 cases and review of the literature. Medicine (Baltimore). 1981;60:413-428. [PubMed] |
| 4. | Shah J, Mandavdhare HS, Birda CL, Dutta U, Sharma V. Thrombotic thrombocytopenic purpura: A rare complication of acute pancreatitis. JGH Open. 2019;3:435-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Arimoto M, Komiyama Y, Okamae F, Ichibe A, Teranishi S, Tokunaga H, Nakaya K, Fujiwara M, Yamaoka M, Onishi S, Miyamoto R, Nakamichi N, Nomura S. A case of thrombotic thrombocytopenic purpura induced by acute pancreatitis. Int J Gen Med. 2012;5:307-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Ali MA, Shaheen JS, Khan MA. Acute pancreatitis induced thrombotic thrombocytopenic purpura. Indian J Crit Care Med. 2014;18:107-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Scully M, Yarranton H, Liesner R, Cavenagh J, Hunt B, Benjamin S, Bevan D, Mackie I, Machin S. Regional UK TTP registry: correlation with laboratory ADAMTS 13 analysis and clinical features. Br J Haematol. 2008;142:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 258] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 8. | Mannucci PM, Canciani MT, Forza I, Lussana F, Lattuada A, Rossi E. Changes in health and disease of the metalloprotease that cleaves von Willebrand factor. Blood. 2001;98:2730-2735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 319] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 9. | Hosler GA, Cusumano AM, Hutchins GM. Thrombotic thrombocytopenic purpura and hemolytic uremic syndrome are distinct pathologic entities. A review of 56 autopsy cases. Arch Pathol Lab Med. 2003;127:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 155] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 10. | Swisher KK, Doan JT, Vesely SK, Kwaan HC, Kim B, Lämmle B, Kremer Hovinga JA, George JN. Pancreatitis preceding acute episodes of thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: report of five patients with a systematic review of published reports. Haematologica. 2007;92:936-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Thachil J. Lessons from acute pancreatitis-induced thrombotic thrombocytopenic purpura. Eur J Intern Med. 2009;20:739-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Bernardo A, Ball C, Nolasco L, Moake JF, Dong JF. Effects of inflammatory cytokines on the release and cleavage of the endothelial cell-derived ultralarge von Willebrand factor multimers under flow. Blood. 2004;104:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 409] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Rock GA, Shumak KH, Buskard NA, Blanchette VS, Kelton JG, Nair RC, Spasoff RA. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. Canadian Apheresis Study Group. N Engl J Med. 1991;325:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1380] [Cited by in RCA: 1297] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 14. | Patel J, Patel P, Ahmed Z. An improbable and unusual case of thrombotic thrombocytopenia purpura. J Community Hosp Intern Med Perspect. 2016;6:32258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Coppo P, Froissart A; French Reference Center for Thrombotic Microangiopathies. Treatment of thrombotic thrombocytopenic purpura beyond therapeutic plasma exchange. Hematology Am Soc Hematol Educ Program. 2015;2015:637-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Zhan H, Streiff MB, King KE, Segal JB. Thrombotic thrombocytopenic purpura at the Johns Hopkins Hospital from 1992 to 2008: clinical outcomes and risk factors for relapse. Transfusion. 2010;50:868-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Dubois L, Gray DK. Case series: splenectomy: does it still play a role in the management of thrombotic thrombocytopenic purpura? Can J Surg. 2010;53:349-355. [PubMed] |
| 18. | Kremer Hovinga JA, Studt JD, Demarmels Biasiutti F, Solenthaler M, Alberio L, Zwicky C, Fontana S, Taleghani BM, Tobler A, Lämmle B. Splenectomy in relapsing and plasma-refractory acquired thrombotic thrombocytopenic purpura. Haematologica. 2004;89:320-324. [PubMed] |