Published online Apr 26, 2022. doi: 10.12998/wjcc.v10.i12.3754
Peer-review started: November 4, 2021
First decision: December 27, 2021
Revised: January 8, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 26, 2022
Processing time: 168 Days and 5.6 Hours
The quality of life in patients who develop low anterior resection syndrome (LARS) after surgery for mid-low rectal cancer is seriously impaired. The underlying pathophysiological mechanism of LARS has not been fully investigated.
To assess anorectal function of mid-low rectal cancer patients developing LARS perioperatively.
Patients diagnosed with mid-low rectal cancer were included. The LARS score was used to evaluate defecation symptoms 3 and 6 mo after anterior resection or a stoma reversal procedure. Anorectal functions were assessed by three-dimensional high resolution anorectal manometry preoperatively and 3-6 mo after surgery.
The study population consisted of 24 patients. The total LARS score was decreased at 6 mo compared with 3 mo after surgery (P < 0.05), but 58.3% (14/24) lasted as major LARS at 6 mo after surgery. The length of the high-pressure zone of the anal sphincter was significantly shorter, the mean resting pressure and maximal squeeze pressure of the anus were significantly lower than those before surgery in all patients (P < 0.05), especially in the neoadjuvant therapy group after surgery (n = 18). The focal pressure defects of the anal canal were detected in 70.8% of patients, and those patients had higher LARS scores at 3 mo postoperatively than those without focal pressure defects (P < 0.05). Spastic peristaltic contractions from the new rectum to anus were detected in 45.8% of patients, which were associated with a higher LARS score at 3 mo postoperatively (P < 0.05).
The LARS score decreases over time after surgery in the majority of patients with mid-low rectal cancer. Anorectal dysfunctions, especially focal pressure defects of the anal canal and spastic peristaltic contractions from the new rectum to anus postoperatively, might be the major pathophysiological mechanisms of LARS.
Core Tip: Rectal cancer is one of the most common malignant tumors in the world. Most patients with mid-low rectal cancer treated by anterior resections suffer anterior resection syndrome (ARS), which seriously impairs the quality of life and mental status. Therefore, the factors impacting anorectal function and its underlying mechanism need to be adequately investigated. Three-dimensional high-resolution anorectal manometry (3D HR-ARM), a more detailed instrument than traditional one, has rarely been used in these patients. In this study, we compared the perioperative anorectal functions of mid-low rectal cancer patients by 3D HR-ARM. Based on these data, focal pressure defects of the anal canal and spastic peristaltic contractions from the new rectum to anus postoperatively might be the major pathophysiological mechanisms of low ARS.
- Citation: Pi YN, Xiao Y, Wang ZF, Lin GL, Qiu HZ, Fang XC. Anorectal dysfunction in patients with mid-low rectal cancer after surgery: A pilot study with three-dimensional high-resolution manometry. World J Clin Cases 2022; 10(12): 3754-3763
- URL: https://www.wjgnet.com/2307-8960/full/v10/i12/3754.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i12.3754
Colorectal cancer is one of the most common malignant tumors in the world. Rectal cancer, especially the mid-low type, in which the distal margin to the anal edge is ≤ 10 cm, is much more common in China[1]. Most patients treated by anterior resections suffer diverse problems related to abnormal defecation, such as frequent bowel movements, urgency, sensation of incomplete evacuation, and fecal incontinence, together referred to as anterior resection syndrome (ARS)[2]. The social activities, quality of life, and mental status of patients with ARS are seriously compromised[2].The efficacy of rectal irrigation, biofeedback, pelvic floor rehabilitation, and sacral nerve stimulation in patients with ARS has been examined but the number of studies and the respective sample sizes are small[3-5]. Therefore, the factors impacting anorectal function and its underlying mechanism remain to be adequately investigated.
Given the variable manifestations of ARS, “gold standard” diagnostic criteria are lacking[2]. Methods including anorectal manometry, transanal ultrasonography, and magnetic resonance imaging have been used to investigate the association between anorectal structure and function in anorectal organic diseases[6,7]. Three-dimensional high-resolution anorectal manometry (3D HR-ARM) uses miniaturized semiconductor sensors that surround a solid-state catheter and thus offers a novel means to serially measure the cross-section of the distal rectum and anal sphincter[8]. Using this solid catheter, the structural changes corresponding to anal sphincter function, including sphincter defects, can be detected. We therefore speculated that 3D HR-ARM could be used to evaluate anorectal structure and function in detail in patients who underwent anterior resection surgery.
The aim of this study was to evaluate anorectal function and to explore the structural factors associated with postoperative anorectal dysfunction in patients with mid-low rectal cancer, by following them from the time of diagnosis to 6 mo after anterior resection or a stoma reversal procedure.
Patients diagnosed with mid-low rectal cancer at Peking Union Medical College Hospital from September 2012 to November 2013 who were older than 18 years of age were enrolled in this study, and the tumor distal margin to the anal edge was ≤ 10 cm, which was measured by the endoscopists during colonoscopic examination. Patients with a history of perianal diseases or surgery, intestinal diseases, or other diseases that might have affected intestinal or defecation function were excluded. All patients enrolled in the study underwent anterior resections (Dixon procedure or intersphincteric resection) with or without a temporary diverting stoma.
All procedures were performed in accordance with ethical standards of the Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the local Ethics Committee (S-482). All patients provided informed consent prior to enrollment in the study.
ARS was evaluated in questionnaires completed by the patient with the face-to-face assistance of a well-trained investigator. The questions were designed to obtain general information, rectal cancer- and surgery-related information, and an assessment of defecation symptoms.
The low ARS (LARS) score has been validated in China and other countries and its reliability was consistently demonstrated[9-11]. With a total score of 42 points, it includes five items: (1) Frequency of uncontrolled intestinal flatus (i.e., gas incontinence; scores 0, 4, and 7 points); (2) fluid incontinence (0, 3, and 3 points); (3) bowel frequency (4, 2, 0, and 5 points); (4) clustering (0, 9, and 11 points); and (5) urgency (0, 11, and 16 points). The total LARS score ranges from no low LARS (0-20 points) to minor LARS (21-29 points) and major LARS (30-42 points). LARS score was assessed at 3 and 6 mo after the initial anterior resection or the stoma reversal procedure following a temporary diverting stoma, and both are referred as “after surgery” in the following text.
Anorectal functions were evaluated using the ManoScan 3D HR-ARM system, which includes the ManoScan 360 A300 acquisition system, Manoview analysis software (version 2.2) (Sierra Scientific Instruments, Los Angeles, CA, United States), and solid-state electrodes (diameter 10.75 mm, length 6.4 cm, 256 data acquisition points) to generate a 3D pressure topographic profile of the anorectum. The solid-state electrodes connect with a balloon, and the balloon pressure is acquired with internal sensors within the acquisition module. 3D HR-ARM was performed preoperatively and 3-6 mo after surgery.
The patient was asked to evacuate his/her rectum at least 2 h before the measurement. The patient was placed in the left lateral position; after a digital rectal examination and informing about the anorectal manometry procedure, the electrode was placed. Then, the patient was told to keep calm and quiet for at least 2 min, during which time the resting pressure of the anus was recorded, and the mean value was calculated as the mean resting pressure. The length of high pressure zone of the anal sphincter is the length of the anal canal with mean resting pressure above 20 mmHg. The patient was then instructed to voluntarily squeeze the perianal muscles as hard as possible for at least 30 s three times separated by a 1-min rest. The average value was recorded as the maximal squeeze pressure of the anus. The perception of rectal filling and the capacity of the new rectum were measured by inflating a balloon with air in 10-mL increments. The thresholds of the patient's first perception of rectal filling, urgency to defecate, and maximal tolerable volume were assessed.
All statistical analyses were performed using SPSS version 17 (SPSS Inc, Chicago, IL, United States). Numerical variables with a normal distribution are presented as the mean ± SD, and those with a non-normal distribution as the median and interquartile range. Categorical variables are expressed as n (%). Fisher exact tests were used to assess between-group differences in categorical variables for the number of patients less than 40, and a Student’s t test or the Mann-Whitney U test was used to assess between-group differences in numerical variables with or without a normal distribution. A paired t-test was used to compare preoperative and postoperative anorectal functions evaluated by 3D HR-ARM, and McNemar-Bowker test or Wilcoxon signed-ranks test was used to compare the LARS scores between 3 and 6 mo after surgery. Pearson correlations or Spearman rank correlations were used to calculate the correlation coefficients. A P value < 0.05 was considered statistically significant.
Twenty-four patients with mid-low rectal cancer who fulfilled the inclusion criteria were enrolled in the study after providing informed written consent, including 14 males and 10 females, with an average age of 57.7 ± 10.4 years. Mid-rectal cancer was diagnosed in 14 and low rectal cancer in 10 patients. The cTNM stage and the major therapies are shown in Table 1. Twenty-two patients had the Dixon procedures, and two had the intersphincteric resections; among them, 18 patients had a temporary diverting stoma and a stoma reversal procedure.
| Item | Number | |
| Sex | Male | 14 |
| Female | 10 | |
| cTNM stage | T2 | 8 |
| T3 | 15 | |
| T4 | 1 | |
| N0 | 11 | |
| N1 | 11 | |
| N2 | 2 | |
| Preoperative neoadjuvant therapy | Yes | 18 |
| No (surgery only) | 6 | |
| Anterior resection | Dixon | 22 |
| Intersphincteric resection | 2 | |
| Diverting stoma | Yes | 18 |
| No | 6 | |
| Postoperative chemotherapy | 5 | |
At 3 mo after surgery, 87.5% of patients (21/24) had LARS, and 83.3% (20/24) had major LARS. At 6 mo after surgery, 83.3% (20/24) still had LARS, and 58.3% (14/24) had major LARS (Table 2). There were significant differences in total LARS score and scores of four items except clustering between 3 mo and 6 mo after surgery (P < 0.05). There were no significant differences in LARS scores among patients with different sexes, ages, and tumor TNM stages.
| Item (scale point) | 3 mo after surgery, n (%) | 6 mo after surgery, n (%) | P value |
| Frequency of uncontrolled intestinal flatus | 0.005 | ||
| No, never (0) | 4 (16.7) | 10 (41.7) | |
| Yes, less than once per wk (4) | 1 (4.2) | 8 (33.3) | |
| Yes, at least once per wk (7) | 19 (79.2) | 6 (25.0) | |
| Frequency of uncontrolled intestinal fluid | 0.003 | ||
| No, never (0) | 5 (20.8) | 13 (54.2) | |
| Yes, less than once per wk (3) | 8 (33.3) | 8 (33.3) | |
| Yes, at least once per wk (3) | 11 (45.8) | 3 (12.5) | |
| Bowel frequency | 0.037 | ||
| > 7 times/d (4) | 7 (29.2) | 2 (8.3) | |
| 4-7 times/d (2) | 16 (66.7) | 18 (75.0) | |
| 1-3 times/d (0) | 1 (4.2) | 4 (16.7) | |
| Less than once per day (5) | 0 (0) | 0 (0) | |
| Clustering | 0.500 | ||
| No, never (0) | 1 (4.2) | 3 (12.5) | |
| Yes, less than once per wk (9) | 0 (0) | 0 (0) | |
| Yes, at least once per wk (11) | 23 (95.8) | 21 (87.5) | |
| Urgency | 0.040 | ||
| No, never (0) | 2 (8.3) | 3 (12.5) | |
| Yes, less than once per wk (11) | 1 (4.2) | 8 (33.3) | |
| Yes, at least once per wk (16) | 21 (87.5) | 13 (54.2) | |
| LARS score1 | 39 (1.5) | 31 (7.5) | < 0.001 |
| No LARS (0-20) | 3 (12.5) | 4 (16.7) | |
| Minor LARS (21-29) | 1 (4.2) | 6 (25.0) | |
| Major LARS (30-42) | 20 (83.3) | 14 (58.3) | |
At both 3 mo and 6 mo after surgery, the tumor location (the tumor distal margin to the anal edge) were negatively correlated with LARS score (r = -0.499, P = 0.013; r = -0.584, P = 0.003) and urgency score (r = -0.444, P = 0.030; r = -0.425, P = 0.038). And at 6 mo after surgery, tumor location was negatively correlated with clustering score (r = -0.559, P = 0.005). Patients who received preoperative neoadjuvant therapy (chemoradiotherapy) had a higher uncontrolled intestinal flatus score (χ2 = 5.614, P = 0.035) at 3 mo after surgery.
The time from surgery to 3D HR-ARM examination was 117-178 d. There were no significant differences in anorectal function at the baseline between the surgery only group and the neoadjuvant therapy group (P < 0.05). Compared with the values before surgery, the length of the high-pressure zone of the anal sphincter after surgery was significantly shorter (P < 0.05), and the mean resting pressure and maximal squeeze pressure of the anus were significantly lower (P < 0.05) in all the patients, especially in the neoadjuvant therapy group after surgery. However, in the surgery only group, only the mean resting pressure was significantly lower (P < 0.05) postoperatively (Table 3). Because some patients could not cooperate well while inflating the balloon in the rectum, we list the number of patients who successfully acquired the first perception of rectal filling volume, urgency to defecate volume, and maximal tolerable volume after surgery in Table 3, and these data were not compared before and after surgery.
| Parameter | Total (n = 24) | Surgery only group (n = 6) | Neoadjuvant group (n = 18) | |||
| Before surgery | After surgery | Before surgery | After surgery | Before surgery | After surgery | |
| Length of high pressure zone of anal sphincter (cm) | 3.6 ± 0.7 | 2.8 ± 1.2a | 3.6 ± 0.5 | 3.6 ± 1.1 | 3.6 ± 0.7 | 2.6 ± 1.2a |
| Mean resting pressure of the anus (mmHg) | 107.6 ± 32.3 | 67.6 ± 28.1a | 102.9 ± 38.9 | 75.2 ± 37.2a | 109.2 ± 30.9 | 65.1 ± 25.2a |
| Maximal squeeze pressure (mmHg) | 259 ± 80.0 | 207.8 ± 63.3a | 274.9 ± 109.6 | 249.8 ± 89.7 | 253.7 ± 70.8 | 193.8 ± 47.2a |
| First perception of rectal filling volume (mL) | 35.6 ± 19.7 | 39.2 ± 25.8 (n = 12) | 20.0 ± 14.1 | 25.0 ± 7.1 (n = 2) | 43.0 ± 27.5 | 42.0 ± 27.4 (n = 10) |
| Urgency to defecate volume (mL) | 60.0 ± 24.3 | 51.4 ± 31.9 (n = 7) | 35.0 ± 21.2 | 35.0 ± 7.1 (n = 2) | 66.0 ± 18.2 | 58.0 ± 36.3 (n = 5) |
| Maximal tolerable volume (mL) | 112.1 ± 42.1 | 71.4 ± 21.2 (n = 7) | 123.3 ± 64.3 | 60.0 ± 26.5 (n = 3) | 110.0 ± 11.6 | 80.0 ± 14.1 (n = 4) |
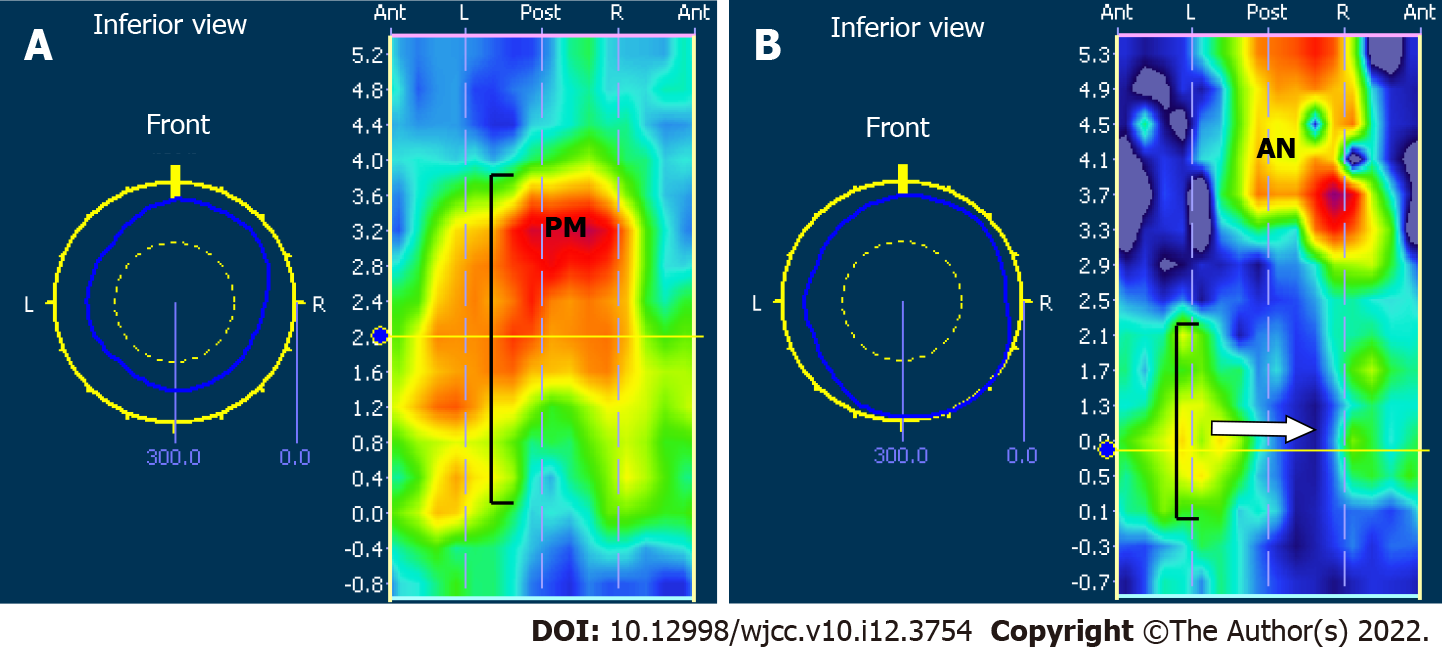
A focal pressure defect in a 3D section of the anal canal was determined in 70.8% (17/24) of the patients postoperatively (Figure 1), including 15 (88.2%) who had received preoperative neoadjuvant therapy. The mean resting pressure of the anus in these 17 patients with focal pressure defects was 60.0 ± 27.9 mmHg. Patients with preoperative neoadjuvant therapy and a lower tumor location were more likely to have a focal pressure defect in a 3D section of the anal canal (P = 0.038, P = 0.019), but there were no significant correlations between the occurrence of focal pressure defects and sex, age, or tumor TNM stages (P > 0.05).
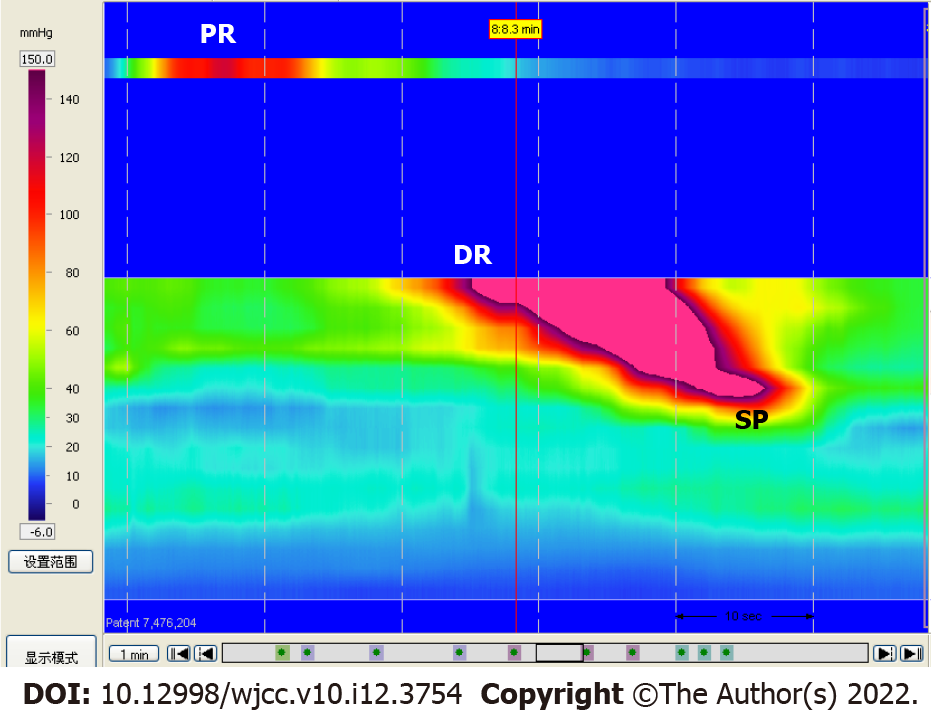
Spastic peristaltic contractions from the new rectum to anus were detected in 45.8% (11/24) of the patients during 3D HR-ARM (Figure 2). The occurrence of spastic peristaltic contractions after surgery did not correlate significantly with sex, age, original tumor location, tumor TNM stages, or preoperative neoadjuvant therapy.
The postoperative mean resting pressure of the anus was negatively correlated with uncontrolled intestinal flatus score and uncontrolled fluid incontinence score (r = -0.507, P = 0.011; r = -0.472, P = 0.020) at 3 mo after surgery. Both at 3 mo and 6 mo after surgery, the postoperative maximal squeeze pressure of the anus was negatively correlated with LARS score (r = -0.461, P = 0.023; r = -0.453, P = 0.026) and clustering score (r = -0.405, P = 0.050; r = -0.539, P = 0.007). There were no significant correlations between the length of the high pressure zone of the anal sphincter, first perception of rectal filling volume, urgency to defecate volume, or maximal tolerable volume (for those cases with detected data) and LARS symptom scores.
Patients who had focal pressure defects in the anal canal and spastic peristaltic contractions from the new rectum to anus postoperatively had a higher LARS score than those without these dysfunctions at 3 mo after surgery [39 (2.0) vs 24 (23), P = 0.025; 39 (2.0) vs 39 (18.5), P = 0.017].
Patients who had focal pressure defects in the anal canal had a higher uncontrolled intestinal flatus score (χ2 = 7.309, P = 0.014), higher uncontrolled fluid incontinence score (P = 0.014), and higher urgency score (χ2 = 7.034, P = 0.017) at 3 mo after surgery than those without focal pressure defects. Patients with focal pressure defects had a higher bowel frequency score and urgency score (χ2 = 6.203, P = 0.019; χ2 = 5.905, P = 0.049) at 6 mo after surgery. Patients who had spastic peristaltic contractions postoperatively had a higher bowel frequency score (χ2 = 6.375, P = 0.023; χ2 = 6.044, P = 0.029) at both 3 mo and 6 mo after surgery.
In this study, we found that LARS score decreased in the early phase after surgery over time, but 58.3% of patients still had major LARS at 6 mo after surgery. The mean resting pressure of the anus decreased in all patients after surgery, 70.8% of patients had focal pressure defects of the anal canal, and 45.8% had spastic peristaltic contractions from the new rectum to anus; these patients were more likely to have higher scores of LARS than patients without these dysfunctions at 3 mo after surgery.
ARS had been seen in up to 90% rectal cancer patients who received radical surgeries[2]. The LARS score is a widely validated score to evaluate ARS. It is simpler to use and better reflects the impact of ARS on the patient’s quality of life, enabling clinicians to rapidly assess postoperative bowel function[9-12]. A meta-analysis showed that the estimated prevalence of major LARS at 1 year and more after rectal radial resection was about 41%, and within 1 year after surgery the prevalence might be higher[13]. Our study found that major LARS was seen in more than half of the patients at 6 mo after surgery. Three years after surgery, 94.2% and 70.6% of patients who underwent coloanal anastomosis with low-lying rectal cancer still had moderate to severe incontinence and major LARS, respectively[12]. The impact of ARS on quality of life still persisted at 11.1 years (range, 7.1-16.1 years) after surgery[14].
Among the diverse clinical manifestations of ARS, the most common is fecal incontinence (97%), followed by increased bowel frequency (80%), urgency (67%), evacuatory dysfunction (47%), and gas-stool discrimination (34%)[15]. Loss of rectal reservoir, anal sphincter damage, autonomic denervation, colon and neorectal motility increase, anastomotic technique, pelvic radiotherapy, rectal-anus sensitivity reduction, and anal resting pressure reduction have been recognized as the pathophysiological mechanisms of ARS[2,16].
Currently, there have been no “gold standard” objective methods to evaluate the anorectal function. Anorectal manometry has been widely used to evaluate anorectal function in patients with defecation dysfunction and fecal incontinence, and 3D HR-ARM has been confirmed to be superior in detecting sphincter dysfunction to traditional linear ARM and HR-ARM[8]. In this study, with 3D HR-ARM we not only found a decreased mean resting pressure of the anus in all patients, but also worse parameters of anorectal manometry in patients with preoperative neoadjuvant therapy after surgery. These results are consistent with the mainstream view that preoperative neoadjuvant radiotherapy is a risk factor for postoperative bowel dysfunction[17]. It had been found that the new rectum of patients with preoperative neoadjuvant therapy was less sensitive to mechanical and temperature stimulation than that of patients who underwent direct surgery[18], even though there was evidence that the short term benefits of neoadjuvant treatment including tumor control, downstaging, improved bowel symptoms, and increased length of the high-pressure zone of the anal sphincter were demonstrated in patients with mid-low rectal cancer 6 wk after adjuvant radiotherapy[19,20].
Except preoperative adjuvant radiotherapy, tumor location was another important risk factor for LARS[13,21]. We found that patients with a lower tumor location had a higher LARS score, and that tumor location was related to the presence of focal pressure defects in the anal canal. Additionally, Battersby et al[22] first developed a Pre-Operative LARS score to predict bowel dysfunction severity prior to anterior resection, and the key predictive factors for LARS were age (at surgery), tumor height, total vs partial mesorectal excision, stoma, and preoperative radiotherapy.
Decreased anal mean resting pressure had been demonstrated to be involved in the patho
Studies focused on the motility of the new rectum were rare, except that Emmertsen et al[24] reported a significantly higher postprandial response of the rectum and neorectal pressure in LARS patients. Interestingly, in this study we captured spastic peristaltic contractions from the new rectum to anus by 3D HR-ARM in nearly half of the patients after surgery, which were related to higher bowel frequency score and higher LARS score. This indicates that the new rectum still preserves the spastic peristaltic contractions of the sigmoid colon, which results in frequent bowel movements and even clustering defecation. We gained enlightenment from this result to teach the patients to start the training exercise of the new rectum for storage and sensation as early as possible after surgery, which could be helpful to alleviate their frequent bowel movements.
The limitations of this pilot study were the small sample size and the short follow-up duration. Considering the discomfort of patients, we performed the 3D HR-ARM once during 3-6 mo after surgery. After this study, the patients entered into the routine follow-up and individualized therapy for the LARS symptoms. In this study, we did not collect the data of sexual practices and anal sex practices and did not include patients with upper rectal cancer, because it is considered that anterior resection for those patients less affects the anorectal function.
In this pilot prospective study, we found that more than half of the patients with mid-low rectal cancer have major LARS at 6 mo after surgery, the mean resting pressure of the anus decreases in all patients, and the anorectal dysfunctions, especially anal focal pressure defects and spastic peristaltic contractions from the new rectum to anus, have correlations with LARS scores after surgery. The anal focal pressure defects and spastic peristaltic contractions from the new rectum to anus might be the major pathophysiological mechanisms of LARS. Further studies are needed to validate our findings regarding LARS and explore effective interventions based on the pathophysiology.
Low anterior resection syndrome (LARS) seriously impairs the quality of life and mental status of rectal cancer patients after radical surgeries, but the underlying mechanism is not clearly understood.
More and more rectal cancer patients have benefited from integrative treatment of surgery and chemo-radiotherapy and their survival rates have been improved. Improving the quality of life and alleviating the defecation related symptom are becoming much more important.
The aim of this research was to fully assess anorectal function of rectal cancer patients perioperatively.
Mid-low rectal cancer patients were assessed with LARS score after surgery and three-dimensional high resolution anorectal manometry before and after surgery.
Twenty-four patients were included in this study. Their LARS scores decreased after surgery over time. The anorectal function detected by three-dimensional high resolution anorectal manometry after surgery was worse than that before surgery in all patients, especially in the neoadjuvant therapy group. The focal pressure defects of the anal canal and spastic peristaltic contractions from the new rectum to anus were detected in 70.8% and 45.8% of the patients, which were associated with higher LARS scores and rarely been reported before.
Anorectal function worsens after surgery in mid-low rectal cancer patients. The focal pressure defects of anal canal and spastic peristaltic contractions from the new rectum to anus postoperatively might be involved in the pathophysiological mechanisms of LARS.
More studies need to be done to confirm our finding that the anal focal pressure defects and spastic peristaltic contractions from the new rectum to anus might be involved in the pathophysiological mechanisms of LARS, and effective interventions should be explored to alleviate the suffering of rectal cancer patients after surgery.
The authors thank Dr. Tao Xu at the Department of Epidemiology and Statistics, Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences & School of Basic Medicine, Peking Union Medical College, China for assistance with the statistical analyses. The abstract was presented as a poster at the joint 2017 meeting of the Asian Neurogastroenterology and Motility Association (ANMA) and the Japanese Society of Neurogastroenterology and Motility (JSNM) held in Osaka, Japan March 23-25, 2017.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casella C, Italy; Mayol J, Spain S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Zheng RS, Sun KX, Zhang SW, Zeng HM, Zou XN, Chen R, Gu XY, Wei WW, He J. [Report of cancer epidemiology in China, 2015]. Zhonghua Zhong Liu Za Zhi. 2019;41:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 277] [Reference Citation Analysis (0)] |
| 2. | Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol. 2012;13:e403-e408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 435] [Article Influence: 33.5] [Reference Citation Analysis (1)] |
| 3. | Rosen HR, Kneist W, Fürst A, Krämer G, Hebenstreit J, Schiemer JF. Randomized clinical trial of prophylactic transanal irrigation versus supportive therapy to prevent symptoms of low anterior resection syndrome after rectal resection. BJS Open. 2019;3:461-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Wu XD, Fu CF, Chen YL, Kong LH, Pan ZZ, Zheng MC. [Intervention effect of biofeedback combined with pelvic floor muscle exercise on low anterior resection syndrome in patients with low anus-preserving rectal cancer]. Zhonghua Yi Xue Za Zhi. 2019;99:2337-2343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 5. | Nishigori H, Ishii M, Kokado Y, Fujimoto K, Higashiyama H. Effectiveness of Pelvic Floor Rehabilitation for Bowel Dysfunction After Intersphincteric Resection for Lower Rectal Cancer. World J Surg. 2018;42:3415-3421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Andrianjafy C, Luciano L, Bazin C, Baumstarck K, Bouvier M, Vitton V. Three-dimensional high-resolution anorectal manometry in functional anorectal disorders: results from a large observational cohort study. Int J Colorectal Dis. 2019;34:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Rezaie A, Iriana S, Pimentel M, Murrell Z, Fleshner P, Zaghiyan K. Can three-dimensional high-resolution anorectal manometry detect anal sphincter defects in patients with faecal incontinence? Colorectal Dis. 2017;19:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Li Y, Yang X, Xu C, Zhang Y, Zhang X. Normal values and pressure morphology for three-dimensional high-resolution anorectal manometry of asymptomatic adults: a study in 110 subjects. Int J Colorectal Dis. 2013;28:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Emmertsen KJ, Laurberg S. Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255:922-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 751] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 10. | Hou XT, Pang D, Lu Q, Yang P, Jin SL, Zhou YJ, Tian SH. Validation of the Chinese version of the low anterior resection syndrome score for measuring bowel dysfunction after sphincter-preserving surgery among rectal cancer patients. Eur J Oncol Nurs. 2015;19:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 11. | Juul T, Ahlberg M, Biondo S, Emmertsen KJ, Espin E, Jimenez LM, Matzel KE, Palmer G, Sauermann A, Trenti L, Zhang W, Laurberg S, Christensen P. International validation of the low anterior resection syndrome score. Ann Surg. 2014;259:728-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 219] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 12. | Cheong C, Oh SY, Choi SJ, Suh KW. Ultralow Anterior Resection and Coloanal Anastomosis for Low-Lying Rectal Cancer: An Appraisal Based on Bowel Function. Dig Surg. 2019;36:409-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Croese AD, Lonie JM, Trollope AF, Vangaveti VN, Ho YH. A meta-analysis of the prevalence of Low Anterior Resection Syndrome and systematic review of risk factors. Int J Surg. 2018;56:234-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 222] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 14. | Pieniowski EHA, Palmer GJ, Juul T, Lagergren P, Johar A, Emmertsen KJ, Nordenvall C, Abraham-Nordling M. Low Anterior Resection Syndrome and Quality of Life After Sphincter-Sparing Rectal Cancer Surgery: A Long-term Longitudinal Follow-up. Dis Colon Rectum. 2019;62:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 15. | Keane C, Wells C, O'Grady G, Bissett IP. Defining low anterior resection syndrome: a systematic review of the literature. Colorectal Dis. 2017;19:713-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 16. | Buzatti KCLR, Petroianu A. Pathophysiological aspects of the low anterior resection syndrome for treatment of rectal cancer. Rev Col Bras Cir. 2017;44:397-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Contin P, Kulu Y, Bruckner T, Sturm M, Welsch T, Müller-Stich BP, Huber J, Büchler MW, Ulrich A. Comparative analysis of late functional outcome following preoperative radiation therapy or chemoradiotherapy and surgery or surgery alone in rectal cancer. Int J Colorectal Dis. 2014;29:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Bregendahl S, Emmertsen KJ, Fassov J, Krogh K, Zhao J, Gregersen H, Laurberg S. Neorectal hyposensitivity after neoadjuvant therapy for rectal cancer. Radiother Oncol. 2013;108:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Pi Y, Xiao Y, Wang Z, Liu F, Lin G, Qiu H, Fang X. [Effects of neoadjuvant chemoradiotherapy on anorectal function in patients with mid and low rectal cancer]. Zhonghua Yi Xue Za Zhi. 2014;94:1857-1860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Song JS, Park IJ, Kim JH, Lee HR, Kim JR, Lee JL, Yoon YS, Kim CW, Lim SB, Yu CS, Kim JC. Peri-treatment change of anorectal function in patients with rectal cancer after preoperative chemoradiotherapy. Oncotarget. 2017;8:79982-79990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Ihn MH, Kang SB, Kim DW, Oh HK, Lee SY, Hong SM. Risk factors for bowel dysfunction after sphincter-preserving rectal cancer surgery: a prospective study using the Memorial Sloan Kettering Cancer Center bowel function instrument. Dis Colon Rectum. 2014;57:958-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Battersby NJ, Bouliotis G, Emmertsen KJ, Juul T, Glynne-Jones R, Branagan G, Christensen P, Laurberg S, Moran BJ; UK and Danish LARS Study Groups. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. Gut. 2018;67:688-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Mion F, Garros A, Brochard C, Vitton V, Ropert A, Bouvier M, Damon H, Siproudhis L, Roman S. 3D High-definition anorectal manometry: Values obtained in asymptomatic volunteers, fecal incontinence and chronic constipation. Results of a prospective multicenter study (NOMAD). Neurogastroenterol Motil. 2017;29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Emmertsen KJ, Bregendahl S, Fassov J, Krogh K, Laurberg S. A hyperactive postprandial response in the neorectum--the clue to low anterior resection syndrome after total mesorectal excision surgery? Colorectal Dis. 2013;15:e599-e606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |