Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3609
Peer-review started: December 29, 2021
First decision: January 25, 2022
Revised: February 3, 2022
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: April 16, 2022
Processing time: 100 Days and 5.3 Hours
The recognized pattern of cervical lymph node metastasis (CLNM) of papillary thyroid carcinoma involves a stepwise route. Contralateral lymph node skip metastasis is very rare. In addition, the patient in our case report also suffered from a breast carcinoma accompanied by left supraclavicular lymphadenopathy, which made it difficult to distinguish the origin of the CLNM. Based on this case, we recommended that more detailed physical and imaging examinations are needed for patients with uncommon cervical lymphatic metastasis of primary cancer.
A 53-year-old women was admitted to the hospital for a neck mass in the left cervical region that had existed for 2 mo. The neck mass was suspected to be an enlarged lateral LN originating from papillary thyroid microcarcinoma of the contralateral thyroid lobe, according to ultrasound and ultrasound-guided fine needle aspiration biopsy. The patient underwent total thyroidectomy and radical cervical LN dissection. Postoperative pathology confirmed the diagnosis of papillary thyroid microcarcinoma with contralateral lymphatic skip metastasis. Unfortunately, a breast cancer was discovered 4 mo later, which was accom
We present a rare case of papillary thyroid microcarcinoma with contralateral lymphatic skip metastasis and breast cancer with supraclavicular lymphatic metastasis.
Core Tip: The recognized pattern of cervical lymph node metastasis (CLNM) of papillary thyroid carcinoma involves a stepwise route. Contralateral lymph node skip metastasis is very rare. In addition, the patient in our case report also suffered from a breast carcinoma accompanied by left supraclavicular lymphadenopathy, which made it difficult to distinguish the origin of the CLNM. Based on this case, we recommended that more detailed physical and imaging examinations are needed for patients with uncommon cervical lymphatic metastasis of primary cancer.
- Citation: Ding M, Kong YH, Gu JH, Xie RL, Fei J. Papillary thyroid microcarcinoma with contralateral lymphatic skip metastasis and breast cancer: A case report. World J Clin Cases 2022; 10(11): 3609-3614
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3609.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3609
Thyroid cancer and breast cancer (BC) are very common in women[1] and both can involve cervical lymph node metastasis (CLNM). The most common type of thyroid cancer is papillary carcinoma, and papillary thyroid microcarcinoma (PTMC) accounts for the majority of cases. When it comes to multiple primary cancers, such as PTMC and BC, the origin of CLNM needs to be distinguished. CLNM in PTMC is usually related to a relatively poor prognosis, especially for lateral CLNM. The recognized pattern of CLNM of PTMC involves a stepwise route. The first site involved is the central compartment, followed by the ipsilateral lateral lymph node (LN) compartment. The contralateral and the mediastinal LN compartments then follow suit[2,3]. Contralateral LN skip metastasis is very rare. CLNM in BC is usually indicative of a terminal stage.
Here, we report a rare case of PTMC with contralateral lymphatic skip metastasis and BC with supraclavicular lymphatic metastasis.
A 53-year-old woman presented to the hospital with a neck mass in her left lateral cervical region.
The patient’s symptom started 2 mo prior and she had not received any treatment in that period.
The patient had a free medical history.
The patient denied any relevant personal family history, particularly for thyroid issues.
A nodule of approximately 0.8 cm in diameter was found in the right lobe of the thyroid gland. It was not tender and easily moved upward and downward when she swallowed. A firm mass of roughly 1.0 cm was palpated in the left lateral cervical region.
Thyroid function and autoimmune antibody were all in normal ranges, except for the anti-thyroid peroxidase (TPO) antibody (787.40 IU/mL; normal range: 0-9 IU/mL). Routine blood indexes were all within normal range. Tests for serum tumor markers, including carcinoembryonic antigen, alpha-fetoprotein, carbohydrate antigen 19-9, CA242, cytokeratin 19 fragment and squamous cell carcinoma antigen, were all negative.
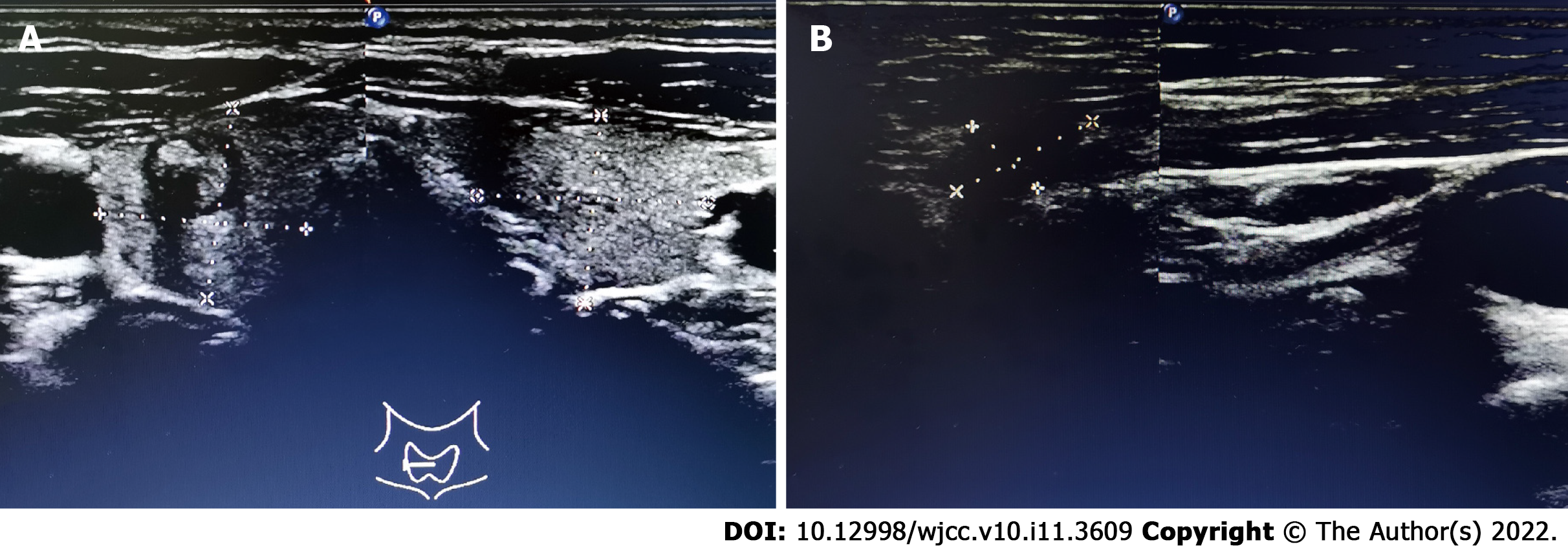
Ultrasound (US) examination showed a 7.8 mm × 7.4 mm heterogeneous hypoechoic nodule in the right lobe of the thyroid gland (Thyroid Imaging Reporting and Data System category 4B). In addition, several abnormal LNs were detected in level V; the biggest one being approximately 14.0 mm × 7.0 mm in size (Figure 1).
US-guided fine needle aspiration biopsy was taken for further evaluation. It indicated that the nodule in the right lobe was PTMC, and the LN was malignant. We preliminarily concluded that this patient suffered from PTMC accompanied by CLNM.
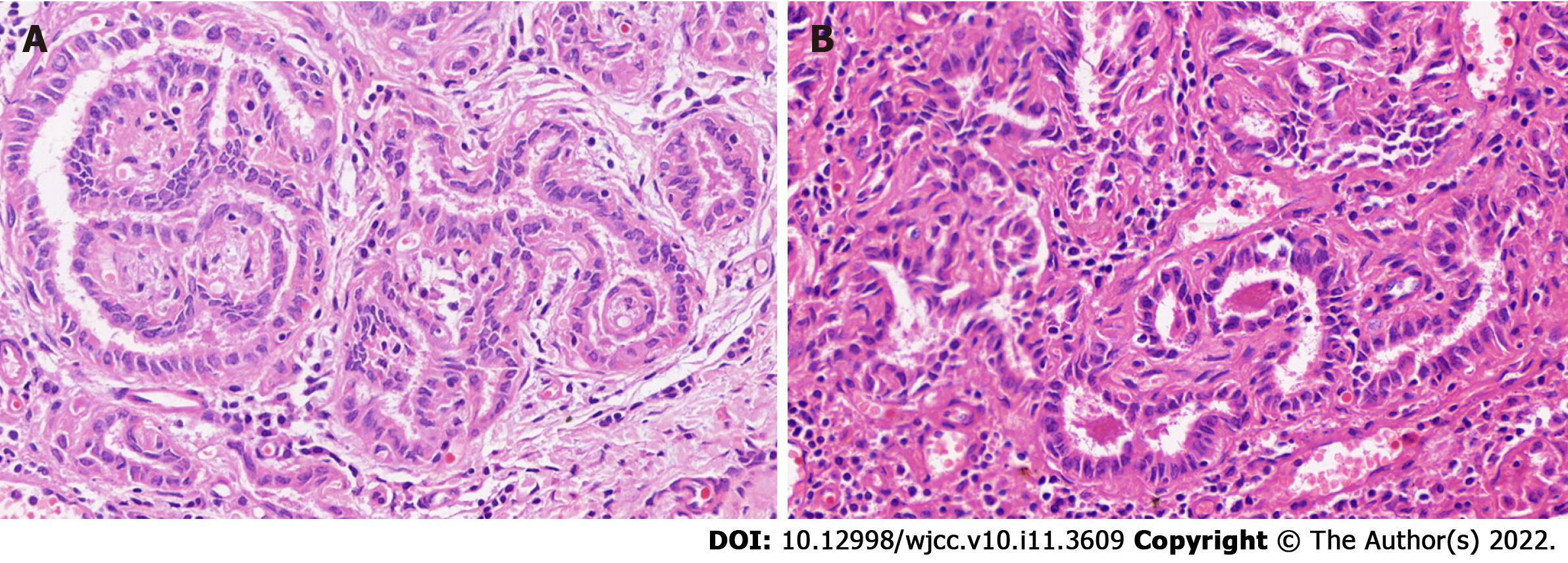
The patient underwent total thyroidectomy and radical cervical neck dissection (bilateral central LN and left lateral LN). Postoperative pathology found isolated PTMC in the right lobe, which was also supported by the findings of immunohistochemical analysis [CD56 (partly positive), HBME-1 (negative), galectin-3 (positive), CK19 (partly positive), TPO (negative), Ki67 index (1%)]. Surprisingly, the bilateral central cervical LNs (n = 16) were all disease-free. Metastasis was found, involving one LN in the left level V and one LN in levels III and IV (Figure 2). Immunohistochemical analysis of the metastatic LN in left levels III and IV were positive for galectin-3 and CK19, and were negative for CD56, HBME-1 and TPO. The Ki-67 index of 1% indicted that the left lateral CLNM in levels III and IV had originated from the thyroid.
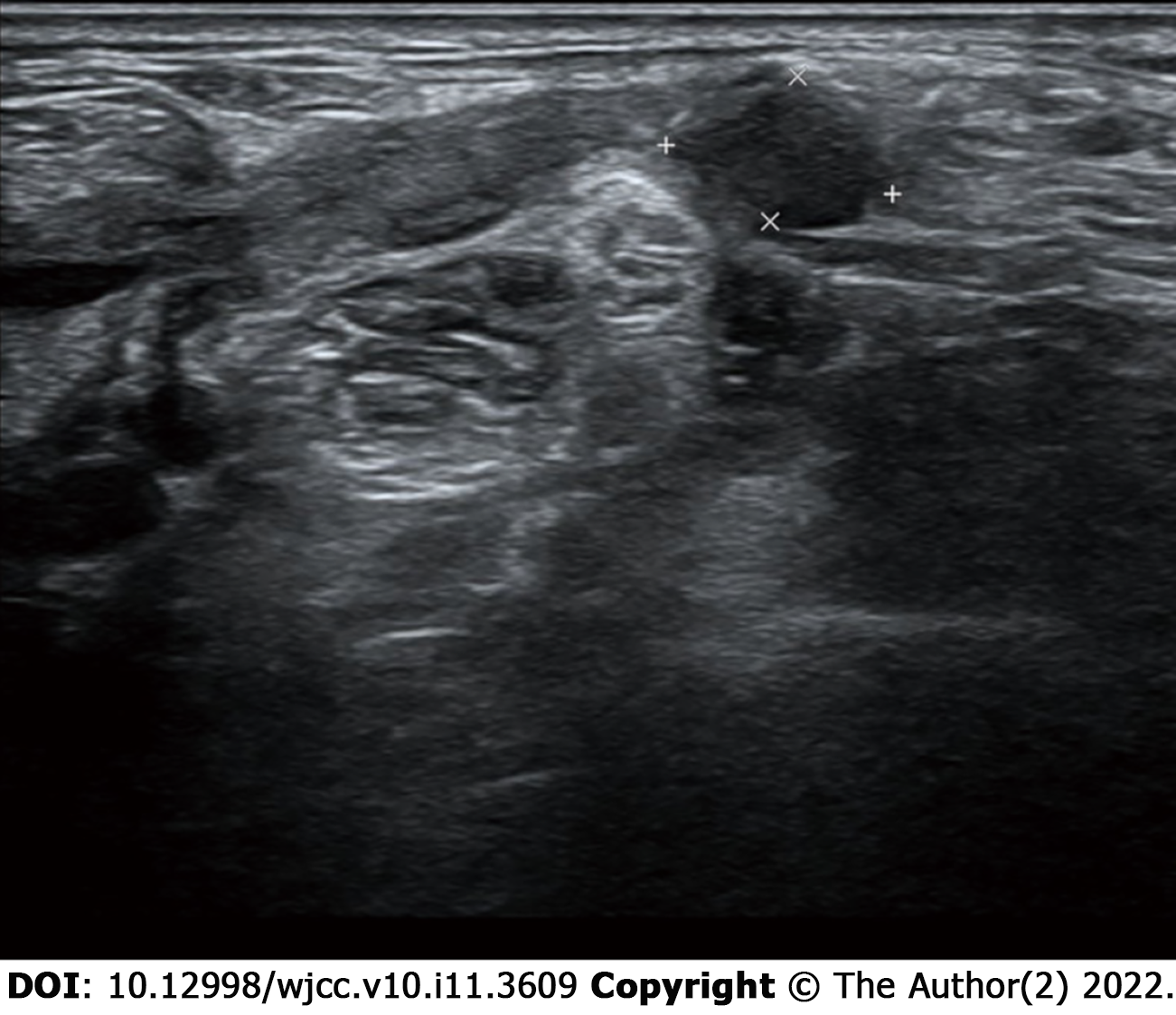
No local relapse was found by US at the next 3-mo follow-up. The patient intended to receive iodine-131 therapy; however, she was admitted to our hospital for a large mass in her left breast 1 mo later. Mammography showed a mass of approximately 3.7 cm ´ 2.6 cm in the left breast, which was highly suspicious of malignancy (Breast Imaging Reporting and Data System 5). US also showed several abnormally enlarged LNs in the left axillary, supraclavicular and level V. US-guided core needle biopsy was taken for the breast mass, which revealed infiltrative carcinoma originating from the breast by pathology and immunohistochemical analysis. US-guided fine needle aspiration biopsy was taken for the suspicious LNs, including the left axillary, supraclavicular and level V, which were all determined to be malignant. It was unknown whether the CLNM in the supraclavicular and level V area originated from the thyroid or breast. The patient underwent another thyroid US. Several hypoechogenic structures could be detected in levels IV and V with irregular shape, obscure boundary and unclear lymphatic hilus. The findings were highly suspicious of metastatic LNs, and we first considered origination from the breast (Figure 3). No distant metastasis was found upon further examination.
PTMC with contralateral lymphatic skip metastasis and BC with supraclavicular lymphatic metastasis.
Given that the BC was in an advanced stage (cT2N3M0), the patient underwent four cycles of neoadjuvant chemotherapy with epirubicin (145 mg/m2) and cyclophosphamide (900 mg/m2), followed by four cycles with docetaxel (150 mg/m2). Clinical responses in the breast and LNs were assessed by US after the neoadjuvant chemotherapy treatment, and she was determined to have achieved partial remission. Then, she underwent a left modified radical mastectomy. Postoperative pathology of the breast revealed invasive ductal carcinoma accompanied by a few invasive micropapillary carcinomas and intermediate grade ductal carcinoma in situ. In addition, three of the seventeen LNs were metastatic. The immunohistochemistry profile of the specimen was as follows: estrogen receptor (+, strong, 95%); progesterone receptor (+, strong, 80%); human epidermal growth factor receptor-2 (1+); Ki-67 (15%); androgen receptor (60%); E-cadherin (+); GATA3 (+); mucin (+); cytokeratin 5/6 (-); epidermal growth factor receptor (-); D2-40 (-); and P63 (-).
The patient is currently receiving postoperative radiotherapy for control of the metastatic lesions. At the 6-mo follow-up, US assessment revealed no local recurrence.
We have reported, here, the first case of a rare contralateral LN skip metastasis of PTMC accompanied by BC. Bruno et al[4] reported a similar case of contralateral LN skip metastasis in Germany. However, those authors identified the unusual pathway of CLNM, mainly via the detection of high thyroglobulin levels in the wash-out liquid of fine-needly aspiration biopsy. In our case, it was mainly based on the postoperative immunohistochemical findings.
We raised two hypotheses for this kind of phenomenon. First, there may be unknown cervical LN drainage pathways. Second, there may be occult PTMC in the left lobe. A previous study reported cases of occult PTMC without detection of the primary tumor[5,6]. In one, Yamashita et al[5] hypothesized that the primary tumor was PTMC of less than 5 mm, as it was difficult to prepare slices of pathological specimens thinner than 5 mm.
Our patient, described herein, had developed cancers of PTMC and BC. However, the US-fine needle aspiration biopsy of LNs in the supraclavicular and level V areas failed to show the origin of her metastasis, which was crucial information. We hypothesized that they had originated from the BC instead of PTMC, for the following reasons. First, the most common sites of lateral CLNM in PTMC are levels II to IV. CLNM in level V is comparatively rare[7,8]. Second, the metastatic LNs of PTMC may show specific features, such as microcalcifications and cystic appearance, on US[9], which was not observed in our patient. Third, the size of the metastatic cervical LNs obviously decreased after neoadjuvant chemotherapy for BC.
Although most PTMC diagnoses have an excellent prognosis due to its indolent nature, some tend to be aggressive and are related to a poor prognosis[10]. The PTMC in our patient was T1aN1bM0 (IVa) stage according to the American Joint Committee on Cancer/Union for International Cancer Control TNM staging system[11]. Therefore, radical therapy was recommended. Because PTMC usually has a high incidence of multifocality and the presence of contralateral abnormal LNs[10], we could not exclude the existence of occult PTMC in the left lobe preoperatively. The patient underwent total thyroidectomy instead of lobectomy for complete resection of malignant tumors that allowed subsequent radiotherapy. Furthermore, prophylactic central compartment bilateral neck dissection was conducted for the presence of clinically involved lateral LNs (cN1b) as recommended by the 2015 American Thyroid Association guidelines[11]. However, a longer follow-up time was needed to learn the long-term prognosis of this patient. The BC was in a terminal stage as soon as it was discovered. In fact, it may have been discovered earlier if a more detailed examination had been performed at her first visit. The level V metastatic LNs discovered in the first surgery were not evaluated by further immunohistochemistry and were assumed to also be contralateral skip metastatic LNs. However, we cannot exclude that it may have originated from BC.
Here, we describe a case of PTMC with contralateral lymphatic skip metastasis and BC with supraclavicular lymphatic metastasis, which has rarely been reported in the literature. Both thyroid cancer and BC may involve CLNM. The characteristics of US, more detailed immunohistochemistry examination findings, and knowledge of the sites of CLNM may help to distinguish the origin of CLNM. In addition, when it comes to uncommon CLNM of the primary cancer, a more detailed examination such as by head-neck computed tomography, chest computed tomography and physical examination is strongly recommended.
We would like to thank Liu-Shu Jiang and Jun Liu for assisting with data collection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Shanghai Association of Chinese Integrative Medicine Thyroid Professional Committee.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gassler N, Raiter A S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15294] [Article Influence: 3058.8] [Reference Citation Analysis (4)] |
| 2. | Attard A, Paladino NC, Lo Monte AI, Falco N, Melfa G, Rotolo G, Rizzuto S, Gulotta E, Salamone G, Bonventre S, Scerrino G, Cocorullo G. Skip metastases to lateral cervical lymph nodes in differentiated thyroid cancer: a systematic review. BMC Surg. 2019;18:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Park JH, Lee YS, Kim BW, Chang HS, Park CS. Skip lateral neck node metastases in papillary thyroid carcinoma. World J Surg. 2012;36:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 4. | Bruno R, Giannasio P, Chiarella R, Capula C, Russo D, Filetti S, Costante G. Identification of a neck lump as a lymph node metastasis from an occult contralateral papillary microcarcinoma of the thyroid: key role of thyroglobulin assay in the fine-needle aspirate. Thyroid. 2009;19:531-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Yamashita G, Kondo T, Okimura A, Nakatsugawa M, Hirano H, Takeda A, Kikawada N, Aihara Y, Chiba Y, Ogawa Y, Tsukahara K. Occult Papillary Thyroid Carcinoma without Detection of the Primary Tumor on Preoperative Ultrasonography or Postoperative Pathological Examination: A Case Report. Case Rep Oncol. 2020;13:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Liu H, Lv L, Yang K. Occult thyroid carcinoma: a rare case report and review of literature. Int J Clin Exp Pathol. 2014;7:5210-5214. [PubMed] |
| 7. | Gong Y, Yang J, Yan S, Su A, Liu F, Gong R, Zhu J, Li Z. Pattern of and clinicopathologic risk factors for lateral lymph node metastases in papillary thyroid carcinoma patients with lateral cervical lymphadenopathy. Medicine (Baltimore). 2018;97:e12263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Caron NR, Tan YY, Ogilvie JB, Triponez F, Reiff ES, Kebebew E, Duh QY, Clark OH. Selective modified radical neck dissection for papillary thyroid cancer-is level I, II and V dissection always necessary? World J Surg. 2006;30:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 9. | Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, Hartl DM, Lassau N, Baudin E, Schlumberger M. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3590-3594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 314] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Yu XM, Lloyd R, Chen H. Current treatment of papillary thyroid microcarcinoma. Adv Surg. 2012;46:191-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 9633] [Article Influence: 1070.3] [Reference Citation Analysis (1)] |