Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3527
Peer-review started: September 11, 2021
First decision: January 25, 2022
Revised: January 31, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 209 Days and 4.1 Hours
The protective effect of tracheal stents is reported to relieve airway obstruction and reduce side effects of rapid progression of malignant tracheoesophageal fistula (MTEF) after immunotherapy in this case with 10 mo follow-up.
Two kinds of silicone stents were placed in the main airway of a 58-year-old male to relieve the airway obstruction caused by advanced esophageal carcinoma. The patient then received four doses of toripalimab. Subsequently, rapid, progressive deterioration of the original fistula was found. Although the fistula enlarged rapidly after immunotherapy, it remained covered completely, and likely because of this, his condition remained stable. Therefore, immunotherapy could be continued to treat the primary tumor. Despite these efforts, the patient died of the advancement of his esophageal cancer.
Appropriately-sized tracheal stent placement combined with immune checkpoint inhibitors may improve the quality of life and survival of patients with MTEF.
Core Tip: A 58-year-old male was diagnosed with advanced esophageal carcinoma and malignant tracheoesophageal fistula. For treatment, two kinds of silicone stents were placed in the main airway, followed by administration of four doses of toripalimab. Follow-up scans showed the original fistula to have rapidly increased in size between the upper trachea and esophagus. The fistula was still covered due to the appropriately-sized stents, which were likely protective, as no serious lung infections occurred and the patient remained stable. Accordingly, immunotherapy could be continued to treat the primary tumor. Unfortunately, however, the patient died of the esophageal cancer in February of 2021.
- Citation: Li CA, Yu WX, Wang LY, Zou H, Ban CJ, Wang HW. Double tracheal stents reduce side effects of progression of malignant tracheoesophageal fistula treated with immunotherapy: A case report. World J Clin Cases 2022; 10(11): 3527-3532
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3527.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3527
Malignant tracheoesophageal fistula (MTEF) is a devastating complication of esophageal cancer and tracheocarcinoma, which leads to a shorter life-span and decreased quality of life. MTEF develops in approximately 5%-15% of patients with an esophageal malignancy, with less than 1% of those having bronchogenic carcinoma[1,2]. Airway stent insertion provides an effective approach to improve symptoms and quality of life[3]. Toripalimab, a selective monoclonal antibody to the immune-checkpoint protein programmed cell death protein-1 (PD-1), is used for patients with advanced or metastatic esophageal squamous cell carcinoma[4].
In this report, we described the progression of MTEF in a patient caused by advanced esophagus squamous cell carcinoma invading the trachea, who was treated with toripalimab after tracheal stent placement.
A 58-year-old male presented with an 18-d history of choking after drinking and eating. He was hospitalized in Dongzhimen Hospital in Beijing on April 28, 2020.
The patient had previously been diagnosed with advanced esophageal squamous cell carcinoma (c-T4bN0M1, stage 4B). The patient received radiation as the first-line therapy, without surgery or chemotherapy. He was then treated with afatinib for 4 d as a trial treatment. Subsequently, he began choking after drinking and eating, and developed dysphagia 18 d before admission. After anti-infective and nutritional support treatments, the patient's symptoms were not significantly relieved.
The patient had no remarkable disease history.
The patient reported a personal history of smoking and drinking for more than 30 years. The patient reported his familial history did not include any tumors.
The patient’s physical examination was unremarkable.
Tests of peripheral blood tumor markers showed that pro-gastrin-releasing peptide (commonly known as pro-GRP) was 93.58 pg/mL and carcinoembryonic antigen (commonly known as CEA) was 5.6 ng/mL, both of which were higher than the normal range. All other markers tested were within normal range. White blood cell count was 3.8 × 109/L, hemoglobin was 125 g/L, and C-reactive protein was 4.69 mg/L.
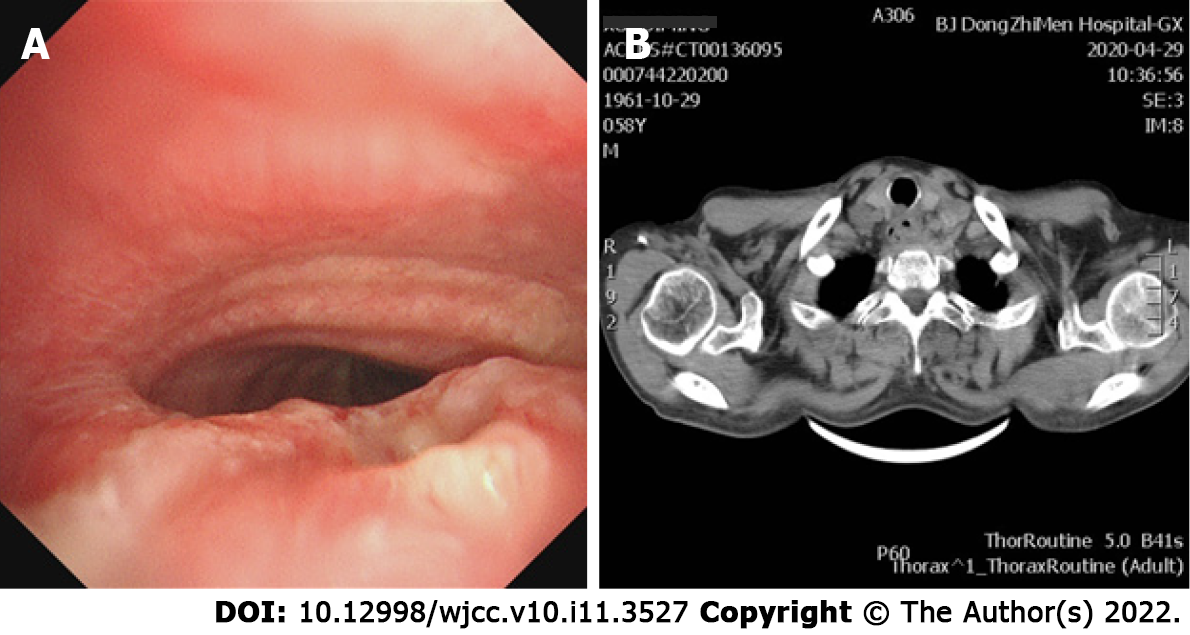
Bronchoscopy and computer tomography imaging found a 5 mm malignant tracheoesophageal fistula in the main airway, located between the upper trachea and esophagus (Figure 1).
Malignant tracheoesophageal fistula
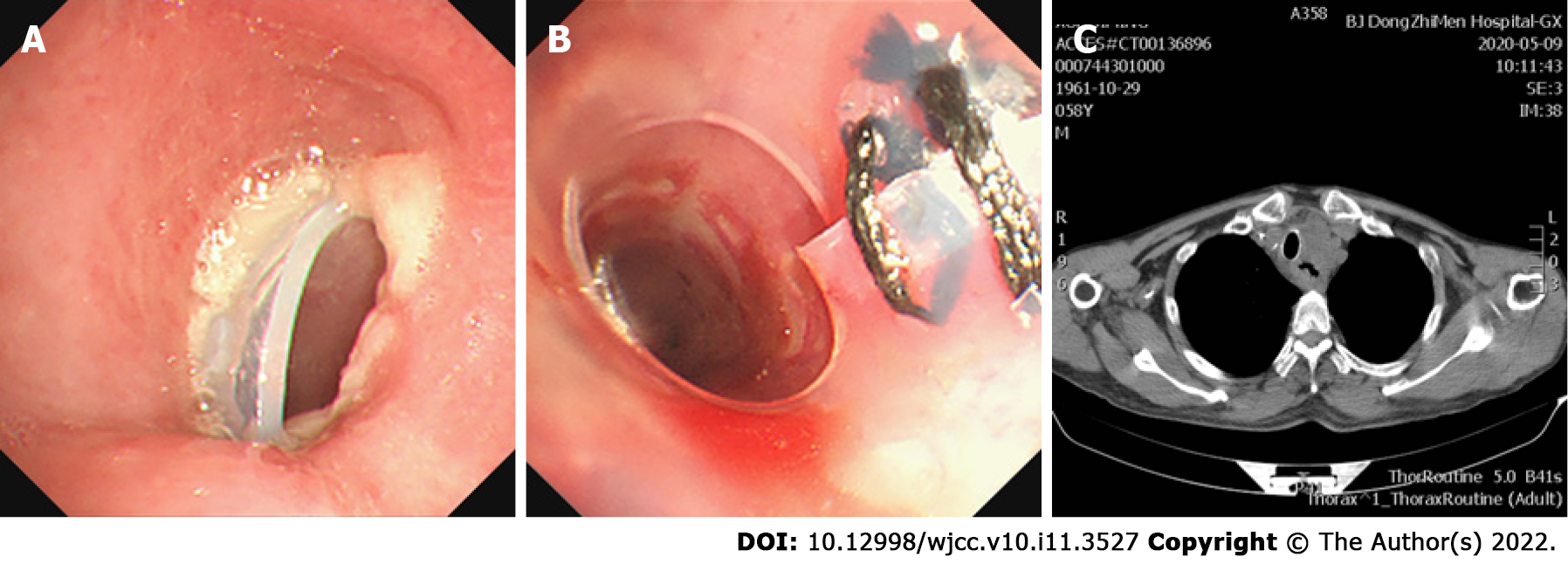
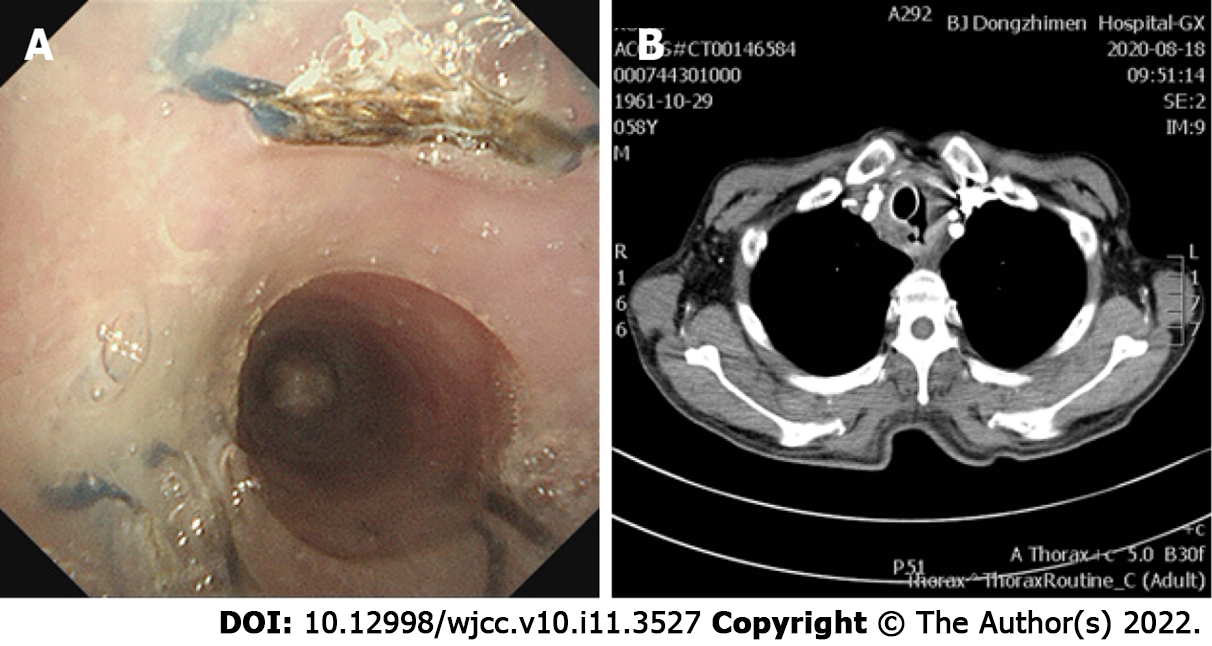
Given that the airway was significantly obstructed by the tumor, and performance status and Eastern Cooperative Oncology Group (commonly known as ECOG) classification had worsened from 1 to 3-4, mechanical debulking was performed on the tumor arising from the membranous trachea. Initially, a Y-shaped silicone stent of 16 mm × 13 mm × 13 mm in diameter and 80 mm × 30 mm × 15 mm in length (TracheobronxaneTM Dumon® TD; Novatech SA, La Ciotat, France) was placed in the main trachea, with projections into both the left and right main bronchus by rigid bronchoscopy. However, the upper edge of the stent blocked the airway wall, so that the trachea was significantly narrowed. To open the trachea, a straight silicone stent 16 mm in diameter and 30-mm length, partially incised along the long axis, was sutured to reduce the lumen diameter and inserted into the Y-type Dumon stent in the form of a telescope (Figure 2). After placement of the stent, the fistula was completely enclosed, without significant stenosis of the trachea. One month after stenting, the patient received the first dose of toripalimab (240 mg, intravenous drip, every 3 wk). After the fourth dose of toripalimab, the patient presented with hemoptysis. Chest computed tomography and bronchoscopy revealed that the original malignant tracheoesophageal fistula between the upper trachea and esophagus had progressed rapidly in size, measuring 20-30 mm (Figure 3). However, the fistula was still completely covered by the silicone stents.
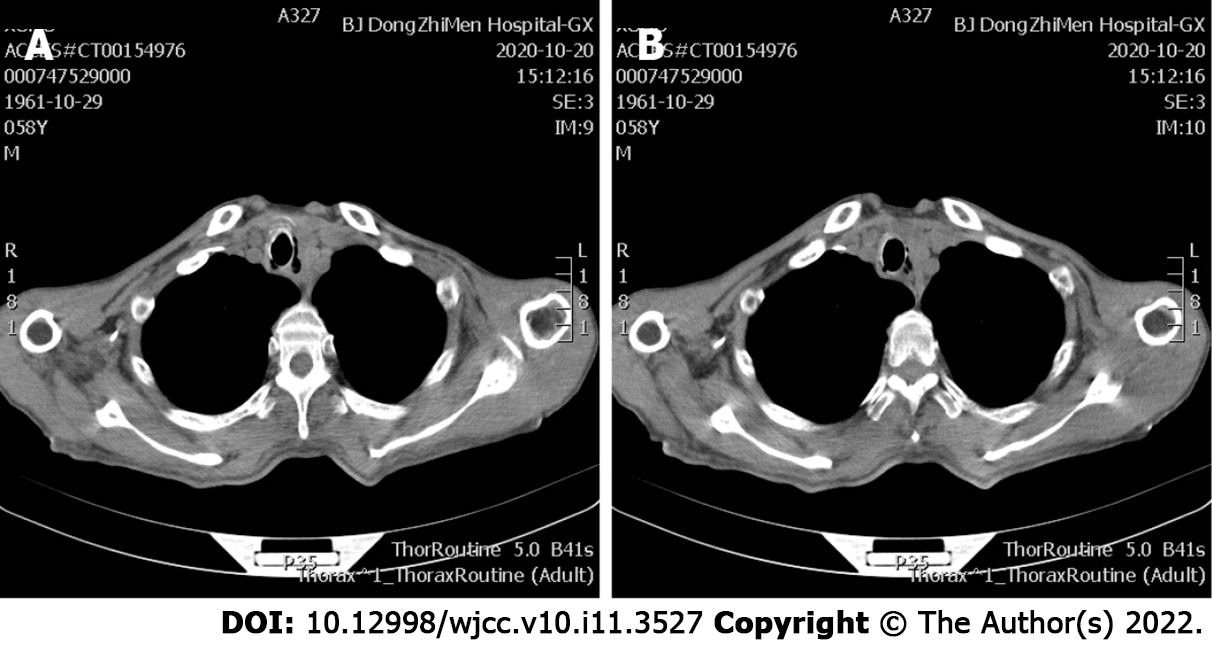
After palliative treatment for infection (piperacillin sodium and sulbactam sodium for injection, 3.75 g, intravenous drip, q8h) and hemostasis (haemocoagulase, 1 U, intravenous injection, q12h), the condition of the patient was stable. Immunotherapy was continued. During a 4-mo follow-up, no complications related to the stent placement were observed (Figure 4). However, after 7 mo total of toripalimab treatment, the patient died of esophageal cancer on February 16, 2021 (Figure 4B).
MTEF develops in approximately 5%-15% of patients with an esophageal malignancy, with less than 1% of those having bronchogenic carcinoma[1,2]. MTEF is a negative predictor of long-term survival, and those patients generally have a very poor prognosis and quality of life. Severe cough, frequent aspiration pneumonia, malnutrition, and life-threatening hemoptysis can lead to rapid deterioration of the patient, and most patients die within 3-4 mo.
MTEF is mostly caused by tumoral invasion or as a complication of cancer therapies. Esophageal cancer invades the trachea directly through its membranous wall or indirectly through metastases from the mediastinal lymph nodes. This leads to tumor necrosis, thus paving the path for MTEF formation[5].
Immediate management of MTEF involves nasogastric tube placement or gastrostomy, to minimize regurgitation. However, this palliative treatment does not improve the patient's quality of life, due its preclusion of oral eating. Radical resection of the MTEF has been reported but with minimal survival advantage[6] because of the advanced stage of the cancer and the consequent and ongoing nutritional depletion. Mesenchymal stem cell transplantation therapy for MTEF is most useful for smaller bronchopleural and distal fistulas, but the further research is required to gain an accurate understanding of the treatment’s efficacy and safety profiles[7].
Insertion of a tracheal or esophageal stent, to cover the fistula, is also effective, as was the case with our patient. The stent placement relieved dyspnea for our patient, avoided chemical lung infections, and improved the quality of life, which enabled the patient to continue immunotherapy. Together, these factors likely contributed to the increased survival time for our patient, which was greater than the reported median survival time for MTEF (from stent insertion to death) of 163 d[3]. Although, it is important to note that the expanded stent may readily erode and enlarge the size of the fistula[5].
Despite the increased survival time, the patient in the present report experienced rapid progression of MTEF after tracheal stent placement and treatment with toripalimab. Although several clinical studies have reported promising efficacies and manageable safety profiles of immune checkpoint inhibitors (ICIs) on advanced or metastatic esophageal squamous cell carcinoma[8,9], response rates to different anti-PD-1 antibodies in patients with previously treated advanced or metastatic esophageal squamous cell carcinoma were reported to be 14.3%-33.3%[10,11]. However, there have been no reports of immunotherapy and/or stenting increasing the size of the original fistula. Based on our case report, however, there is a potential risk of treating MTEF with immunotherapy and stenting. It is possible that immunotherapy may have hastened the development of a fistula by lysing the tumor. Although the fistula progressed rapidly after immunotherapy, the enlarged fistula remained completely covered, owing to the appropriate size of the stents; in addition, due to the dilation of the trachea by the stents, the airway remained open and protected against lung infections from either the cancer or the esophagobronchial fistula.
This rare case highlights the possibility that toripalimab might exacerbate the progress of a fistula in patients with MTEF. However, using an appropriately sized tracheal stent combined with ICIs therapy may improve the survival of patients with MTEF.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Covantsev S, Russia; Jabbarpour Z, Iran S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Chen YH, Li SH, Chiu YC, Lu HI, Huang CH, Rau KM, Liu CT. Comparative study of esophageal stent and feeding gastrostomy/jejunostomy for tracheoesophageal fistula caused by esophageal squamous cell carcinoma. PLoS One. 2012;7:e42766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Balazs A, Kupcsulik PK, Galambos Z. Esophagorespiratory fistulas of tumorous origin. Non-operative management of 264 cases in a 20-year period. Eur J Cardiothorac Surg. 2008;34:1103-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Wang H, Tao M, Zhang N, Li D, Zou H, Zhang J, Luo L, Ma H, Zhou Y. Airway Covered Metallic Stent Based on Different Fistula Location and Size in Malignant Tracheoesophageal Fistula. Am J Med Sci. 2015;350:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Zheng Y, Liu XB, Sun HB, Xu J, Shen S, Ba YF, Yan M, Qin Z, Liu BX, Wang ZF, Liu SL, Zhang RX, Chen PN, Liang GH, Yuan D, Li ZX, Liu Q, Wang HR, Li HM, Lv H, Ma X, Zhu J, Yu YK, Xing WQ; written on Henan Cancer Hospital Thoracic Oncology Group (HCHTOG). A phase III study on neoadjuvant chemotherapy versus neoadjuvant toripalimab plus chemotherapy for locally advanced esophageal squamous cell carcinoma: Henan Cancer Hospital Thoracic Oncology Group 1909 (HCHTOG1909). Ann Transl Med. 2021;9:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Shamji FM, Inculet R. Management of Malignant Tracheoesophageal Fistula. Thorac Surg Clin. 2018;28:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 6. | Davydov M, Stilidi I, Bokhyan V, Arzykulov G. Surgical treatment of esophageal carcinoma complicated by fistulas. Eur J Cardiothorac Surg. 2001;20:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Huang P, Gao XL, Zhong ZC, Chen WF, Wang YC, Li J. [Bone marrow⁃derived mesenchymal stem⁃cell in treatment of bronchopleural fistula]. Zhonghua Jie He He Hu Xi Za Zhi. 2019;42:137-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Lin S, Kato K, Kim SB, Ajani JA, Zhao K, He Z, Yu X, Shu Y, Luo Q, Wang J, Chen Z, Niu Z, Sun JM, Lin CY, Hara H, Pazo-Cid R, Borg C, Li L, Tao A, Van Cutsem E. RATIONALE 302: Randomized, phase 3 study of tislelizumab versus chemotherapy as second-line treatment for advanced unresectable/metastatic esophageal squamous cell carcinoma. J Clin Oncol. 2021;39:4012-4012. [RCA] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, Doi T, Moriwaki T, Kim SB, Lee SH, Bennouna J, Kato K, Shen L, Enzinger P, Qin SK, Ferreira P, Chen J, Girotto G, de la Fouchardiere C, Senellart H, Al-Rajabi R, Lordick F, Wang R, Suryawanshi S, Bhagia P, Kang SP, Metges JP; KEYNOTE-181 Investigators. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J Clin Oncol. 2020;38:4138-4148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 690] [Article Influence: 138.0] [Reference Citation Analysis (0)] |
| 10. | Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, Lordick F, Kim SB, Tajika M, Kim HT, Lockhart AC, Arkenau HT, El-Hajbi F, Gupta M, Pfeiffer P, Liu Q, Lunceford J, Kang SP, Bhagia P, Kato K. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients With Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol. 2019;5:546-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 373] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 11. | Huang J, Xu B, Mo H, Zhang W, Chen X, Wu D, Qu D, Wang X, Lan B, Yang B, Wang P, Zhang H, Yang Q, Jiao Y. Safety, Activity, and Biomarkers of SHR-1210, an Anti-PD-1 Antibody, for Patients with Advanced Esophageal Carcinoma. Clin Cancer Res. 2018;24:1296-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |