Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3505
Peer-review started: December 15, 2021
First decision: January 26, 2022
Revised: February 3, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 114 Days and 3.2 Hours
Chronic radiative chest wall ulcers are common in patients undergoing radiation therapy. If not treated early, then symptoms such as erosion, bleeding and infection will appear on the skin. In severe cases, ulcers invade the ribs and pleura, presenting a mortality risk. Small ulcers can be repaired with pedicle flaps. Because radioactive ulcers often invade the thorax, surgeons need to remove large areas of skin and muscle, and sometimes ribs. Repairing large chest wall defects are a challenge for surgeons.
A 74-year-old female patient was admitted to our department with chest wall skin ulceration after radiation therapy for left breast cancer. The patient was diagnosed with chronic radioactive ulceration. After multidisciplinary discussion, the authors performed expansive resection of the chest wall ulcers and repaired large chest wall defects using a deep inferior epigastric perforator (DIEP) flap combined with a high-density polyethylene (HDPE) patch. The patient was followed-up 6 mo after the operation. No pigmentation or edema was found in the flap.
DIEP flap plus HDPE patch is one of the better treatments for radiation-induced chest wall ulcers.
Core Tip: In recent years, with the development of microsurgical techniques and breast reconstruction techniques and the wide application of autologous flap transplantation, breast surgeons have provided new ideas for the treatment of chronic radiation-induced ulcers of the chest wall. Deep inferior epigastric perforator flap combined with a high-density polyethylene patch is a promising treatment for chronic radiation-induced ulcers of the chest wall.
- Citation: Huang SC, Chen CY, Qiu P, Yan ZM, Chen WZ, Liang ZZ, Luo KW, Li JW, Zhang YQ, Huang BY. Reconstruction of complex chest wall defects: A case report. World J Clin Cases 2022; 10(11): 3505-3510
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3505.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3505
The incidence of chest wall ulcers due to chronic radiation is about 25%-30%. The radiation dose, radiation time and patient’s condition are the risk factors known to affect its occurrence. It has been reported that radiation doses exceeding 70 Gy/7 wk/30 fractions are prone to cause such ulcers[1]. Radiation-induced ulcers of the chest wall are secondary, progressive and irreversible, with a duration of as short as several months and as long as years or decades. Moreover, they can be classified as a special type of protracted wound. In addition, radiation can cause vascular contracture, reduce the amount of blood supplied to the skin, induce fibrosis of the skin, and directly impair the repair function of the skin tissue[2-5].
Chronic radiation-induced ulcers of the chest wall are often associated with infection and easily cause rib necrosis and osteomyelitis. This seriously affects the quality of life of patients and is a detriment to a patient’s confidence in treating cancer. In the past, surgeons have tried to treat it with debridement and dressing change or skin grafting, but it is difficult to achieve the ideal therapeutic effect.At present, chest wall reconstruction includes two parts: Bone reconstruction of the chest wall, and soft tissue reconstruction of the chest wall. The former uses biological or artificial materials to restore stability of the chest wall, while the latter realizes the tightness of the chest wall by transferring its own tissue flap to cover the defect. In the repair of the chest wall, titanium alloy plates and high-density polyethylene (HDPE) patches are two commonly used materials. Some scholars have pointed out that HDPE patches have sufficient toughness and can better maintain the stability of the chest wall without interference with radiotherapy[6]. In terms of soft tissue repair, a banded myocutaneous flap is better to repair chest wall defects; however, the excessive muscle harvested during the surgery causes the patient sustains greater trauma, resulting in prolonged recovery time and affecting the subsequent treatment of patients. Due to the development of microsurgical techniques, the application of free flaps to repair chest wall defects has become a clinical hot spot.
In this report, the breast cancer patient suffered from a chronic radiation-induced ulcer of the chest wall combined with necrosis of the second to fifth ribs. The chest wall defect was repaired by deep inferior epigastric perforator (DIEP) flap combined with a HDPE patch, with good postoperative recovery.
A 74-year-old female patient was admitted for a radioactive ulcer on the chest wall for more than 4 mo.
The patient’s chest wall ulcer occurred 1 wk after the 6th radiotherapy treatment and lasted about 4 mo. The area of the ulcer was 4 cm × 6 cm. The surface of the ulcer was dirty, and the basal granulation tissue was sparse. The ulcer surface bled easily, which indicated that it was closely attached to deep tissue. The ulcer was hard and surrounded by scar tissue.
The patient underwent modified radical mastectomy of the left breast in our department 5 mo prior. The patient did not have hypertension, diabetes or hyperlipidemia.
Personal and family history was unremarkable.
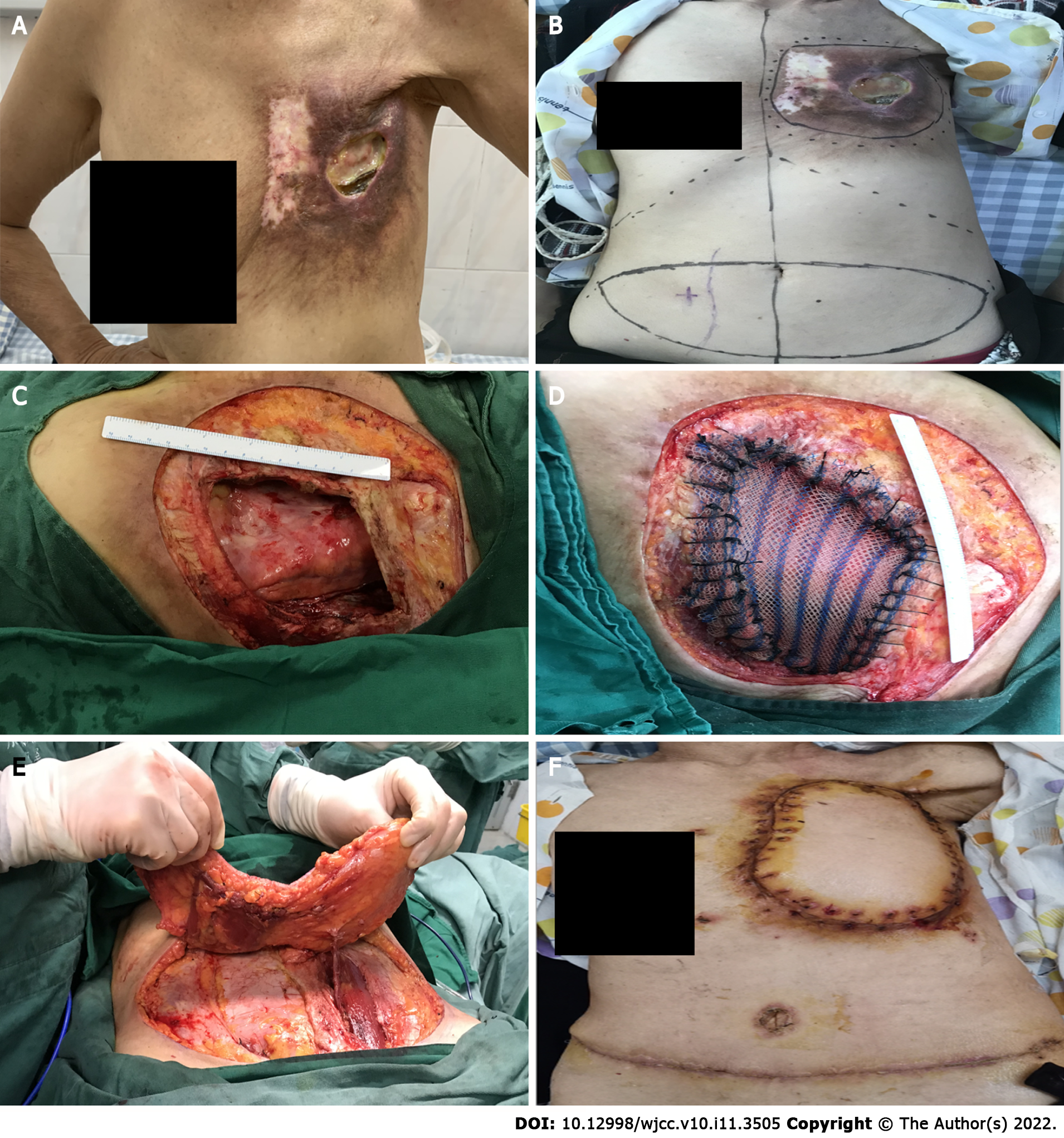
An irregularly shaped ulcer was observed on the surface of the skin of the left chest wall and measuring 4 cm × 6 cm. It was accompanied by red coloration, fishy odor, and pus-like discharge. The skin around the ulcer was red and swollen, hard in consistency and tender (Figure 1A). No significantly enlarged lymph nodes were palpable in the left axilla or supraclavicular region.
All laboratory tests were normal.
Chest computed tomography: Irregular subcutaneous soft tissue of the left anterior chest wall and multiple small nodular high-density shadows in the local area were observed. These were predicted to change after radiotherapy. Slightly disordered structure of the left axilla was also observed but no enlarged lymph nodes were found.
The consultation included specialists in breast surgery, thoracic surgery and radiology. After discussion by several specialists, the decision was made to perform an expanded excision of the ulcer on the chest wall of the patient, including the invaded muscle and ribs. Then, the thoracic defect would be repaired with a patch, and the chest wall defect would be covered with a free flap (Figure 1B).
Radiation-induced ulcer of the left chest wall (Figure 1C).
DIEP flap combined with an HDPE patch was used to repair the chest wall ulcer (Figure 1D and E).
The patient was followed-up for 6 mo after the operation. The skin flap showed no pigmentation and no edema. The skin growth and healing of the donor site were acceptable. The donor site was not complicated with abdominal skin necrosis, abdominal bulge, abdominal weakness, nor abdominal hernia (Figure 1F).
Although radiotherapy equipment, technology and design have been developed to varying degrees in recent years, the occurrence of chronic radiation-induced ulcers of the chest wall are still common when radiation therapy is received after breast cancer surgery.
According to scholars, conservative treatment of radiation-induced ulcers using debridement, dressing change and antibiotics is ineffective for most patients[7]. Many clinicians have tried different methods to treat chronic radiation-induced ulcers of the chest wall. According to Nishimoto et al[8], an ideal therapeutic effect was observed in 4 patients upon transfusing platelet-rich plasma and simultaneous skin grafting. Other studies have reported that chronic radiation-induced ulcers of the chest wall have been cured by intravenous infusion of plasma-purified mannan-binding lectin to patients[9,10]. Researchers have also conducted an animal experiment (in guinea pigs) and administered arginine glutamate after artificially causing radiation-induced ulcers, and the therapeutic effect of the ulcers was adequate[10,11]. However, due to individual differences, the above treatment has not been widely promoted in clinical practice, and surgical treatment is still the main treatment.
Radiation injury causes soft tissue damage and leads to necrosis of the sternum and ribs. Surgical reconstruction of the chest wall includes two parts: Thoracic reconstruction and soft tissue repair. It is generally believed that if the anterolateral chest wall defect is less than 6 cm × 6 cm and the posterior chest wall defect is less than 10 cm × 10 cm, then generally no thoracic reconstruction is required[10,12]. Soft tissue coverage alone can eliminate the abnormal respiratory movement of the chest wall after surgery. However, large defects require thoracic reconstruction to restore the firmness and stability of the thorax in order to protect the viscera and maintain normal respiration.
In the reconstruction of the bony chest wall, two kinds of synthetic materials titanium alloy plates and HDPE soft patch are mostly used. HDPE patches have good toughness and elicit minimal aseptic inflammatory response. In a short time, human tissue can grow on the patch and cover the cavity in the body, which is conducive to wound repair. In addition, the patch is easy to cut and use during surgery. However, compared with a titanium alloy plate, its hardness and support force are not satisfactory. Occasionally, when the patient breathes, the HDPE patch suture loosens, which results in deformation of the chest wall. However, HDPE patches do not interfere with subsequent radiation therapy.
Thorough debridement and myocutaneous flap coverage of the defect are the preferred methods for the surgical treatment of chest wall ulcers. When debridement of thoracic ulcers is performed, it is required to remove the injured skin and subcutaneous soft tissue, necrotic ribs, sternum, clavicle and surrounding muscle fibrous tissue as much as possible. The choice of myocutaneous flaps, such as latissimus dorsi flap, pectoralis major flap and rectus abdominis flap, is preferred[6]. These myocutaneous flaps have the advantages of rich tissue volume, stable vascular pedicle course, and easy operation. However, due to the harvesting of a large amount of autologous muscles from patients, they cause greater trauma to individuals and result in a longer recovery time, which may delay the subsequent treatment time. In contrast, pedicle musculocutaneous flaps are difficult to cover large wounds due to their small incision area.
In this case, DIEP soft tissue was used to repair the chest wall defect. Without harvesting the muscle, there was a sufficient amount of tissue to cover the defect, which was less invasive to the patient and had less recovery time, that did not disrupt subsequent radiotherapy. However, compared with other myocutaneous flaps, DIEP has a relatively small amount of tissue and cannot be used to cover deeper or larger defects. Moreover, the operation of DIEP is relatively complex and requires knowledge and expertise in microvascular anastomosis surgery, which is difficult to promote in clinical practice.
The main characteristics of this case were: (1) Intraoperative use of DIEP does not cut the rectus abdominis muscle, making it less invasive to the patient and shortening the postoperative recovery time; and (2) Intraoperative use of HDPE patches can maintain stability of the chest wall and does not interfere with the patient’s subsequent radiotherapy.
In summary, for patients with smaller chest wall defects accompanied by rib necrosis, DIEP combined with HDPE patch repair can be used to obtain satisfactory clinical efficacy and reduce the occurrence of postoperative complications. This may be a better way to treat large chest wall radiation-induced ulcers.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feizi A, Iran; Kapritsou M, Greece S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Zhou Y, Zhang Y. Single- versus 2-stage reconstruction for chronic post-radiation chest wall ulcer: A 10-year retrospective study of chronic radiation-induced ulcers. Medicine (Baltimore). 2019;98:e14567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Futran ND, Trotti A, Gwede C. Pentoxifylline in the treatment of radiation-related soft tissue injury: preliminary observations. Laryngoscope. 1997;107:391-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Arnold PG, Pairolero PC. Reconstruction of the radiation-damaged chest wall. Surg Clin North Am. 1989;69:1081-1089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Feldmeier JJ. Hyperbaric oxygen therapy and delayed radiation injuries (soft tissue and bony necrosis): 2012 update. Undersea Hyperb Med. 2012;39:1121-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Enomoto M, Yagishita K, Okuma K, Oyaizu T, Kojima Y, Okubo A, Maeda T, Miyamoto S, Okawa A. Hyperbaric oxygen therapy for a refractory skin ulcer after radical mastectomy and radiation therapy: a case report. J Med Case Rep. 2017;11:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Hameed A, Akhtar S, Naqvi A, Pervaiz Z. Reconstruction of complex chest wall defects by using polypropylene mesh and a pedicled latissimus dorsi flap: a 6-year experience. J Plast Reconstr Aesthet Surg. 2008;61:628-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 7. | Fujioka M. Surgical Reconstruction of Radiation Injuries. Adv Wound Care (New Rochelle). 2014;3:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Nishimoto S, Fukuda K, Kawai K, Fujiwara T, Tsumano T, Fujita K, Kakibuchi M. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: A preliminary report. Indian J Plast Surg. 2012;45:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Maaløe N, Bonde C, Laursen I, Christiansen M, Hölmich LR. Mannan-binding lectin and healing of a radiation-induced chronic ulcer--a case report on mannan-binding lectin replacement therapy. J Plast Reconstr Aesthet Surg. 2011;64:e146-e148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Zhou Y, Guan X, Zhang Y. Staged repair of chronic chest wall radiation ulcer. J Tissue Eng. 2014;10:335-338. [DOI] [Full Text] |
| 11. | Khalin I, Kocherga G. Arginine glutamate improves healing of radiation-induced skin ulcers in guinea pigs. Int J Radiat Biol. 2013;89:1108-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Chang RR, Mehrara BJ, Hu QY, Disa JJ, Cordeiro PG. Reconstruction of complex oncologic chest wall defects: a 10-year experience. Ann Plast Surg. 2004;52:471-9; discussion 479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |