Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3436
Peer-review started: September 12, 2021
First decision: October 25, 2021
Revised: November 9, 2021
Accepted: January 13, 2022
Article in press: January 13, 2022
Published online: April 16, 2022
Processing time: 208 Days and 3.8 Hours
Several studies have reported the prognostic value of ultrasound elastography (UE) in patients receiving neoadjuvant chemotherapy (NACT) for breast cancer. However, the assessment of parameters differed between shear-wave elasto
To assess the accuracy of UE for predicting the pathologic complete response (pCR) in breast cancer patients following NACT.
A comprehensive and systematic search was performed in the databases of MEDLINE, EMBASE, SCOPUS, PubMed Central, CINAHL, Web of Science and Cochrane library from inception until December 2020. Meta-analysis was per
A total of 14 studies with 989 patients were included. The pooled sensitivities were 86% [95% confidence interval (CI): 76%-92%] for UE, 77% (95%CI: 68%-84%) for shear-wave elasto
Strain-wave type of UE can accurately predict the pCR following NACT amongst breast cancer patients. Studies exploring its accuracy in different ethnic populations are required to strengthen the evidence.
Core Tip: Several studies have reported the prognostic value of ultrasound elastography (UE) in patients receiving neoadjuvant chemotherapy (NACT) for breast cancer. However, the assessment of parameters differed between shear-wave elastography and strain elastography in terms of measured elasticity parameter and mode of imaging. It is important, therefore, to assess the accuracy of the two modes of elastography. We assessed the accuracy of UE for predicting the pathologic complete response (pCR) in breast cancer patients following NACT. Strain-wave type of UE can accurately predict the pCR following NACT amongst breast cancer patients. Studies exploring its accuracy in different ethnic populations are required to strengthen the evidence.
- Citation: Chen W, Fang LX, Chen HL, Zheng JH. Accuracy of ultrasound elastography for predicting breast cancer response to neoadjuvant chemotherapy: A systematic review and meta-analysis. World J Clin Cases 2022; 10(11): 3436-3448
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3436.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3436
Neoadjuvant chemotherapy (NACT) has been established as the standard mode of treatment for inflammatory or locally advanced breast cancer. Pathologic complete response (pCR) has been utilized as a surrogate marker for detecting the prognosis or long-term survival following NACT in breast cancer patients[1], with several studies showing a response rate of almost 70% and pCR rate of about 30%[1,2]. Some factors were found to be associated with an increased risk of developing resistance to chemotherapy[3]. Hence, early prediction of the response to NACT in patients with breast cancer is critical.
Ultrasonic elastography (UE) is one of the most commonly used non-invasive imaging methods based on the mechanical properties of the tissue to assess the differences in breast lesion stiffness and elasticity (both quantitatively and qualitatively)[4]. It detects and quantifies the differences in tissue stiffness. Therefore, it can be used as an excellent imaging technique to differentiate benign and malignant breast masses[4]. There are two types of UE employed currently for examining the breast lesions, i.e., shear-wave elastography and strain elastography. Both techniques characterize the breast lesions based on the level of stiffness. Mean stiffness can be used as an effective preoperative predictor of progression of the disease in invasive breast cancer, and maximal stiffness has been used as a predictor of histopathological severity of breast lesions[4].
Breast cancer treatment with NACT might increase the probability of down-staging of the tumors. However, pCR to NACT is highly variable, and the current protocols to predict pCR to NACT are not sensitive enough. Formulating a tailored strategy using validated biomarkers to predict the degree of response to NACT has become a priority nowadays in the research of breast cancer. Several studies have reported the prognostic value of UE in patients receiving NACT for breast cancer. Breast masses with higher aggressive pathological properties can have higher stiffness value, suggesting that UE might provide very useful information to determine the prognosis of the patient[5]. However, the assessment of parameters is different for shear-wave elastography and strain elastography in terms of measured elasticity parameter and mode of imaging. To the best of our knowledge, no meta-analysis has yet assessed the accuracy of the two modes of elastography in predicting the response to NACT in breast cancer patients. The aim of the current study was to systematically search the literature for all studies assessing the accuracy of UE for predicting response to NACT in breast cancer, and pool the data for meta-analysis.
The inclusion criteria were as follows: Studies evaluating the predictive accuracy of UE for pCR following NACT in breast cancer patients; studies using histopathological examination as the reference standards for finding the pCR for inclusion in our review; prospective and retrospective studies.
The exclusion criteria were as follows: Studies not reporting the values necessary for pooling the sensitivity and specificity; unpublished studies.
We had a comprehensive search strategy to screen the databases such as MEDLINE, EMBASE, SCOPUS, PubMed Central, CINAHL, Web of Science, and Cochrane library. We did not have any language restriction and time limit for the search was between the inception of database till December 2020. The following search terms were used: “Ultrasound Elastography”, “Neoadjuvant Chemotherapy”, “Breast Cancer”, “Breast Carcinoma”, “Validation Studies”, “Diagnostic Accuracy Studies”, “Pathologic Complete Response”, and “Remission”. We also hand-searched the bibliographies of the included studies and checked for any missed-out studies matching our eligibility criteria. The details for different search strategies employed for different databases are provided in the Supplementary Material.
Two investigators (LF and HC) were responsible for performing the primary search of the articles by screening the title and abstract, and downloading the relevant full-text publications. The same set of investigators also independently read the retrieved full-texts and checked whether the studies were meeting the eligibility criteria of our review. Disagreements were resolved with assistance from a third author (WC), which helped in reaching a consensus for study selection. We achieved an overall agreement of 97% with a kappa statistic of 0.87.
The responsibility of data extraction from the final included full-text articles was assigned to the primary investigator. Data was extracted using a structured pre-defined form and directly transferred to the STATA software (StataCorp, CollegeStation, TX, United States). This data extraction form consists of the following components: Author and year of publication, country, design, participants, total sample size, study setting, details of UE, reference test, average age, cut-off (mean stiffness/strain ratio), sensitivity, and specificity. The third investigator ensured the data quality by double-checking data entries before performing the meta-analysis.
Two independent investigators (LF and HC) rated the included studies based on the level of bias risk using the quality assessment of diagnostic accuracy studies-2 (QUADAS-2) tool. The studies were rated for the following domains: Patient selection, conduct & interpretation of the index and reference tests, and flow and timing of outcome assessment[6]. Discrepancies and disagreements during the rating of studies were resolved by the third investigator who helped in achieving the consensus and rated all the studies as unclear, low, or high quality (based on the risk of bias).
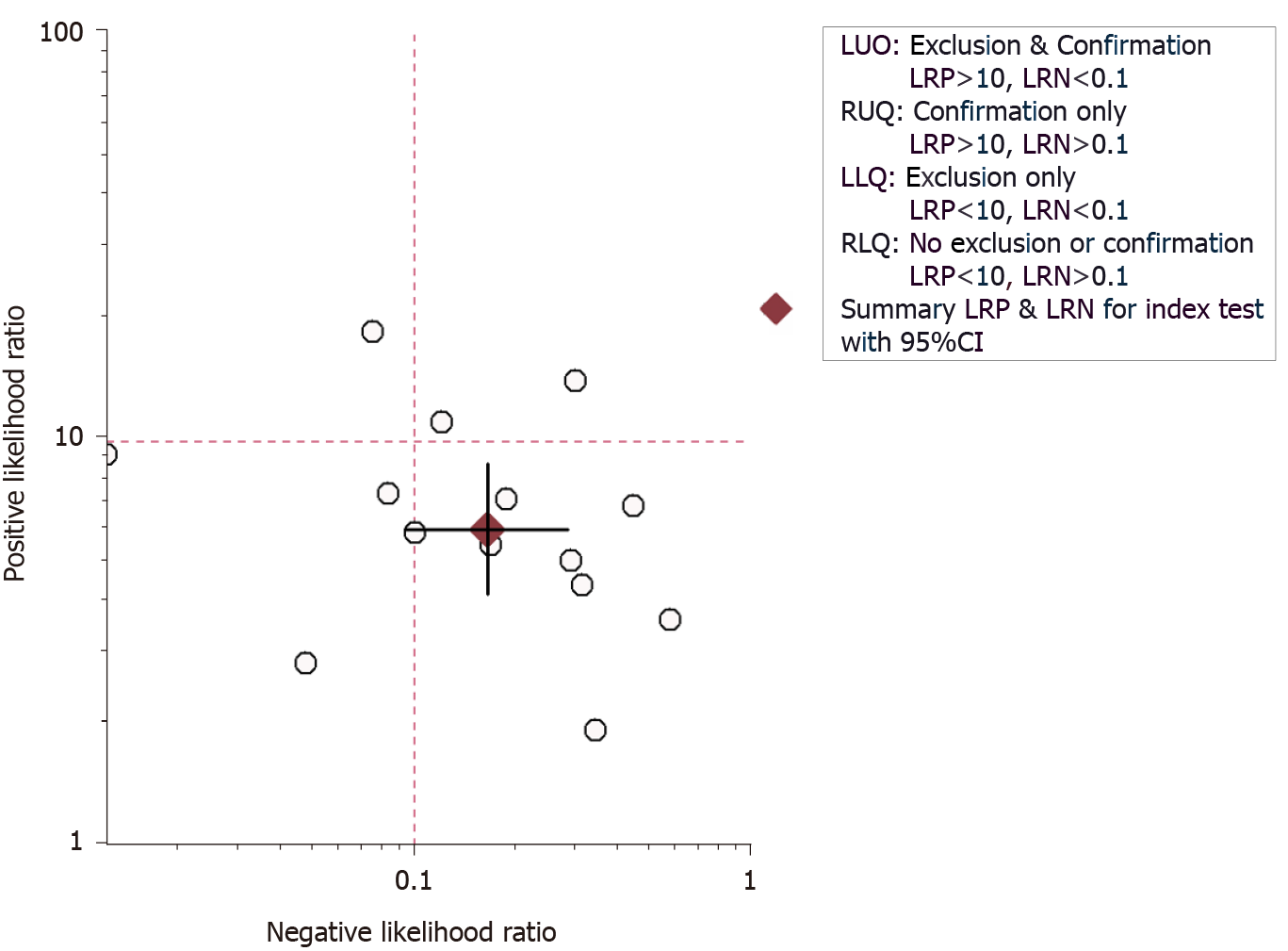
Meta-analysis was performed using the STATA version 14 software (StataCorp, TX, United States). Sensitivity and specificity were pooled by bivariate method for predicting the pCR following NACT in breast cancer patients using UE. We also estimated other important accuracy parameters such as positive and negative likelihood ratios (LRP and LRN, respectively) and diagnostic odds ratio (DOR) for the predictive utility of UE. We also reported these results separately for shear wave and strain wave elastography. We have reported these results using the following plots: Forest plot to depict pooled specificity and sensitivity, LR scattergram to depict the LRP and LRN, and Fagan plot to depict the pre- and post-test probabilities. LR scattergram consists of the following four quadrants with its interpretation: Left upper quadrant (LRP value > 10, LRN value < 0.1) indicative of confirmation & exclusion diagnostic criterion, right upper quadrant (LRP value > 10, LRN value > 0.1) indicative of confirmation diagnostic criterion, left lower quadrant (LRP value < 10, LRN value < 0.1) indicative of exclusion diagnostic criterion, and right lower quadrant (LRP value < 10, LRN value > 0.1) indicative of neither confirmation nor exclusion diagnostic criterion. “Summary receiver operator characteristic curve” (sROC) was used to report the summary predictive accuracy of UE for pCR.
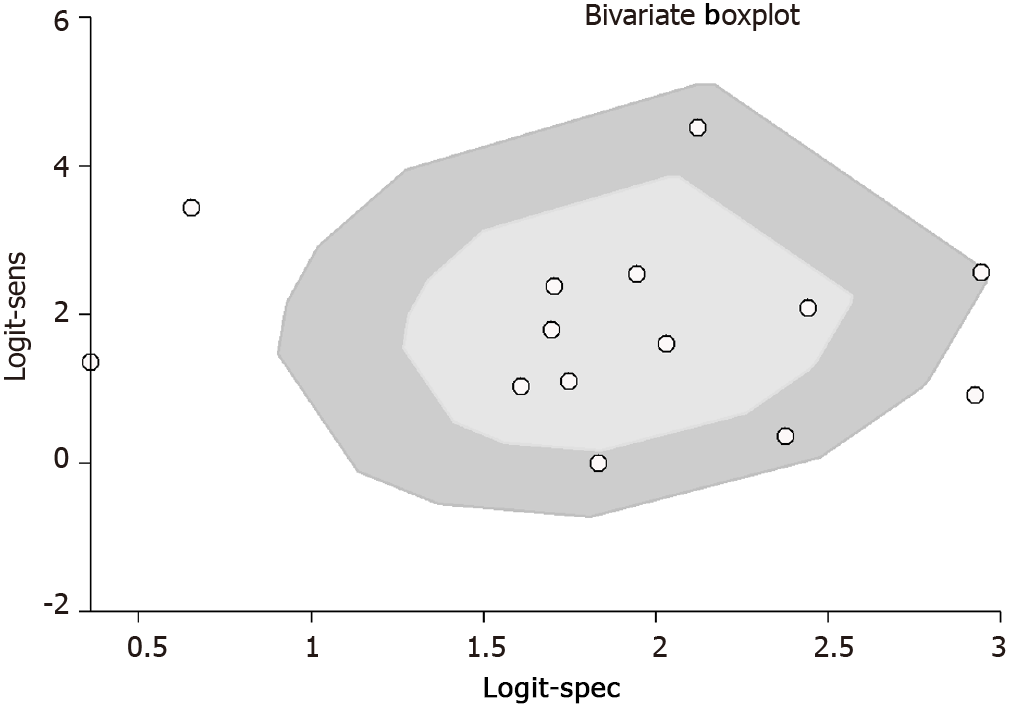
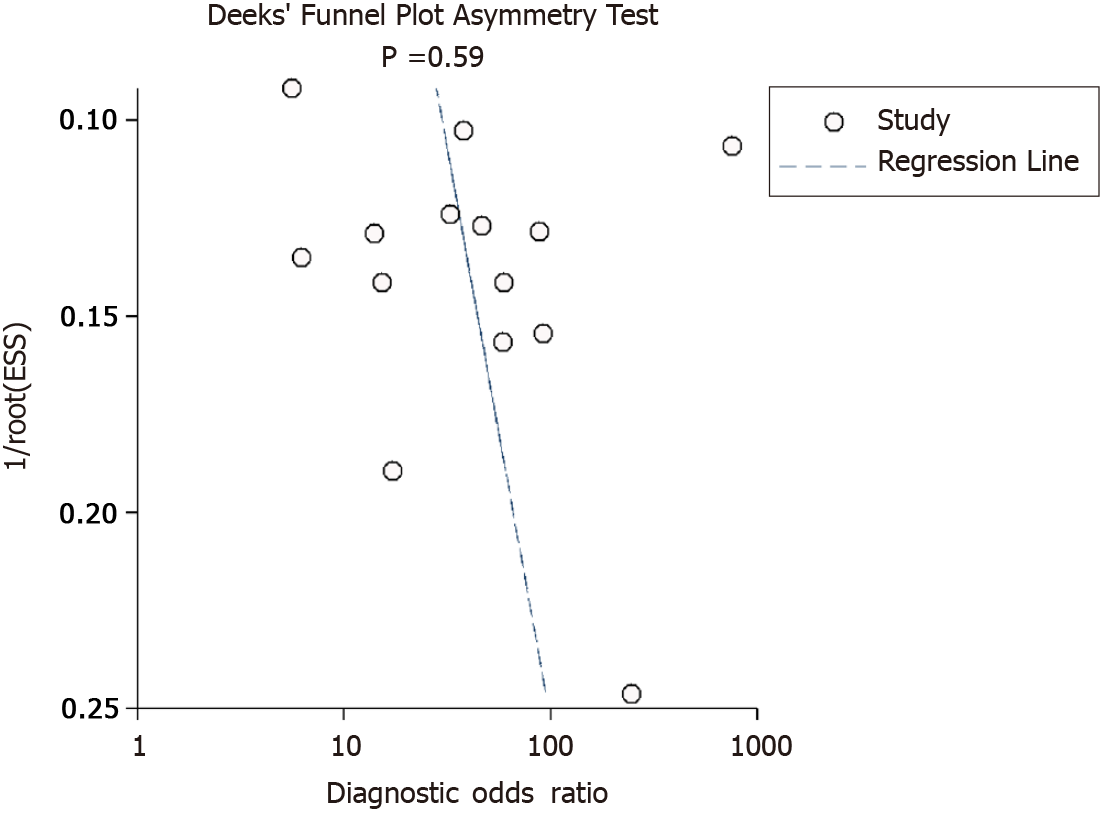
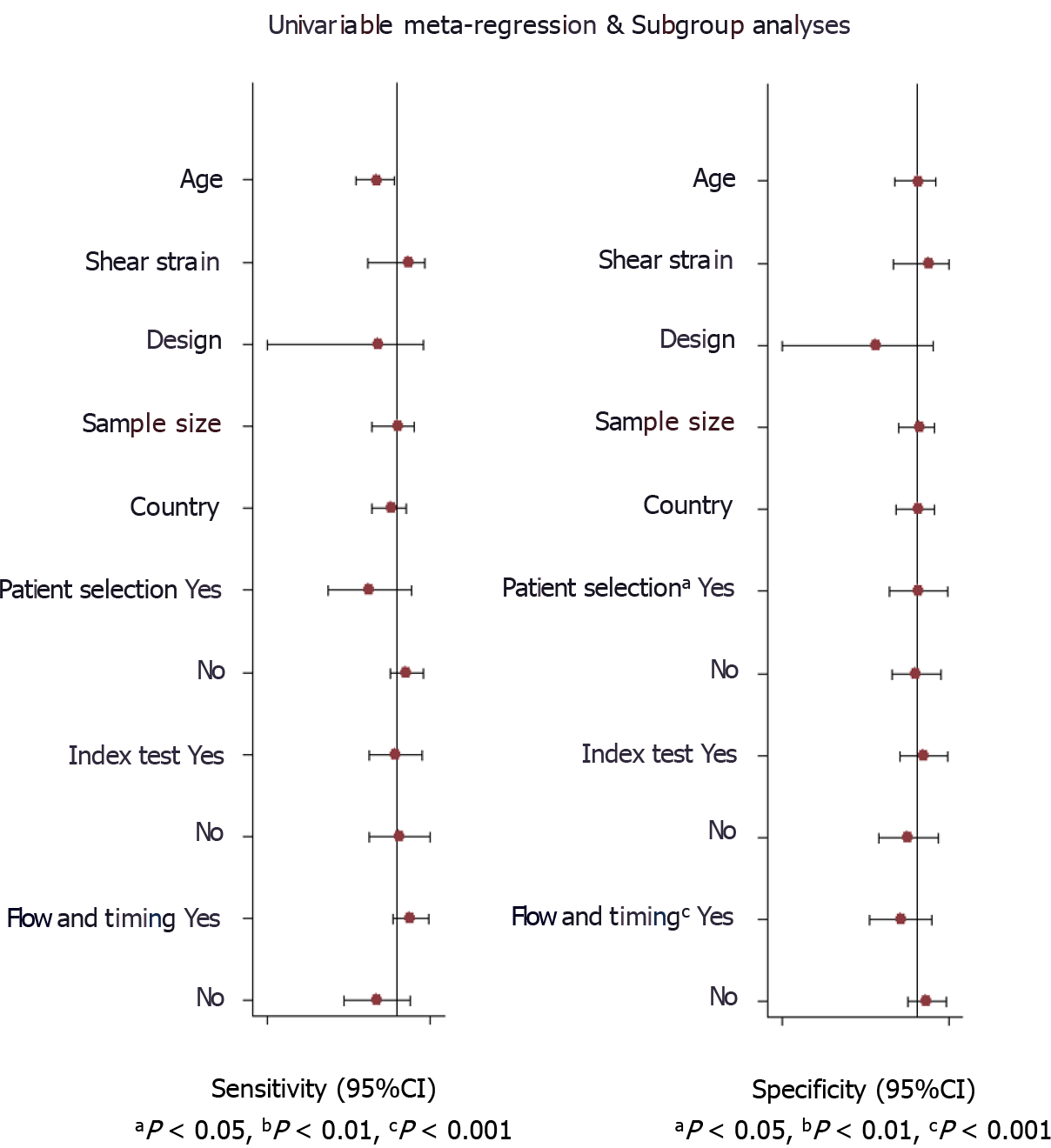
Evaluation of heterogeneity was done by chi-square and I2 statistic. It is represented graphically by a bivariate box plot. Additional meta-regression analysis was performed to identify the source of the high heterogeneity found in our results. The covariates used in the meta-regression were study design, sample size, mean age, shear or strain wave UE, country, cut-off, and quality related factors. Deeks’ test was performed to assess the possibility of publication bias and it is graphically represented by funnel plot.
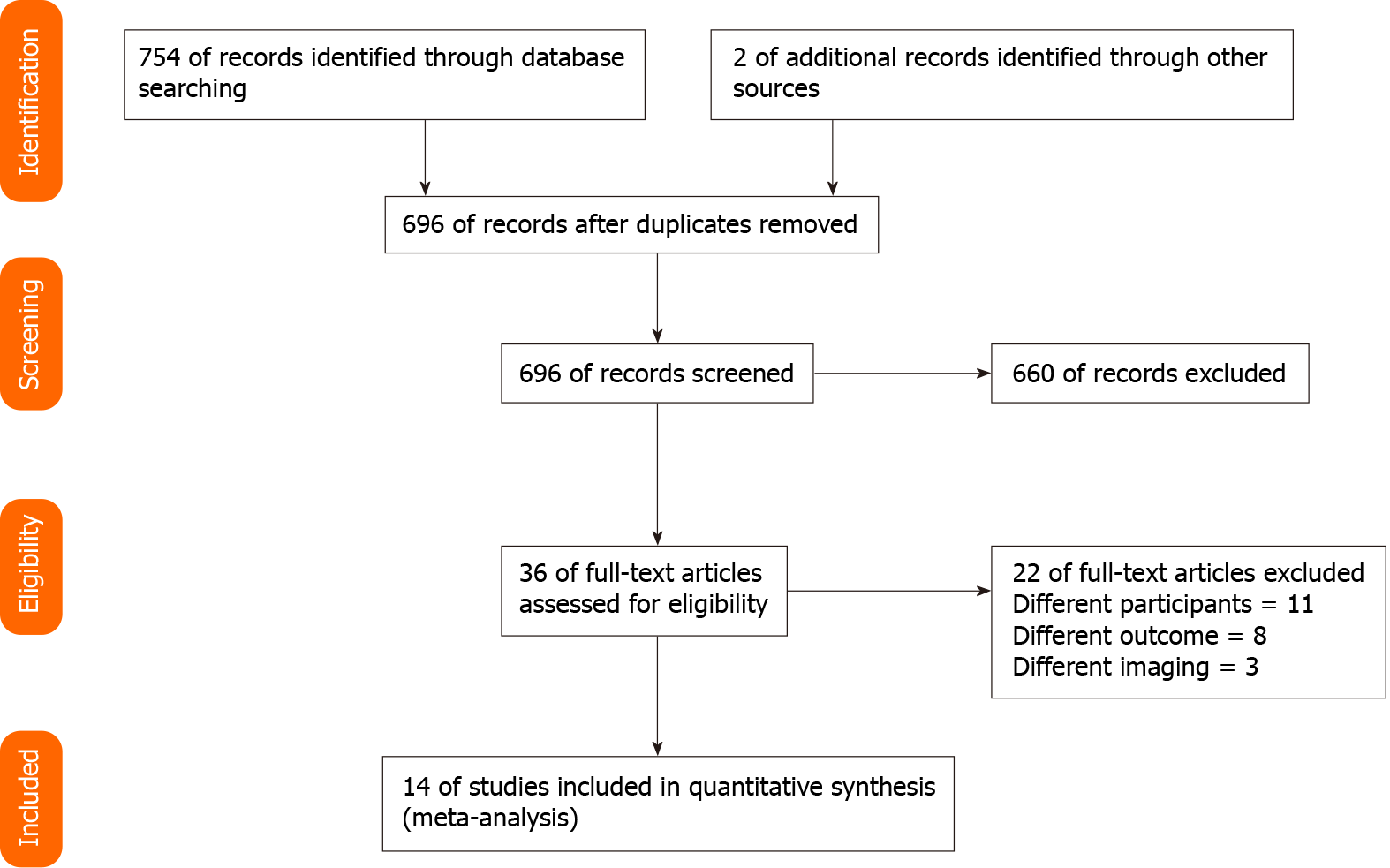
We found a total of 754 records, amongst which 36 were found to be relevant for the full-text retrieval. Full-texts of two additional articles were retrieved after going through the bibliography of the selected articles. Finally, 14 studies with 989 participants have met the eligibility criteria and were included in our review (Figure 1)[7-20].
Of 14 studies included in the analysis, 12 were prospective in nature. Six studies were conducted in China. The average age of the patients was 39 to 55 years. We analyzed data from 989 patients to assess the predictive accuracy of UE for pCR after receiving NACT (samples size of individual studies ranged from 15 to 134 patients). In total, seven studies assessed the accuracy of only shear wave elastography, six assessed the accuracy of only strain wave elastography, while only one assessed the accuracy of both shear wave and strain wave elastography. All the studies performed histopathological examination following surgical resection as the reference standard (Table 1).
| No | Ref. | Country | Study design | Sample size | Study participants | Type of ultrasound elastography | Cut-off | Reference standard | Mean age (in years) |
| 1 | Evans et al[7], 2018 | United Kingdom | Prospective | 64 | Patients with breast cancer receiving NACT | Shear wave elastography | Mean stiffness = 50 kPA | Assessment of any invasive cancer cells in the tumour bed at surgical resection after 6 cycles of NACT and an assessment of nodal metastases at axillary surgery | 52 |
| 2 | Evans et al[8], 2018 | United Kingdom | Prospective | 80 | Patients with breast cancer receiving NACT | Shear wave elastography | Mean stiffness = 83 kPA | Assessment of any invasive cancer cells in the tumour bed at surgical resection after 6 cycles of NACT and an assessment of nodal metastases at axillary surgery | 53 |
| 3 | Falou et al[9], 2013 | Canada | Prospective | 15 | Locally advanced breast cancer patients receiving NACT | Strain wave elastography | Mean strain ratio = 81 | Histopathological examination following mastectomy | 45 |
| 4 | Fang et al[10], 2019 | China | Prospective | 60 | Breast cancer patients with stage IIa-IIIc (T1-T4; N0-N3; M0) and underwent surgery after receiving NACT | Strain wave elastography | Mean strain ratio = 5.4 | Pathological examination after surgical resection | 39 |
| 5 | Fernandes et al[11], 2019 | Canada | Prospective | 92 | Patients with biopsy confirmed locally advanced breast cancer receiving NACT | Strain wave elastography | Elastography score = 4 | Histopathological examination | 55 |
| 6 | Hayashi et al[12], 2012 | Japan | Retrospective | 55 | Histologically confirmed invasive breast cancer before NACT, and they underwent surgery after completion of NACT | Strain wave elastography | Elastography score = 4 | Pathologic response was assessed in surgical specimens of the breast with reference to the standards of the Japanese Breast Cancer Society | 52 |
| 7 | Jing et al[13], 2016 | China | Prospective | 62 | Patients with diagnosis of breast carcinoma by ultrasound-guided core needle biopsy who received neoadjuvant chemotherapy followed by surgical excision | Shear wave elastography | Stiffness threshold-36.1% | Pathologic assessments involved a 2-step process. First, samples from core needle biopsies were examined to record the histologic and biologic characteristics of the tumours. These findings were usually combined with the clinical features of the patients to predict the response to neoadjuvant chemotherapy. Second, pathologic responses to neoadjuvant chemotherapy were evaluated according to the Miller-Payne grading criteria | 49 |
| 8 | Katyan et al[14], 2019 | India | Prospective | 86 | TNM stage III and T3N0 subset of stage IIb breast cancer patients receiving NACT | Strain wave elastography | Strain ratio = 0.1 | Histopathological examination | NA |
| 9 | Lee et al[15], 2015 | Korea | Retrospective | 71 | Women with stage II-III invasive breast cancers who received NACT | Shear wave elastography | Mean stiffness = 98.1 kPA | Histopathological examination | NA |
| 10 | Ma et al[16], 2017 | China | Prospective | 71 | Women confirmed with invasive breast cancer by ultrasound guided core needle biopsy and underwent NACT and subsequent surgical excision | Shear and strain wave eastography | Stiffness threshold-30.4%; Strain ratio = 6.7 | Histopathological examination | 47.3 |
| 11 | Ma et al[17], 2020 | China | Prospective | 43 | Breast cancer patients who were confirmed to be HER-2 positive by biopsy and puncture and underwent NACT | Shear wave elastography | Mean stiffness = 30 kPA | Histopathological examination after surgical resection | NA |
| 12 | Maier et al[18], 2020 | Germany | Prospective | 134 | Histologically confirmed unilateral or bilateral breast cancer and indication for NACT | Shear wave elastography | Shear wave velocity = 3.35 | Pathological examinations and immunohistochemistry from the core-cut biopsy before and from the surgical specimen after NACT | 52.1 |
| 13 | Wang et al[19], 2019 | China | Prospective | 65 | Patients confirmed via biopsy to have breast cancer prior to receiving NACT treatment and they received no other treatment | Strain wave elastography | Strain ratio = 8 | Histopathology results of the lesion samples isolated in the surgery were compared with those of the biopsy specimens obtained prior to treatment to determine the response to NACT | 48.3 |
| 14 | Zhang et al[20], 2020 | China | Prospective | 91 | Patients diagnosed with invasive breast cancer by ultrasound-guided core needle biopsy and received NACT and subsequent surgical intervention | Shear wave elastography | Stiffness threshold–41.4% | Histopathological examination | 46.9 |
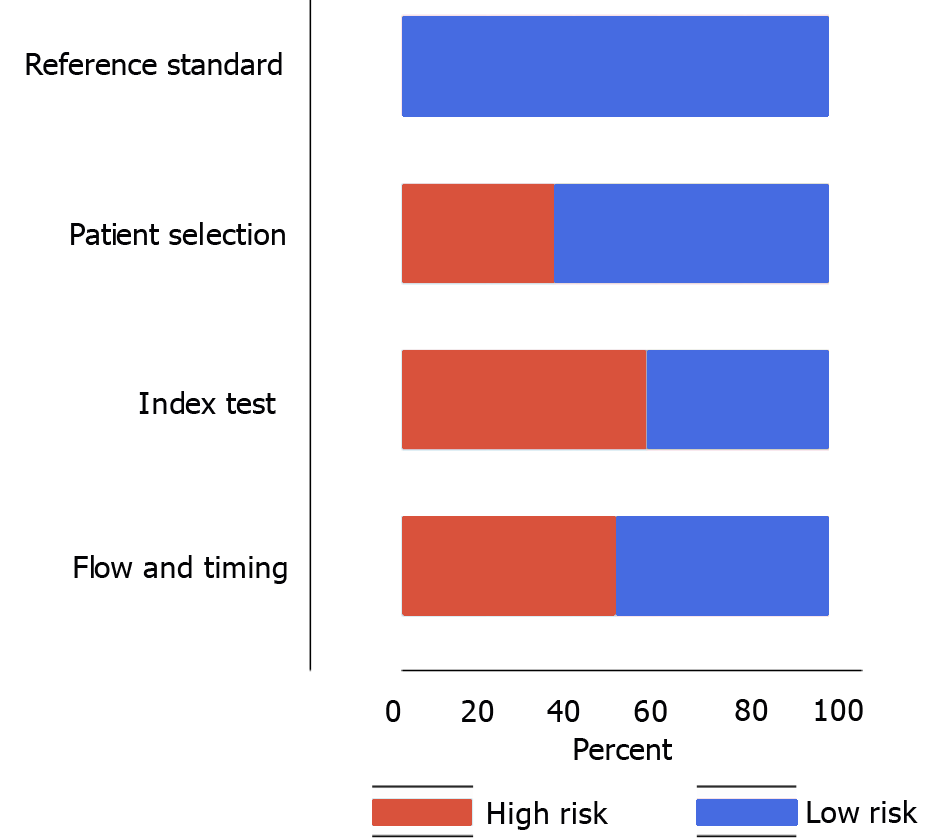
Risk of bias according to the QUADAS tool is shown in Figure 2. Five out of fourteen studies had a high risk with respect to patient selection domain, eight had a high risk of conduct and interpretation of index test bias, and seven had a high risk of patient flow and interval between index tests and reference standards bias. None of the studies had a high risk of bias with respect to the conduct and interpretation of reference standard.
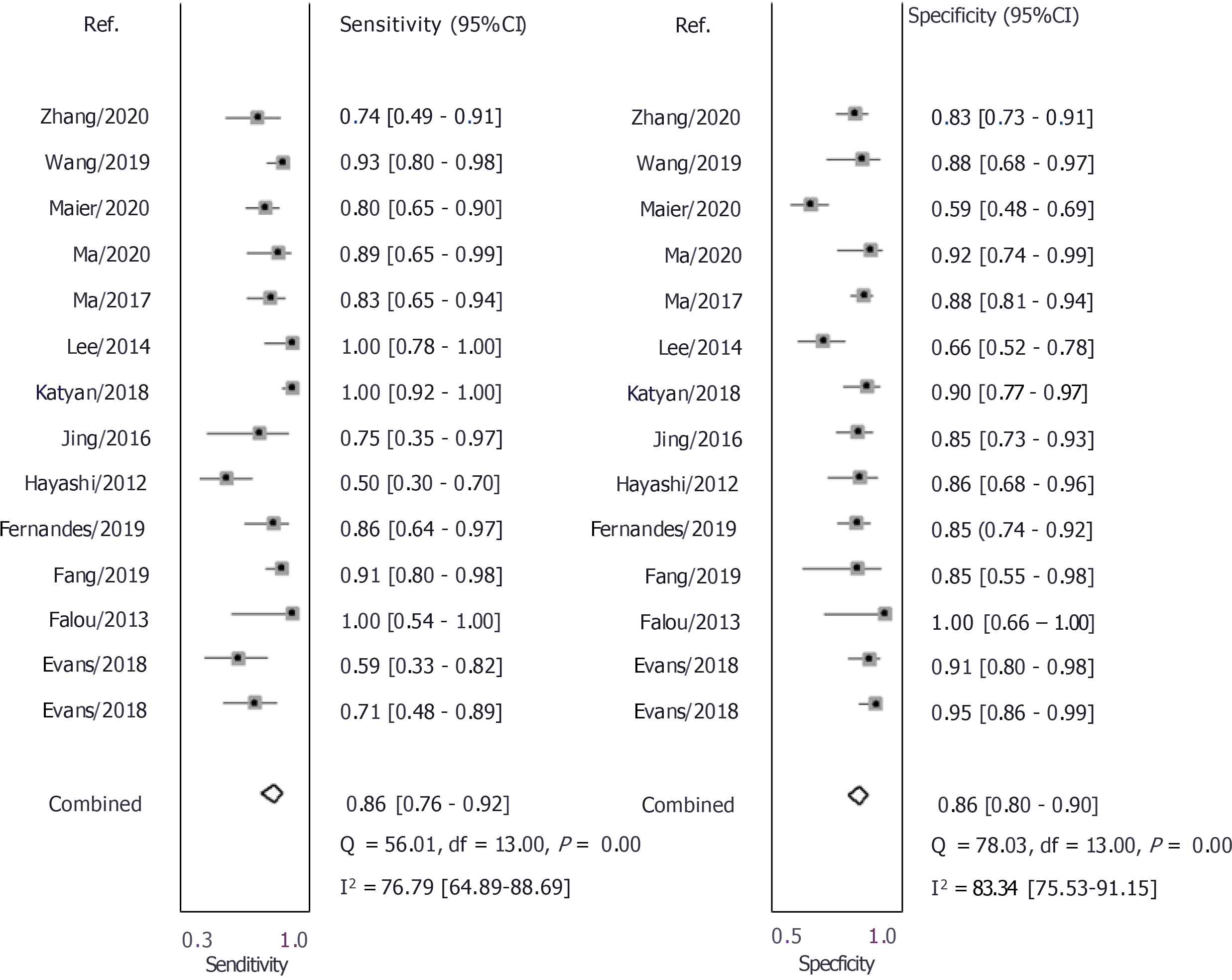
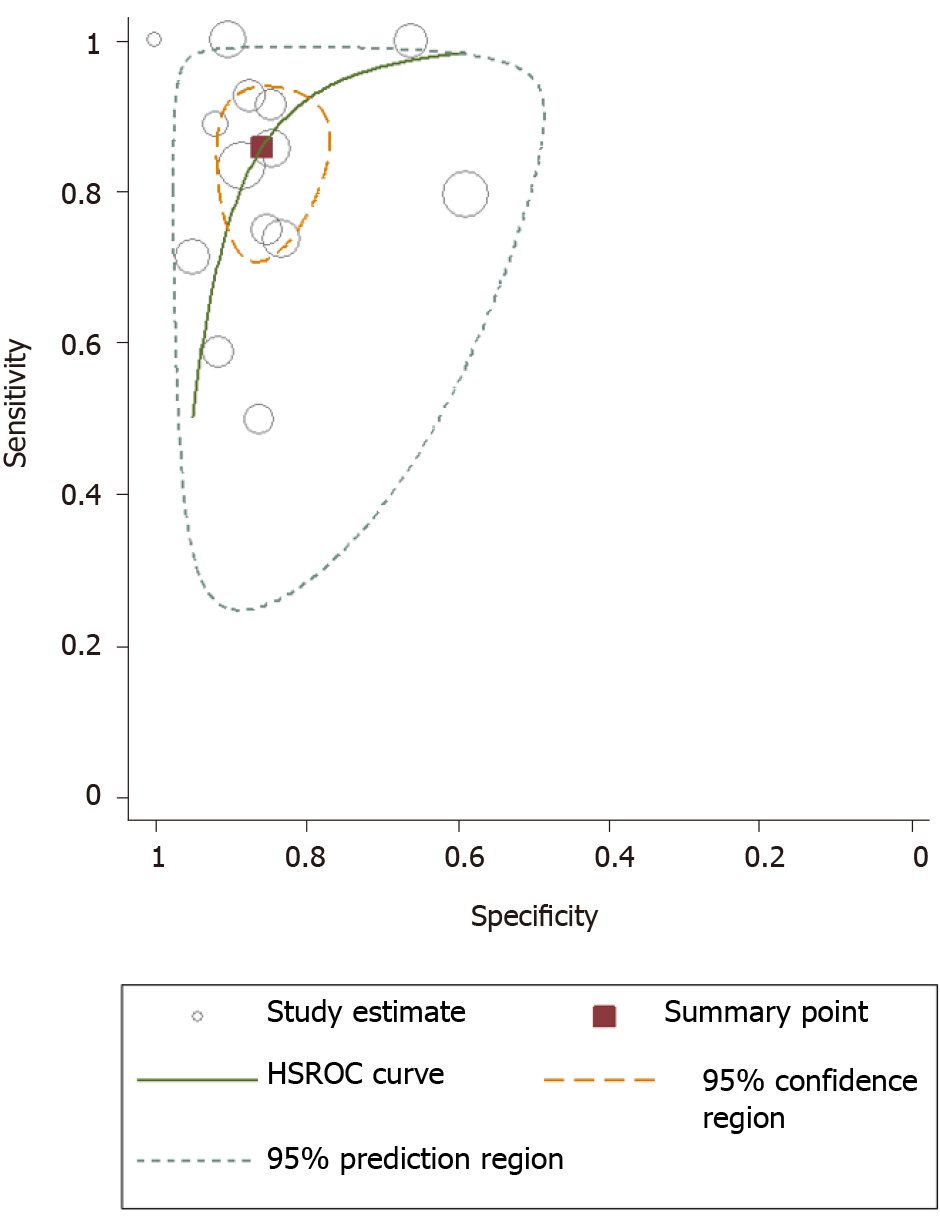
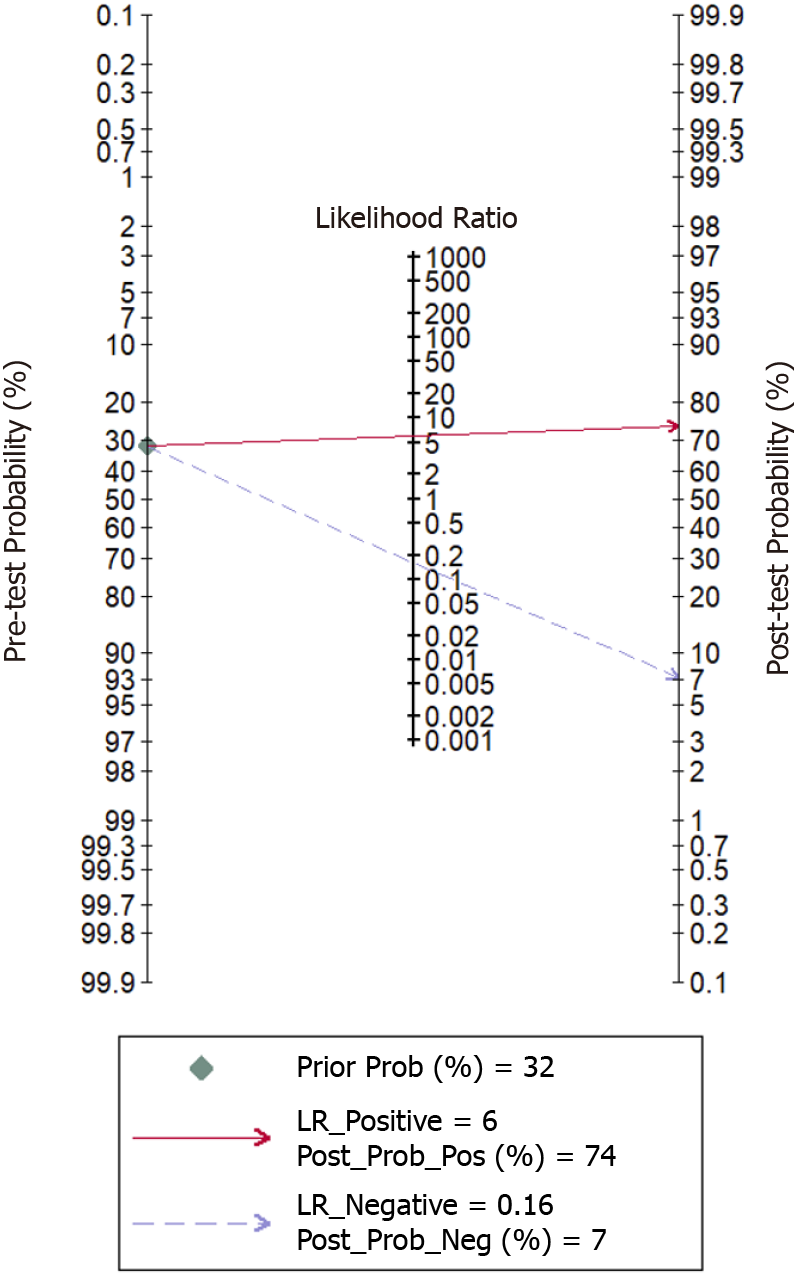
As shown in Figures 3 and 4, the pooled sensitivity and specificity of UE for pCR amongst patients with breast cancer following NACT were 86% [95% confidence interval (CI): 76%-92%] and 86% (95%CI: 80%-90%), respectively. The DOR was 37 (95%CI: 17-77). The LRP was 6 (95%CI: 4-9) and the LRN 0.16 (0.09-0.30). The LRP and LRN in the right lower quadrant of the LR scattergram (Figure 5) indicate that UE cannot be used for confirmation or exclusion of pCR following NACT. As shown in Fagan’s nomogram (Figure 6), UE had a good clinical utility for predicting pCR following NACT (positive = 74%; negative = 7%), as it differs significantly from the pre-test probability (32%). Significant heterogeneity was found with a chi-square P < 0.001 and I2 > 75%. Bivariate box plot further confirmed the presence of heterogeneity (Figure 7).
Deek’s test for publication bias indicated the absence of publication bias (P = 0.59). This was further confirmed by the symmetrically shaped funnel plot (Figure 8). Meta-regression analysis was performed to assess the source of heterogeneity using the covariates. As shown in Figure 9, in the sensitivity model, patient selection (P < 0.05) (P < 0.05) could be a source of heterogeneity. Patient selection (P < 0.05) as well as flow and timing of tests (P < 0.001) were potential sources of heterogeneity in the specificity model, and the mean age was responsible for heterogeneity in the joint model (P < 0.001).
We next performed a subgroup analysis based on the type of elastography used for predicting pCR after NACT. Eight studies used shear-wave elastography for assessing its prognostic utility. Our results show a pooled sensitivity of 77% and pooled specificity of 84% with a DOR of 17, LRP of 4.8, and LRN of 0.27. Seven studies used strain-wave elastography for assessing its prognostic utility. Our results indicate a pooled sensitivity of 93% and pooled specificity of 87% with a DOR of 87, LRP of 7.4, and LRN of 0.08.
Major objectives of performing the NACT are to attain operability, and ensure breast conservation and historical prognostic information. In recent years, the approach shifted towards personalization of the therapy, investigation of new therapies, and identification of response biomarkers. More advanced and accurate prediction of pCR will allow to identify high-risk groups and prevent adverse outcomes by providing more specific management. Developing a fast, easy, and effective screening tool will reduce the financial burden on healthcare system, prevent life-threatening complications, and reduce mortality. However, the utility of UE has not been synthesized to predict the risk of pCR. The main goal of this review was to determine the predictive performance of shear and strain wave ultrasound elastography for the pCR.
A total of 14 studies reporting the utility of UE for predicting pCR following NACT were identified by our systematic search strategy. Most of the studies were prospective and had a low risk of bias. UE had an equal pooled sensitivity and specificity of 86%. Other diagnostic accuracy parameters also were moderate. LR scattergram showed that, since LRN and LRP occupied the right lower quadrant, UE cannot be used for confirming or excluding SAP. The clinical utility of UE was relatively acceptable, with a significant rise in the post-imaging probability compared to the pre-imaging probability on Fagan’s nomogram.
Since there are two techniques of UE (shear and strain wave elastography), we determined the better technique by performing a separate subgroup analysis and calculating pooled sensitivity and specificity for each of them. We found strain wave elastography as the better technique with a pooled sensitivity of 93% and specificity of 87% compared to shear wave elastography (77% and 84%). This means that strain elastography can help in effectively ruling out the pCR patients correctly as it had a sensitivity more than 90%. Strain elastography, therefore, has a major advantage over shear wave elastography, as studies report its good predictive performance for ruling out the patients with pCR.
The accuracy parameters obtained in this review could not be compared, since no similar reviews were conducted in the past. Nevertheless, our results are almost similar to the accuracy of UE in predicting malignant liver lesions or axillary lesions[21,22]. There is a need for additional studies comparing the prognostic performance of this imaging technique with magnetic resonance imaging and other forms of ultrasonography, in order to identify the method with the highest accuracy that can be used in the clinical practice. Further large-scale longitudinal studies are also needed to assess the predictive accuracy of strain wave elastography as only few studies reported this outcome.
It is important to interpret these results with caution, as there are several differences in the methods and quality of our included studies, which can ultimately affect the final pooled estimates. First, we evaluated and found a significant heterogeneity (significant chi-square test and higher I2 statistic values). Hence, we performed meta-regression and found the factors responsible for this higher heterogeneity. Quality related factors and mean age were found to be the significant covariates responsible for such heterogeneity. We confirmed that there was no publication bias in the studies reporting our study outcome using Deek’s test and funnel plot.
Our study has certain strengths. This is the first meta-analysis assessing the predictive ability of UE for pCR amongst breast cancer patients, with a larger number of studies (14 studies) included. Lack of significant publication bias adds credibility of the results in the meta-analysis. However, there are several limitations to our study. First, there was a significant between-study variability in our analysis. This can limit the prospect to infer or interpret the pooled findings. However, we explored the source of heterogeneity using meta-regression analysis to overcome this limitation. Second, the predictive accuracy of UE depends on several other factors such as the ethnicity, timing of the index test and outcome assessment, and disease severity. However, we could not evaluate their influence in our analysis. We have also not pre-registered this review online. Finally, the number of subjects/participants included was relatively small.
Despite these limitations, our study findings provide useful information for the clinicians and oncologists and may have significant implications for developing treatment strategies for breast cancer patients following NACT. Although UE had moderate sensitivity and specificity, strain-wave type of UE had a very high accuracy to rule out the patients with pCR. It should be useful, therefore, as an effective prognostic tool following the administration of NACT, because it may allow for identification of the patients at risk of developing incomplete pathological response. Applying this imaging technique could reduce the time spent undertaking various invasive diagnostic procedures and could also reduce the healthcare costs involved in the process. However, ultrasonography-based imaging techniques have substantial overlap between benign and malignant features, mainly for small lesions. A palpation imaging technique could help compensate for this deficiency by comprehensively analyzing the 2-D and 3-D tumor characteristics[23]. At the same time, it may be difficult to diagnose intraductal lesions and calcification in breast masses using palpation imaging. This, in turn, can be overcome via ultrasound or mammography. Hence, future studies perhaps should attempt to combine palpation imaging, ultrasonography, and mammography to analyze ambiguous clinical cases in order to improve breast lesion diagnosis. Additional large-scale setting-specific longitudinal studies are merited to establish the best imaging methods to assess all the patients administered with NACT.
Several studies have reported the prognostic value of ultrasound elastography (UE) in patients receiving neoadjuvant chemotherapy (NACT) for breast cancer. However, the assessment of parameters is different for shear-wave elastography and strain elastography in terms of measured elasticity parameter and mode of imaging.
To the best of our knowledge, no meta-analysis has been conducted to assess the accuracy of the two modes of elastography in predicting the response to NACT.
The aim of the current study was to systematically search the literature for all studies assessing the accuracy of UE for predicting response to NACT in breast cancer, and pool the data for meta-analysis.
A comprehensive and systematic search was performed in the databases of MEDLINE, EMBASE, SCOPUS, PubMed Central, CINAHL, Web of Science, and Cochrane library from inception until December 2020.
We found that UE had an equal pooled sensitivity and specificity of 86% for predicting the pathologic complete response (pCR) in breast cancer patients following NACT. We also found that strain wave elastography was the better technique with a pooled sensitivity of 93% and specificity of 87% compared to shear wave elastography (77% and 84%). This means that strain elastography can help in effectively ruling out the patients correctly as it had a sensitivity more than 90%.
Strain-wave type of UE can accurately predict the pCR following NACT amongst breast cancer patients.
Additional large-scale setting-specific longitudinal studies are merited to establish the best imaging methods to assess all the patients administered with NACT.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheungpasitporn W, Sun C S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wu RR
| 1. | Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L, Valagussa P, Swain SM, Prowell T, Loibl S, Wickerham DL, Bogaerts J, Baselga J, Perou C, Blumenthal G, Blohmer J, Mamounas EP, Bergh J, Semiglazov V, Justice R, Eidtmann H, Paik S, Piccart M, Sridhara R, Fasching PA, Slaets L, Tang S, Gerber B, Geyer CE Jr, Pazdur R, Ditsch N, Rastogi P, Eiermann W, von Minckwitz G. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3169] [Cited by in RCA: 3205] [Article Influence: 291.4] [Reference Citation Analysis (2)] |
| 2. | Crippa F, Agresti R, Sandri M, Mariani G, Padovano B, Alessi A, Bianchi G, Bombardieri E, Maugeri I, Rampa M, Carcangiu ML, Trecate G, Pascali C, Bogni A, Martelli G, de Braud F. ¹⁸F-FLT PET/CT as an imaging tool for early prediction of pathological response in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy: a pilot study. Eur J Nucl Med Mol Imaging. 2015;42:818-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Pavlopoulou A, Oktay Y, Vougas K, Louka M, Vorgias CE, Georgakilas AG. Determinants of resistance to chemotherapy and ionizing radiation in breast cancer stem cells. Cancer Lett. 2016;380:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Hayashi M, Yamamoto Y, Iwase H. Clinical imaging for the prediction of neoadjuvant chemotherapy response in breast cancer. Chin Clin Oncol. 2020;9:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Youk JH, Gweon HM, Son EJ. Shear-wave elastography in breast ultrasonography: the state of the art. Ultrasonography. 2017;36:300-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, Leeflang MM, Sterne JA, Bossuyt PM; QUADAS-2 Group. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6953] [Cited by in RCA: 9589] [Article Influence: 684.9] [Reference Citation Analysis (0)] |
| 7. | Evans A, Whelehan P, Thompson A, Purdie C, Jordan L, Macaskill J, Henderson S, Vinnicombe S. Identification of pathological complete response after neoadjuvant chemotherapy for breast cancer: comparison of greyscale ultrasound, shear wave elastography, and MRI. Clin Radiol. 2018;73:910.e1-910.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Evans A, Whelehan P, Thompson A, Purdie C, Jordan L, Macaskill J, Waugh S, Fuller-Pace F, Brauer K, Vinnicombe S. Prediction of Pathological Complete Response to Neoadjuvant Chemotherapy for Primary Breast Cancer Comparing Interim Ultrasound, Shear Wave Elastography and MRI. Ultraschall Med. 2018;39:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (2)] |
| 9. | Falou O, Sadeghi-Naini A, Prematilake S, Sofroni E, Papanicolau N, Iradji S, Jahedmotlagh Z, Lemon-Wong S, Pignol JP, Rakovitch E, Zubovits J, Spayne J, Dent R, Trudeau M, Boileau JF, Wright FC, Yaffe MJ, Czarnota GJ. Evaluation of neoadjuvant chemotherapy response in women with locally advanced breast cancer using ultrasound elastography. Transl Oncol. 2013;6:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Fang C, Yang TWYZJXW. Value of tissue elastography in the prediction of efficacy of neoadjuvant chemotherapy in breast cancer. J BUON. 2019;24:555-559. [PubMed] |
| 11. | Fernandes J, Sannachi L, Tran WT, Koven A, Watkins E, Hadizad F, Gandhi S, Wright F, Curpen B, El Kaffas A, Faltyn J, Sadeghi-Naini A, Czarnota G. Monitoring Breast Cancer Response to Neoadjuvant Chemotherapy Using Ultrasound Strain Elastography. Transl Oncol. 2019;12:1177-1184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Hayashi M, Yamamoto Y, Ibusuki M, Fujiwara S, Yamamoto S, Tomita S, Nakano M, Murakami K, Iyama K, Iwase H. Evaluation of tumor stiffness by elastography is predictive for pathologic complete response to neoadjuvant chemotherapy in patients with breast cancer. Ann Surg Oncol. 2012;19:3042-3049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Jing H, Cheng W, Li ZY, Ying L, Wang QC, Wu T, Tian JW. Early Evaluation of Relative Changes in Tumor Stiffness by Shear Wave Elastography Predicts the Response to Neoadjuvant Chemotherapy in Patients With Breast Cancer. J Ultrasound Med. 2016;35:1619-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Katyan A, Mittal MK, Mani C, Mandal AK. Strain wave elastography in response assessment to neo-adjuvant chemotherapy in patients with locally advanced breast cancer. Br J Radiol. 2019;92:20180515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (1)] |
| 15. | Lee SH, Chang JM, Han W, Moon HG, Koo HR, Gweon HM, Kim WH, Noh DY, Moon WK. Shear-Wave Elastography for the Detection of Residual Breast Cancer After Neoadjuvant Chemotherapy. Ann Surg Oncol. 2015;22 Suppl 3:S376-S384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Ma Y, Zhang S, Li J, Kang Y, Ren W. Comparison of strain and shear-wave ultrasounic elastography in predicting the pathological response to neoadjuvant chemotherapy in breast cancers. Eur Radiol. 2017;27:2282-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Ma BX, Fan ZN, Wu G. [The application of 3-dimensional shear wave elastography in the therapeutic effect evaluation of neoadjuvant chemotherapy for Her-2 positive breast cancer patients]. Zhonghua Zhong Liu Za Zhi. 2020;42:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Maier AM, Heil J, Harcos A, Sinn HP, Rauch G, Uhlmann L, Gomez C, Stieber A, Funk A, Barr RG, Hennigs A, Riedel F, Schäfgen B, Hug S, Marmé F, Sohn C, Golatta M. Prediction of pathological complete response in breast cancer patients during neoadjuvant chemotherapy: Is shear wave elastography a useful tool in clinical routine? Eur J Radiol. 2020;128:109025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Wang B, Jiang T, Huang M, Wang J, Chu Y, Zhong L, Zheng S. Evaluation of the response of breast cancer patients to neoadjuvant chemotherapy by combined contrast-enhanced ultrasonography and ultrasound elastography. Exp Ther Med. 2019;17:3655-3663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Zhang J, Tan X, Zhang X, Kang Y, Li J, Ren W, Ma Y. Efficacy of shear-wave elastography vs dynamic optical breast imaging for predicting the pathological response to neoadjuvant chemotherapy in breast cancer. Eur J Radiol. 2020;129:109098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Hu X, Huang X, Chen H, Zhang T, Hou J, Song A, Ding L, Liu W, Wu H, Meng F. Diagnostic effect of shear wave elastography imaging for differentiation of malignant liver lesions: a meta-analysis. BMC Gastroenterol. 2019;19:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Tang GX, Xiao XY, Xu XL, Yang HY, Cai YC, Liu XD, Tian J, Luo BM. Diagnostic value of ultrasound elastography for differentiation of benign and malignant axillary lymph nodes: a meta-analysis. Clin Radiol. 2020;75:481.e9-481.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Ding Y, Sun C, Zhou Q, Cheng C, Yan C, Wang B. Use of Palpation Imaging in Diagnosis of Breast Diseases: A Way to Improve the Detection Rate. Med Sci Monit. 2020;26:e927553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |