Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3414
Peer-review started: September 20, 2021
First decision: January 10, 2022
Revised: January 14, 2022
Accepted: March 6, 2022
Article in press: March 6, 2022
Published online: April 16, 2022
Processing time: 199 Days and 22.9 Hours
Patients with recurrent or locally advanced head and neck squamous cell carcinoma (HNSCC) typically have limited treatment options and poor prognosis.
To evaluate the efficacy and safety of two drugs with potent radio-sensitization properties including gemcitabine and nedaplatin as concurrent chemoradiotherapy regimens in treating HNSCC.
This single-arm prospective study enrolled patients with HNSCC to receive gemcitabine on days 1 and 8 and nedaplatin on days 1 to 3 for 21 days. Intensity-modulated radiation therapy with a conventional fraction was delivered 5 days per week. Objective response rate (ORR), disease control rate, and toxicity were observed as primary endpoints. Overall survival (OS) and progression free survival were recorded and analyzed as secondary endpoints.
A total of 24 patients with HNSCC were enrolled. During the median 22.4-mo follow-up, both ORR and disease control rate were 100%. The one-year OS was 75%, and one-year progression-free survival (PFS) was 66.7% (median PFS was 15.1 mo). Recurrent HNSCC patients had a poorer prognosis than the treatment-naïve patients, and patients who achieved complete response had better survival than those in the PR group (all P < 0.05). The most common grade 1-4 (100%) or grade 3-4 toxicities (75%) were hematological, and the most common grade 3-4 non-hematological toxicity was mucositis in 17 (71%) patients.
Gemcitabine plus nedaplatin with concurrent chemoradiotherapy is a therapeutic option for HNSCC with predictable tolerability. Considering the high adverse event rate, the optimized dose and schedule must be further explored.
Core Tip: Our article focuses on the comprehensive treatments of head and neck squamous cell carcinoma, especially for recurred tumors after surgery or tumor lesions that could not be surgically removed.
- Citation: Huo RX, Jin YY, Zhuo YX, Ji XT, Cui Y, Wu XJ, Wang YJ, Zhang L, Zhang WH, Cai YM, Zheng CC, Cui RX, Wang QY, Sun Z, Wang FW. Concurrent chemoradiotherapy using gemcitabine and nedaplatin in recurrent or locally advanced head and neck squamous cell carcinoma. World J Clin Cases 2022; 10(11): 3414-3425
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3414.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3414
Head and neck cancers including lip, oral cavity, pharynx, nasopharynx and cervical esophagus cancer, generally have similar biological characteristics and utilize the same therapies. Of these, head and neck squamous cell carcinoma (HNSCC) is the most common cancer type, accounting for 85% of these cases. Annually, there are over 550000 new patients and 380000 deaths globally due to the disease[1]. In China, the annual incidence and mortality of head and neck cancer are 586600 and 431200 patients, respectively[2]. Smoking and drinking increase the risk of morbidity[1]. The cure rate is high for early-stage patients who undergo the main therapies including surgery, radiation, and/or chemotherapy. The 5-year survival rate of patients with oral cavity and pharynx cancer is approximately 80%[2]. Most cases, however, are diagnosed in advanced stages where locoregional recurrences and distant metastases lead to poor survival rates, and where therapeutic options are limited[3]. The median overall survival (OS) rate for patients with recurrent or metastatic HNSCC (RMHNSCC) is approximately 6 months, and the 1-year survival rate is approximately 20%[4]. Potential disfigurement and functional loss require careful deliberation when considering surgical resection, while the continued development of chemoradiotherapy approaches provide further options[5].
The standard management of locally advanced head and neck squamous cell carcinoma has evolved[6]. Radiation, especially combined with chemotherapy, is the standard treatment for organ and functional preservation and effectiveness[7]. A high-dose cisplatin regimen (100 mg/m2) or carboplatin/ fluorouracil combination regimen demonstrates superior OS to that of standard radiotherapy[6,8,9]. The Radiation Therapy Oncology Group (RTOG) 97-03 study demonstrated that in the context of concurrent radiation and chemotherapy, the 2-year disease-free survival and OS rates were significantly better in the TP (docetaxel/cisplatin) group than those in the PF group[10]. Neuropathy or anaphylaxis caused by docetaxel and hyperglycemia or hypertension connected with dexamethasone limit the application of docetaxel-based chemotherapy. Cisplatin (DDP), a common radiotherapy sensitizer, is highly emetic and may increase the risk of malnutrition in HNSCC patients. Though cetuximab, a representative targeted therapy, is regarded as a less toxic alternative, no prospective large trials have compared cetuximab to cisplatin in combination with radiotherapy. Recently, anti-PD-1 immunotherapy has been approved for the treatment of platinum-refractory recurrent and/or metastatic HNC based on an associated increase in OS and a decrease in toxicities[5]. However, due to their high costs and the rare but fatal adverse effects associated with their use, anti-PD-1 drugs are not extensively used. Therefore, new chemotherapy drugs with high efficacy and acceptable toxicity in treating HNSCC need to be explored.
Gemcitabine (GEM) (dFdC) is an antimetabolite that mechanistically functions via the incorporation of dFdC triphosphate adducts into DNA, resulting in chain termination and inhibition of DNA synthesis. The primary mechanism of GEM-induced radiosensitization is depletion of dATP caused by inhibition of ribonucleotide reductase[11]. In a phase II trial, single-agent GEM in patients with RMHNSCC resulted in a response rate (RR) of 13% amongst 54 patients[12]. In 110 Locally advanced HNSCC patients receiving weekly low-dose GEM with concomitant radiotherapy, the response rate was 78%, and the therapy was tolerated[13]. Additionally, GEM has demonstrated favorable safety and efficacy to treat esophageal squamous cell carcinoma. A phase II clinical trial evaluated weekly GEM and docetaxel regimens as secondline treatments in patients with metastatic esophageal squamous cell carcinoma (ESCC). The disease control rate (DCR) was 88%, with an overall response rate (ORR) of 30%[14]. Of note, GEM has synergistic action with cisplatin. In another phase II trial of triweekly GEM and cisplatin in patients with metastatic or recurrent advanced ESCC, the overall response rate was 42.1%[15]. In a Phase II multicenter clinical trial, the efficacy of a nedaplatin-based regimen, which led to less gastrointestinal reaction, was not significantly different from that of the cisplatin-based regimen for HNSCC[16]. Furthermore, nedaplatin showed excellent antitumor activity and low toxicity with concurrent chemoradiotherapy in treating HNSCC patients[17,18]. However, few studies have been conducted to explore the dosage and safety of GEM in combination with nedaplatin as the concomitant chemoradiotherapy regimen.
Our study explored the efficacy and safety of GEM in combination with nedaplatin as the concomitant chemoradiotherapy regimen for the treatment of patients with recurrent or locally advanced squamous cell carcinoma of the head and neck.
Patients with recurrent or locally advanced head and neck squamous cell carcinoma who presented at Department of Oncology, Tianjin Union Medicine Centre, China, between July 2017 and July 2020 were recruited for enrollment. Eligible patients provided written informed consent prior to the onset of study activities. The study was registered at Chinese Clinical Trial Registry (http://www.chictr.org.cn) (ChiCTR2000034141) and was approved by China Ethics Committee of Registering Clinical Trials (ChiECRCT20170178).
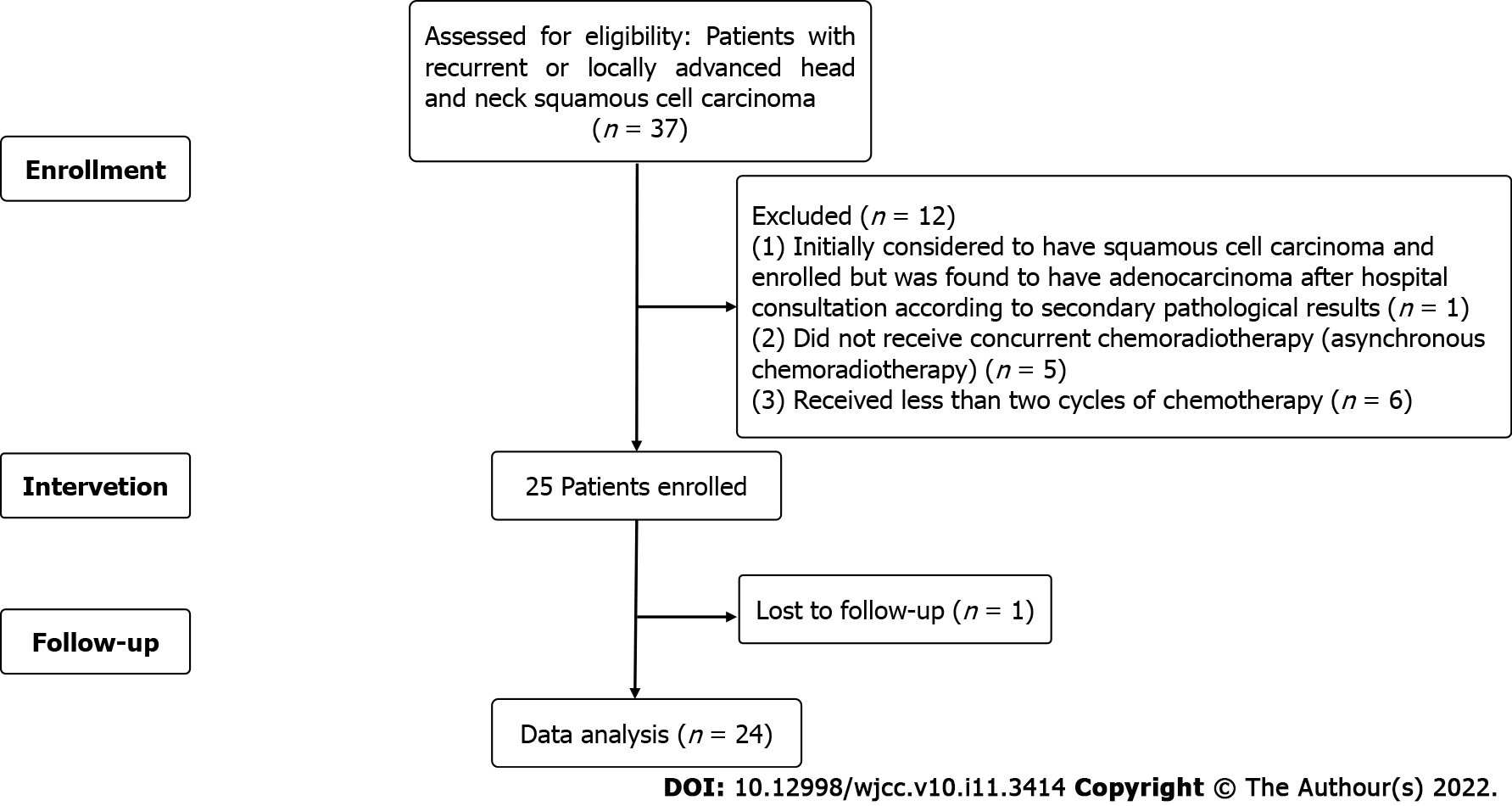
Eligibility criteria was: (1) Confirmed recurrent or locally advanced head and neck squamous cell carcinoma by histology; (2) no previous treatment with GEM; (3) aged 18-70 years; (4) Eastern Cooperative Oncology Group (ECOG) performance status of 0-2; (5) at least one measurable lesion by the RECIST 1.1 criteria; (6) no other active malignancies; (7) previous organ-sparing surgery allowed; (8) life expectancy of at least 3 mo, with adequate bone marrow and normal organ function (renal, heart and hepatic function); (9) at least 4 week treatment-free; (10) at least one year since the last radiotherapy; (11) no other distant organ metastasis; (12) no other serious medical complications; and (13) willing to provide written informed consent. Pregnant women and women with childbearing potential and without access to contraception were excluded. Drop-out criteria was: (1) Did not receive concurrent chemoradiotherapy (asynchronous chemoradiotherapy); (2) received less than two cycles of chemotherapy; and (3) lost to follow-up.
The pre-treatment evaluation included a medical history and physical examination, blood tests (including complete blood count, renal and liver function tests), electrocardiograph, echocardiography, chest and abdominal CT scan, and enhanced CT or Magnetic Resonance Imaging (MRI) of the head and neck. In addition, laryngoscopy or nasopharyngoscopy and biopsy were performed, if deemed necessary. Blood testing continued weekly throughout the study period.
Radiotherapy schemes were determined based upon individual carcinomas. Intensity-modulated radiation therapy as delivered once per day on a linear accelerator with 6 MV energy 5 days per week. The average dose was 2 Gy per fraction. Concurrent chemotherapy was given 2 hours prior to radiotherapy. Patients received intravenous GEM (600 mg/m2) over 30 min on days 1 and 8, and intravenous nedaplatin (25 mg/m2) over 1 h per day on days 1 to 3 for 21 days. Most patients received 2-4 cycles of subsequent chemotherapy after concomitant chemoradiation according to residual disease and their condition. During the subsequent treatment, the dose of GEM was increased to 1000 mg/m2, and the dose of nedaplatin remained unchanged. The dose of GTV was within the range of 64-74 Gy based upon the lesion, Clinical Target Volume was 66-70 Gy, and PTV was 54-60 Gy. The preventive radiotherapy dose to the lymphatic drainage area was 54-60 Gy, after which a shrinking field administration with locally increased dose was given to the focal area. To those who received postoperative radiotherapy previously (60-66 Gy), the second radiotherapy Gross Target Volume dose was not more than 70 Gy after adjustment (64-70 Gy).
Demographic and clinical indices were collected, which included age, sex, social history of smoking or drinking, primary tumor site, T stage, N stage, AJCC stage, degree of differentiation, type of tumor (recurrence/Local advancement), ECOG PS, regression ratio of tumor diameter, progression-free survival (PFS) and OS. PFS was defined as the time from enrollment to the first progression of disease or death. OS was defined as the time from patients’ enrollment to death from any cause. Therapeutic evaluation was conducted according to the RECIST V1.1 criteria. Complete response (CR) was defined as the disappearance of all evidence of disease, with the minor axis of metastatic lymph shrinking to < 1 cm by physical examination, CT/MRI, or direct endoscopy. Partial response (PR) was defined as a reduction in the largest axis diameters of measurable disease (or the minor axis of metastatic lymph node) by 30%, with no progression to other lesions and no new lesions. Tumor progression (PD) was defined as an increase in the largest axis diameter of measurable disease (or the minor axis of metastatic lymph node) by 20% or the appearance of new lesions. Stable disease (SD) was defined as a disease status between PR and PD. The ORR was calculated as the number of patients achieving CR or PR divided by the number of all enrolled patients. DCR was calculated as the number of patients who reached CR or PR or SD divided by the total number of enrolled patients.
The primary endpoints of this study were ORR, DCR and toxicity. The secondary endpoints were PFS and OS.
Toxicity was evaluated according to the RTOG Acute Radiation Injury Scoring Criteria and the National Cancer Institute Common Toxicity Criteria (NCI-CTC) scale version 4.0. If severe adverse reactions (grade 4 hematological toxicity, grade 3-4 non-hematological toxicity) occurred or if PS worsened, the therapy was delayed, and the dose was decreased by approximately 25% in the subsequent course. Any required supportive therapy was determined by the treating physician.
Follow up was conducted monthly either by telephone, in-patient admission or outpatient chart review. Data collected included disease progression, short-term and long-term adverse reactions, and imaging and laboratory findings. Imaging exam was performed 1 mo after radiotherapy, then every 2 chemotherapy cycles, and finally every 3-6 mo.
The data were analyzed in per-protocol set by excluding the participants who dropped out of the study and failed to comply with the study protocol. Demographic data was analyzed via descriptive statistics SPSS Version 23 (SPSS Inc., Chicago, IL, United States). Data were presented as mean ± standard deviation. Qualitative data were described as frequency and proportion. Univariate analysis of prognostic factors, including sex, age, T stage, N stage, AJCC stage, differentiation degree, smoking and drinking history, was performed, and data were compared using the log rank test. Survival curves were drawn by the Kaplan-Meier method. Statistical significance was considered when the P value was ≤ 0.05.
A total of 37 patients was screened for enrollment (Figure 1). Of these, 1 patient, initially considered to have squamous cell carcinoma and enrolled, was found to have adenocarcinoma after hospital consultation according to secondary pathological results, and was excluded from study participation. During treatment, 5 patients did not receive concurrent chemoradiotherapy (asynchronous chemoradiotherapy), 6 patients received less than two cycles of chemotherapy, and one patient was lost to follow-up. Thus, a total of 24 people was included in the statistical analysis.
Patients demographics and disease characteristics are described in Table 1. The male-to-female ratio was 2:1. All patients had stage III-IV with the majority having poorly differentiated squamous cell carcinoma and the most common site being the nasopharynx (37.5%). The majority of patients smoked (70.8%) or consumed alcohol (87.5%). At enrollment, 10 patients (41.7%) had recurrent disease after surgery, four of whom received postoperative radiotherapy with the GTV range of 60-66 Gy. Most (79.2%) patients had not previously received chemotherapy. A minority of patients received subsequent paclitaxel-containing salvage chemotherapy after tumor progression.
| Characteristics | Number (n) | Percentage (%) | Characteristics | Number (n) | Percentage (%) |
| Age, yr | Sex | ||||
| Mean | 59.8 ± 8.08 | Male | 16 | 0.67 | |
| Range | 37.1-66.2 | Female | 8 | 0.33 | |
| T stage | N stage | ||||
| 1 | 1 | 0.042 | 1 | 5 | 0.208 |
| 2 | 9 | 0.375 | 2 | 17 | 0.708 |
| 3 | 4 | 0.167 | 3 | 2 | 0.083 |
| 4 | 10 | 0.417 | Differentiation | ||
| Smoking history | High | 1 | 0.042 | ||
| Nonsmoker | 7 | 0.292 | Moderately | 3 | 0.125 |
| 20-30 yr | 11 | 0.458 | Low | 12 | 0.5 |
| 30-39 yr | 1 | 0.042 | Uncertain | 8 | 0.333 |
| > 40 yr | 5 | 0.208 | Primary tumor site | ||
| History of alcohol use | Nasopharynx | 9 | 0.375 | ||
| Nondrinker | 3 | 0.125 | Larynx | 5 | 0.208 |
| Infrequent (< once/wk) | 8 | 0.333 | Hypopharynx | 3 | 0.125 |
| Light (< 3 times/wk) | 2 | 0.083 | Oral Cavity | 2 | 0.083 |
| Moderate (> 3 times/wk but < once/d) | 6 | 0.25 | Tongue | 2 | 0.083 |
| Heavy (> once/d) | 5 | 0.208 | Esophagus | 2 | 0.083 |
| Disease situation | Nasal cavity | 1 | 0.042 | ||
| Recurrence | 10 | 0.417 | AJCC stage | ||
| Local advancement | 14 | 0.583 | III | 8 | 0.333 |
| ECOG PS | IVA | 13 | 0.542 | ||
| 0 | 0 | 0 | IVB | 3 | 0.125 |
| 1 | 22 | 0.917 | Pre-treatment | ||
| 2 | 2 | 0.083 | Surgery | 10 | 0.417 |
| Radiotherapy | 20 | 0.167 | |||
| Chemotherapy | 5 | 0.208 | |||
All 24 patients were followed up for a median period of 22.4 months (IQR 4.5-37.0). Eight of 24 evaluable cases (33.3%) achieved a CR confirmed by enhanced CT or enhanced MRI and endoscopy, and a PR was observed in sixteen (66.7%) cases with an ORR of 100% and a DCR of 100% (Table 2).
| Indexes | Number (n) | Percentage (%) |
| CR | 8 | 33.3 |
| PR | 16 | 66.7 |
| SD | 0 | 0 |
| ORR | 24 | 100 |
| DCR | 24 | 100 |
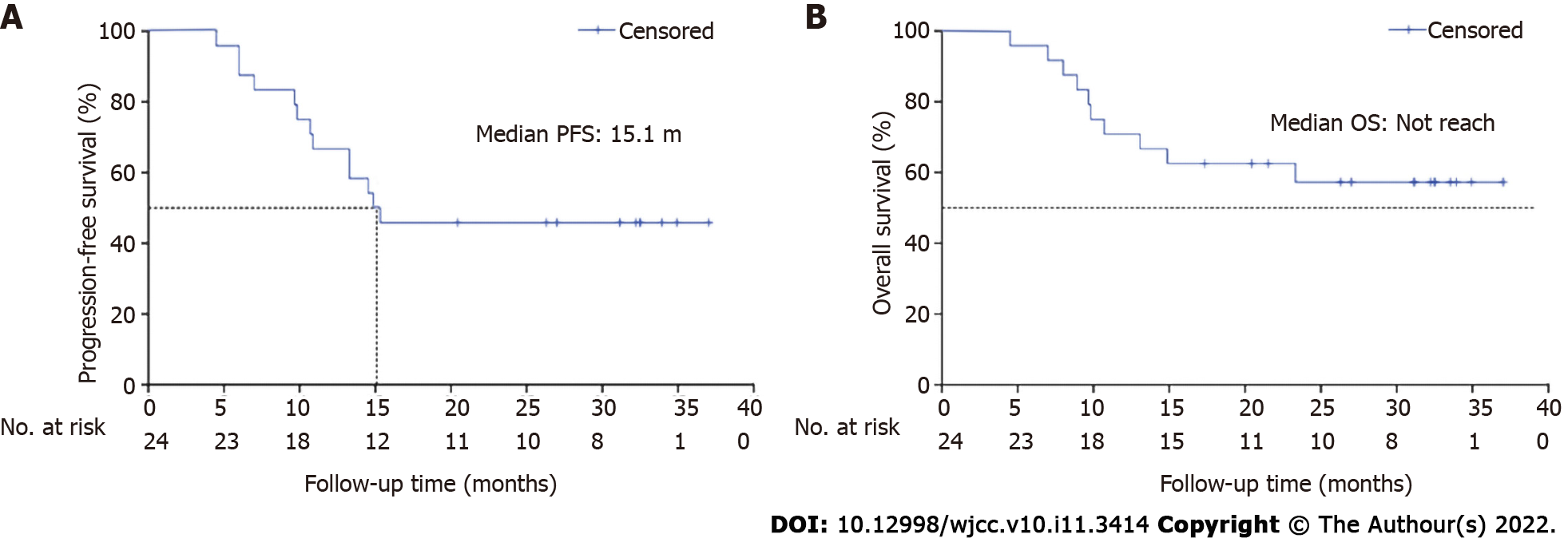
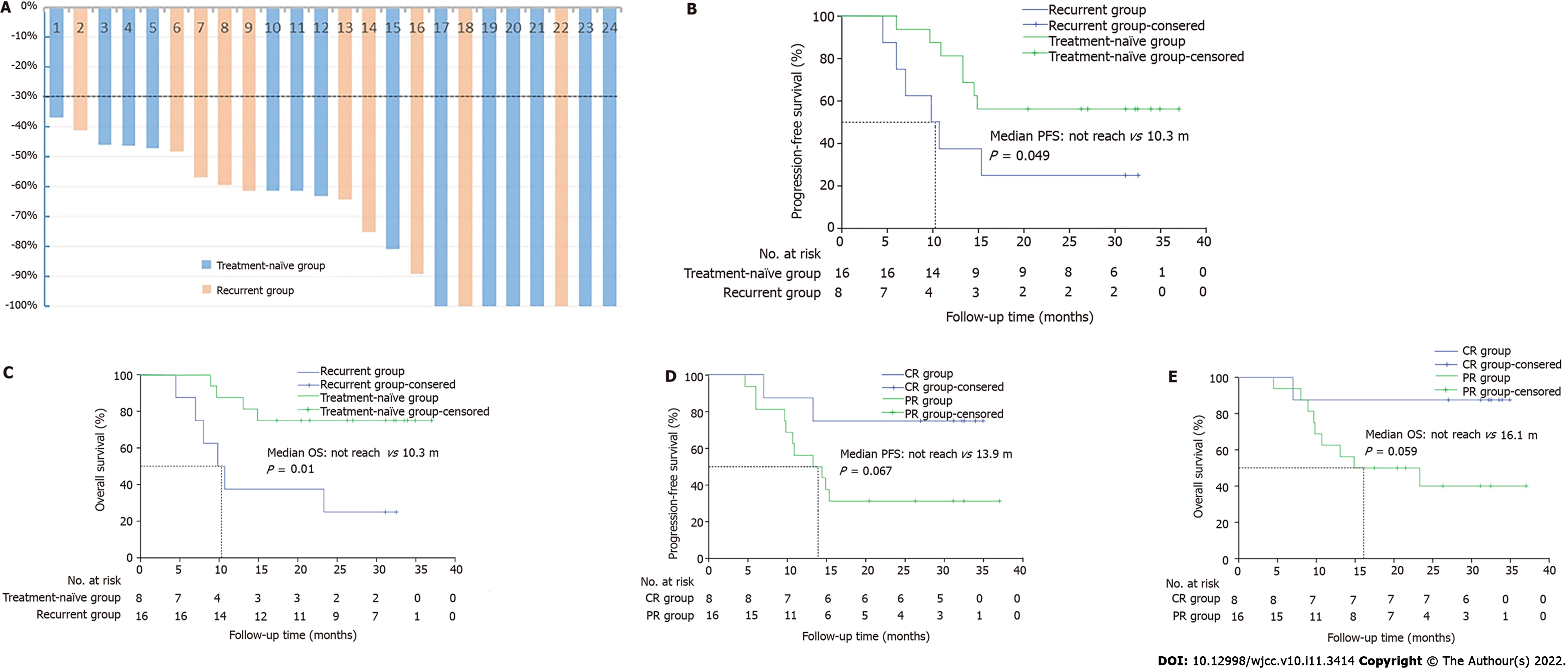
Regression ratios of tumor diameter are shown in Figure 3A. The one-year OS rate was 70.8%, and one-year PFS rate was 66.7%. The median PFS was 15.1 months, while the median OS was not attained. Fourteen patients remained alive at the end of this study, including 11 patients without disease progression (Figure 2).
A subgroup comparison was conducted for the recurrent and the treatment-naïve groups. The median PFS and OS of the recurrent group were both 10.3 months and those for treatment-naïve group had not reached (PPFS=0.069, POS=0.01). The one-year PFS rates of the recurrent and treatment-naïve groups were 37.5% and 81.3%, respectively, and the one-year OS rates of the two groups were 37.5% and 87.5%, respectively (Figure 3B and C).
Another subgroup analysis for the CR and PR groups revealed additional differences (Figure 3D and E). In the PR group, the median PFS and OS time were 13.9 mo and 16.1 months, respectively, and those in the CR group were not reached with the P values in the PFS and OS analysis being 0.067 and 0.059, respectively. The one-year PFS rates of the CR and PR group were 87.5% and 56.3%, respectively, and the one-year OS rate was 87.5% vs 62.5%. In the CR group, one patient died of nasal hemorrhage and one with tumor recurrence in situ; all others remained alive and tumor-free. In the PR group, recurrence in situ was observed in 7 patients (41.2%), and one had distant metastases. Of the 9 patients who died, 3 died from oral and nasal hemorrhage.
Univariate analysis demonstrated no correlation of PFS or OS with age, sex, T stage, N stage, AJCC stage, differentiation degree, smoking or drinking history.
Adverse events were assessed over the course of the study. All patients showed some level of hematological toxicities, most of which required cell-stimulating factor support and/or blood transfusion treatments. Commonly observed adverse events included mucositis, fatigue, nausea, pain, infection, electrolyte disturbances, and dermatitis. Due to grade 3-4 hematological toxicities, only about 25% of patients completed the designed concomitant chemoradiotherapy protocol without interruption. Grade 3-4 toxicities included hematological toxicities (75%), including leukopenia in 18 (75%) patients, neutropenia in 10 (42%) patients, thrombocytopenia in 13 (54%) patients and anemia in 4 (17%) patients. The most common grade 3-4 non-hematological toxicity seen were mucositis in 17 (71%) patients, followed by pain in 5 (21%) patients, electrolyte disturbances in 4 (17%) patients, cardiotoxicity in 3 (12.5%) patients, infection in 3 (12.5%), patients and hemorrhage in 3 (12.5%) patients. Less common long-term adverse events included neuropathy, cervical stenosis and fibrosis (difficulty opening the mouth). Eighteen patients (75%) needed intravenous nutritional support (Table 3).
| Adverse events | Grade | |||||
| 1 | 2 | 3 | 4 | 1-4 | 3-4 | |
| Hematologic | 2 | 5 | 7 | 11 | 24 (100%) | 18 (75%) |
| Leukopenia | 1 | 5 | 14 | 4 | 24 (100%) | 18 (75%) |
| Neutropenia | 7 | 4 | 9 | 1 | 21 (87.5%) | 10 (41.7%) |
| Thrombocytopenia | 6 | 3 | 6 | 7 | 22 (91.7%) | 13 (54.2%) |
| Anemia | 10 | 10 | 4 | 0 | 24 (100%) | 4 (16.7%) |
| Mucositis | 0 | 4 | 2 | 15 | 21 (87.5%) | 17 (70.8%) |
| Fatigue | 10 | 9 | 0 | 0 | 19 (79.2%) | 0 (0%) |
| Nausea | 2 | 10 | 0 | 0 | 12 (50%) | 0 (0%) |
| Pain | 1 | 8 | 3 | 0 | 12 (50%) | 3 (12.5%) |
| Infection | 4 | 6 | 2 | 0 | 12 (50%) | 2 (8.3%) |
| Electrolyte disturbance | 6 | 2 | 4 | 0 | 12 (50%) | 4 (16.7%) |
| Dermatitis | 8 | 1 | 0 | 2 | 11 (45.8%) | 2 (8.3%) |
| Constipation | 10 | 2 | 0 | 0 | 12 (50%) | 0 (0%) |
| Hemorrhage | 1 | 0 | 2 | 1 | 4 (16.7%) | 3 (12.5%) |
| Hepatotoxicity | 14 | 4 | 1 | 0 | 19 (79.2%) | 1 (4.2%) |
| Renal toxicity | 2 | 0 | 0 | 0 | 2 (8.3%) | 0 (0%) |
| Cardiotoxicity | 0 | 0 | 2 | 4 | 6 (25%) | 6 (25%) |
| Xerostomia | 4 | 0 | 0 | 0 | 4 (16.7%) | 0 (0%) |
| Neuropathy | 5 | 1 | 0 | 0 | 6 (25%) | 0 (0%) |
The results of this study showed that both ORR and DCR of patients with HNSCC who received concurrent chemoradiotherapy using gemcitabine and nedaplatin were 100%. The one-year OS rate was 75%, and one-year PFS rate was 66.7%. Recurrent HNSCC patients had a poorer prognosis than treatment-naïve patients, and patients who achieved CR had better survival than those who achieved PR. Both the most common grade 1-4 (100%) or grade 3-4 toxicities (75%) were hematological disorders, and the most common grade 3-4 non-hematological toxicity was mucositis in 17 (71%) patients. Although gemcitabine plus nedaplatin with concurrent chemoradiotherapy for HNSCC is a therapeutic option with predictable tolerability, considering the high adverse event rate, the optimized dose and schedule must be further explored.
GEM is widely applied in induction chemotherapy, concomitant chemoradiotherapy and salvage chemotherapy in treating HNSCC[13,19,20]. GEM has been thought to have radiosensitizing and synergistic action with cisplatin[11] and demonstrates effective cytotoxicity in HNSCC cell lines[8,21]. In our study, we explored the efficiency and safety of GEM combined with nedaplatin in concurrent chemoradiotherapy. Although all patients had stage III or IV disease, it was encouraging to see an ORR of 100% and a DCR of 100%, which were higher and more promising than the values shown in similar studies[13,22]. However, the one-year survival rate was 70.8%, and the one-year PFS rate was 66.7%, which were slightly lower than those reported in other studies[13,22].
Although the median OS has not been reached, we estimated that the median OS would be more than 2 years. There was no significant difference between our PFS and OS survival figures and those reported in similar concomitant chemoradiation studies[2]. Our CR rate was 33.3%, with a PR rate of 66.7%, compared with a CR rate of 55%-83% reported in other studies[11,24,25]. We posited that these results reflected the prognosis of eight patients who experienced tumor recurrence after surgery and chemotherapy, among whom four patients had received radiotherapy before enrollment. This patient subgroup that made up 33.3% of total patients was more refractory to treatment than others. In subgroup analysis, the recurrent group had a poorer prognosis than the treatment-naïve group. In the relapsed subgroup, we still observed a 37.5% CR rate, a 62.5% PR rate, a 100% ORR and a 37.5% one-year survival rate. Because of the small sample size, statistical significance was not reached. A similar CCRT study reported that the one-year OS was 43%, and the ORR was 54.5% in recurrent HNSCC patients [26]. Similar results were obtained in locoregional failure research on HNSCC; the one-year OS was 50%, with a 66.6% ORR in the CCRT subset[27].
In another subgroup analysis, patients who achieved CR appeared to achieve longer PFS and OS time than the PR-achieving group. The reason might be that the tumor biological behavior in the CR group was more sensitive to chemotherapy and radiotherapy, suggesting that CR was a better prognostic factor. Because most patients (75%) in our study received subsequent chemotherapy after concomitant chemoradiation, only one case experienced distant metastasis at the cut-off point.
The most common grade 3-4 treatment-related adverse events (75% hematologic toxicities and 70.8% mucositis) necessitated active treatment with G-CSF, IL-11, TPO, blood infusion and oral/intravenous nutritional support. Considering the higher dose of GEM, the hematologic toxicities were more serious than those described in related studies[7,13,20,25]. The grade 1-2 adverse events detected were anemia, fatigue and hepatotoxicity, all of which were relatively easy to treat. Some rare late adverse events were observed, including xerostomia, open mouth difficulty, radiation myelitis, cervical stenosis and fibrosis. Notably, 4 patients (16.7%) died of mouth or nose hemorrhage without obvious disease progression. The hemorrhage was probably due to carotid blowout syndrome (CBS), of which the incidence was 3% to 4.5% in all postoperative patients and 4.5% to 21.1% in patients who received reirradiation[28]. The mortality of CBS was as high as 75%[29]. Two of the four patients received surgery and second radiotherapy, which were both independent risk factors for CBS[30]. There was no chemotherapy-related deaths occurred in our study. Although there were death cases in both groups, the patients died after completion of treatment and in a period of time after discharge, which could not directly prove that the death was related to treatment. Tumor progression and invasion of blood vessels might also lead to death. Therefore, the death event was not considered as a grade 5 adverse event.
Our exploratory study had several limitations. First, the sample number was too small to conduct a deeper analysis and present a more thorough discussion. Second, the follow-up period was not long enough to adequately evaluate OS data or long-term adverse events. Third, the chemotherapeutic drug doses utilized in this study were not optimized and needed to be explored in future studies in order to reduce severe adverse events.
In summary, the data presented here demonstrated the efficacy and predictable tolerability of GEM plus nedaplatin in concurrent chemoradiotherapy in the treatment of HNSCC. These data served to add to the compendium of treatment modalities regarding first-line treatment for HNSCC patients with recurrent or locally advanced disease. Nevertheless, further studies are required to optimize the dose and schedule of GEM-based chemoradiotherapy to achieve better disease control and survival while minimizing related adverse events.
As one of the most common malignant tumors, head and neck squamous cell carcinoma (HNSCC) seriously affects the survival and quality of life of patients. At present, in addition to surgery, chemoradiotherapy is the main treatment modality. However, the chemotherapy regimens of concurrent chemoradiotherapy are limited. The activity of gemcitabine and nedaplatin in treating HNSCC has been confirmed.
Our study focused on the efficacy and safety of gemcitabine combined with nedaplatin in concurrent chemoradiotherapy for the treatment of recurrent or metastatic HNSCC. This study provided another therapeutic option of concurrent chemoradiotherapy for HNSCC in the future.
The main objective of this study was to evaluate the effect of gemcitabine combined with nedaplatin on PFS and overall survival (OS) in HNSCC patients who received concurrent radiochemotherapy, and to explore the most suitable dose. The protocol and dose used in our study have proved to be effective and safe for these patients. These results can provide reference for concurrent chemoradiotherapy in the future.
This study was a prospective single arm clinical trial. In this study, GN regimen chemotherapy and concurrent radiotherapy were used, imaging and laboratory examination were performed regularly, and RECIST 1.1 was used to evaluate treatment efficacy. The adverse effects were recorded simultaneously. The efficacy evaluation indexes included objective response rate (ORR), disease control rate (DCR), OS and progression free survival (PFS). Kaplan-Meier method was used for survival analysis by SPSS Version 23. These methods can truly and effectively reflect the effectiveness and safety of treatment schemes and are common methods in the world currently. The main objective of this study was to evaluate the effect of gemcitabine combined with nedaplatin on PFS and OS in HNSCC patients who received concurrent radiochemotherapy, and to explore the most suitable dose. The protocol and dose used in our study have proved to be effective and safe for these patients. These results can provide reference for concurrent chemoradiotherapy in the future.
The ORR and DCR were both 100%. The one-year OS was 75%, and one-year PFS was 66.7%. The most common toxicities were hematological diseases, and the most common non-hematological toxicity was mucositis. The results showed the treatment regimen in this trial was effective and the safety was acceptable, which might provide new choice for HNSCC treatment. However, the results need more large-scale randomized clinical trial to confirm.
This study provided a new chemotherapy regimen (GN) in concurrent radiochemotherapy for HNSCC.
It is necessary to explore more effective and safer chemoradiotherapy regimens for the treatment of HNSCC in the future.
We thank Tianjin Union Medical Center for support during the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: The Standing Committee of Sarcoma Professional Committee of China Anti Cancer Association; The Standing Committee of Tumor Standardized Treatment Committee of Chinese Medical Association; The Standing Committee of Tianjin Radiotherapy Professional Committee; The Tianjin Integrated Traditional Chinese and Western Medicine.
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hamaya Y, Japan; Marickar F, India S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Hayes RB, Ahn J, Fan X, Peters BA, Ma Y, Yang L, Agalliu I, Burk RD, Ganly I, Purdue MP, Freedman ND, Gapstur SM, Pei Z. Association of Oral Microbiome With Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018;4:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13201] [Article Influence: 1466.8] [Reference Citation Analysis (3)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12184] [Article Influence: 1523.0] [Reference Citation Analysis (3)] |
| 4. | Leemans CR, Braakhuis BJ, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer. 2011;11:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1786] [Cited by in RCA: 1869] [Article Influence: 133.5] [Reference Citation Analysis (1)] |
| 5. | Algazi AP, Grandis JR. Head and neck cancer in 2016: A watershed year for improvements in treatment? Nat Rev Clin Oncol. 2017;14:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Forastiere AA, Zhang Q, Weber RS, Maor MH, Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, Thorstad W, Wagner H, Ensley JF, Cooper JS. Long-term results of RTOG 91-11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol. 2013;31:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 939] [Cited by in RCA: 872] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 7. | Aguilar-Ponce J, Granados-García M, Villavicencio V, Poitevin-Chacón A, Green D, Dueñas-González A, Herrera-Gómez A, Luna-Ortiz K, Alvarado A, Martínez-Said H, Castillo-Henkel C, Segura-Pacheco B, De la Garza J. Phase II trial of gemcitabine concurrent with radiation for locally advanced squamous cell carcinoma of the head and neck. Ann Oncol. 2004;15:301-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Maseki S, Ijichi K, Nakanishi H, Hasegawa Y, Ogawa T, Murakami S. Efficacy of gemcitabine and cetuximab combination treatment in head and neck squamous cell carcinoma. Mol Clin Oncol. 2013;1:918-924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Denis F, Garaud P, Bardet E, Alfonsi M, Sire C, Germain T, Bergerot P, Rhein B, Tortochaux J, Calais G. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol. 2004;22:69-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 621] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 10. | Garden AS, Harris J, Vokes EE, Forastiere AA, Ridge JA, Jones C, Horwitz EM, Glisson BS, Nabell L, Cooper JS, Demas W, Gore E. Preliminary results of Radiation Therapy Oncology Group 97-03: a randomized phase ii trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. J Clin Oncol. 2004;22:2856-2864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Vanderveken OM, Szturz P, Specenier P, Merlano MC, Benasso M, Van Gestel D, Wouters K, Van Laer C, Van den Weyngaert D, Peeters M, Vermorken J. Gemcitabine-Based Chemoradiation in the Treatment of Locally Advanced Head and Neck Cancer: Systematic Review of Literature and Meta-Analysis. Oncologist. 2016;21:59-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Saddoughi SA, Garrett-Mayer E, Chaudhary U, O'Brien PE, Afrin LB, Day TA, Gillespie MB, Sharma AK, Wilhoit CS, Bostick R, Senkal CE, Hannun YA, Bielawski J, Simon GR, Shirai K, Ogretmen B. Results of a phase II trial of gemcitabine plus doxorubicin in patients with recurrent head and neck cancers: serum C₁₈-ceramide as a novel biomarker for monitoring response. Clin Cancer Res. 2011;17:6097-6105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Halim AA, Wahba HA, El-Hadaad HA, Abo-Elyazeed A. Concomitant chemoradiotherapy using low-dose weekly gemcitabine vs low-dose weekly paclitaxel in locally advanced head and neck squamous cell carcinoma: a phase III study. Med Oncol. 2012;29:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Lee MY, Jung KS, Kim HS, Lee JY, Lim SH, Kim M, Jung HA, Kim SM, Sun JM, Ahn MJ, Lee J, Park SH, Yi SY, Hwang IG, Lee SC, Ahn HK, Lim DH, Lee SI, Park KW. Weekly docetaxel and gemcitabine in previously treated metastatic esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:4268-4274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Huang J, Fan QX, Chen L, Liu AN, Cai RG, Hao XZ, Wang JW, Sun Y. Long-term outcomes of gemcitabine and cisplatin in patients with recurrent or metastatic esophageal squamous cell carcinoma: a phase II trial. Chin Med J (Engl). 2011;124:4012-4017. [PubMed] |
| 16. | Zhang P, Feng FY, Wu LY, Hu Y, Liu JW, Gao YJ, Guan XQ, Nan KJ, Suo AL, Wang XW, Zhang MH, Zhang WD, Li CW, Zhang Y, Zhao JB. [Phase II multicenter clinical trial of nedaplatin in the treatment of malignant tumors]. Zhonghua Zhong Liu Za Zhi. 2006;28:230-234. [PubMed] |
| 17. | Fuwa N, Kodaira T, Furutani K, Tachibana H, Nakamura T, Daimon T. Chemoradiation therapy using radiotherapy, systemic chemotherapy with 5-fluorouracil and nedaplatin, and intra-arterial infusion using carboplatin for locally advanced head and neck cancer - Phase II study. Oral Oncol. 2007;43:1014-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Lai PC, Chiu TH, Huang YT. Overexpression of BDNF and TrkB in human bladder cancer specimens. Oncol Rep. 2010;24:1265-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J, Peng P, Wu X, Lin Q, Xi X, Peng J, Xu M, Chen D, Lu X, Wang R, Cao X, Chen X, Lin Z, Xiong J, Xie C, Li Z, Pan J, Li J, Wu S, Lian Y, Yang Q, Zhao C. Gemcitabine plus cisplatin vs fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet. 2016;388:1883-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 381] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 20. | El Deen DA, Toson EA, El Morsy SM. Gemcitabine-based induction chemotherapy and concurrent with radiation in advanced head and neck cancer. Med Oncol. 2012;29:3367-3373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Zhu MH, Ji SL, Zhang CY, Cui L, Xiong L, Zheng HL. DNA microarray reveals ZNF195 and SBF1 are potential biomarkers for gemcitabine sensitivity in head and neck squamous cell carcinoma cell lines. Int J Clin Exp Pathol. 2014;7:1514-1523. [PubMed] |
| 22. | Ali EM, Abdelraheem AG. Concurrent radiotherapy and chemotherapy for locally advanced squamous cell carcinoma of the head and neck. Head Neck Oncol. 2011;3:48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Specenier PM, Van den Weyngaert D, Van Laer C, Weyler J, Van den Brande J, Huizing MT, Dyck J, Schrijvers D, Vermorken JB. Phase II feasibility study of concurrent radiotherapy and gemcitabine in chemonaive patients with squamous cell carcinoma of the head and neck: long-term follow up data. Ann Oncol. 2007;18:1856-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Iqbal H, Jamshed A, Bhatti AB, Hussain R, Jamshed S, Irfan M, Hameed N, Illyas A. Five-year follow-up of concomitant accelerated hypofractionated radiation in advanced squamous cell carcinoma of the buccal mucosa: a retrospective cohort study. Biomed Res Int. 2015;2015:963574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Chauhan A, Singh H, Sharma T, Manocha KK. Gemcitabine concurrent with radiation therapy for locally advanced head and neck carcinomas. Afr Health Sci. 2008;8:149-155. [PubMed] |
| 26. | Villaflor VM, Haraf D, Salama JK, Kocherginsky M, Langerman A, Gomez-Abuin G, Beniwal P, Blair EA, Stenson KM, Portugal L, Seiwert T, Williams RD, Dekker AJ, Witt ME, Vokes EE, Cohen EEW. Phase II trial of pemetrexed-based induction chemotherapy followed by concomitant chemoradiotherapy in previously irradiated patients with squamous cell carcinoma of the head and neck. Ann Oncol. 2011;22:2501-2507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Datta NR, Nagar YS, Singh S, Naryan L. Loco-regional failures in head and neck cancer: can they be effectively salvaged by nonsurgical therapeutic modalities? Int J Clin Oncol. 2003;8:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Suárez C, Fernández-Alvarez V, Hamoir M, Mendenhall WM, Strojan P, Quer M, Silver CE, Rodrigo JP, Rinaldo A, Ferlito A. Carotid blowout syndrome: modern trends in management. Cancer Manag Res. 2018;10:5617-5628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 29. | Alterio D, Turturici I, Volpe S, Ferrari A, Russell-Edu SW, Vischioni B, Mardighian D, Preda L, Gandini S, Marvaso G, Augugliaro M, Durante S, Arculeo S, Patti F, Boccuzzi D, Casbarra A, Starzynska A, Santoni R, Jereczek-Fossa BA. Carotid blowout syndrome after reirradiation for head and neck malignancies: a comprehensive systematic review for a pragmatic multidisciplinary approach. Crit Rev Oncol Hematol. 2020;155:103088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Chiesa Estomba CM, Betances Reinoso FA, Osorio Velasquez A, Castro Macia O, Gonzalez Cortés MJ, Araujo Nores J. Carotid blowout syndrome in patients treated by larynx cancer. Braz J Otorhinolaryngol. 2017;83:653-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |