Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3379
Peer-review started: October 15, 2021
First decision: December 17, 2021
Revised: December 18, 2021
Accepted: February 17, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 175 Days and 8.4 Hours
Atrial fibrillation (AF) is the most common arrhythmia developing in post-operative patients. Limited data are available regarding pre-operative risk factors and prognostic impact of post-operative AF (POAF) following hip fracture surgery (HFS) in Korean population.
We aimed to investigate the incidence, predictors, and hospital prognosis of POAF in HFS patients.
This study included 245 patients without history of AF who underwent HFS between August 2014 and November 2016. POAF was defined as new-onset AF that occurred during hospitalization after HFS.
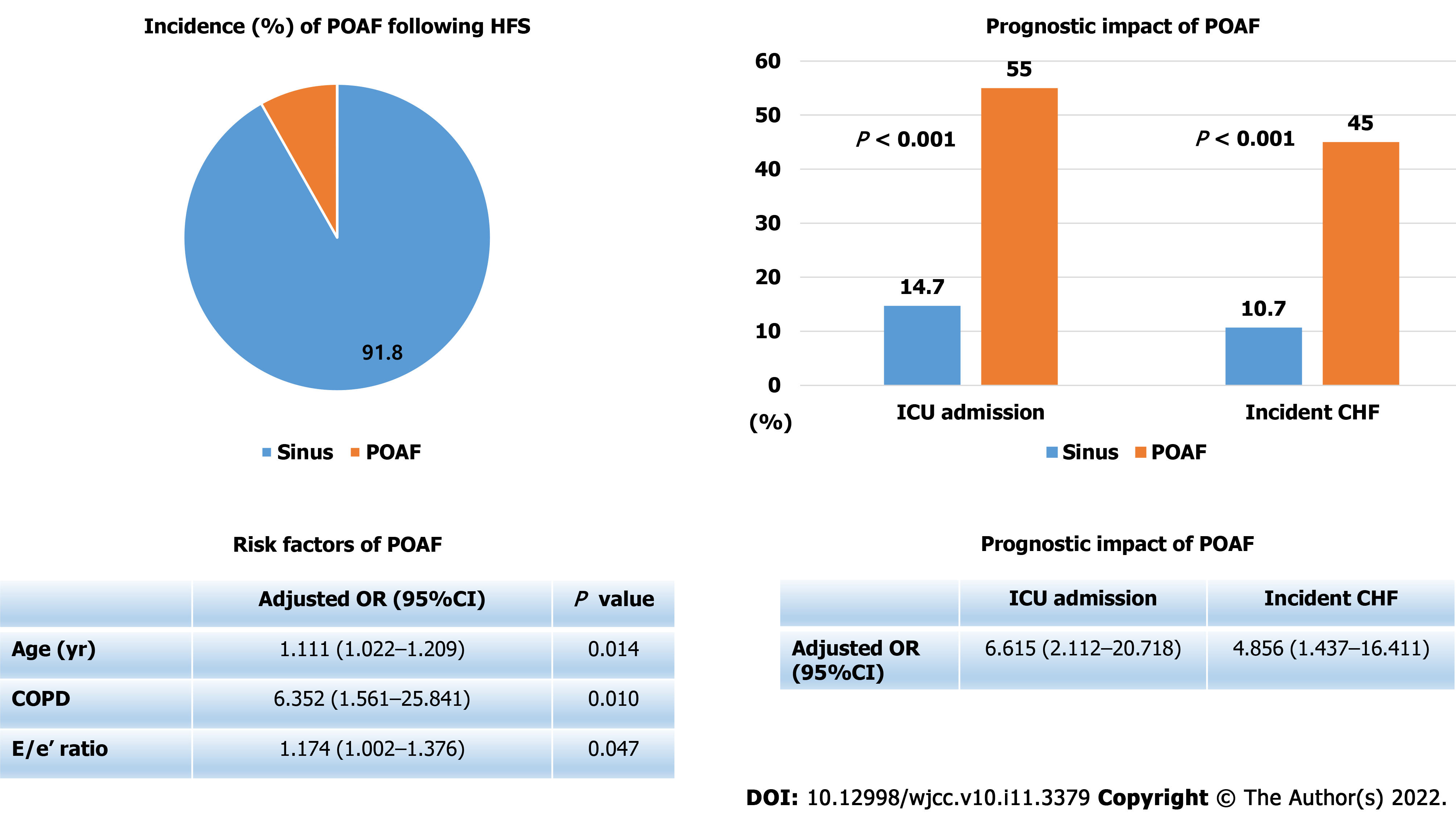
Twenty patients (8.2%) experienced POAF after HFS. POAF developed on median post-operative day 2 (interquartile range, 1–3). Multivariable logistic regression analysis showed that age [odds ratio (OR), 1.111; 95% confidence interval (CI), 1.022–1.209], chronic obstructive pulmonary disease (COPD) (OR, 6.352; 95%CI, 1.561–25.841) and E/e’ ratio (OR, 1.174; 95%CI, 1.002–1.376) were significant predictors of POAF. Patients with POAF had a significantly higher intensive care unit admission rate (55.0% vs 14.7%, P < 0.001) and incidence of congestive heart failure (45.0% vs 10.7%, P < 0.001). In multivariable logistic regression analysis, POAF was significantly associated with increased incidence of congestive heart failure (OR, 4.856; 95%CI, 1.437–16.411) and intensive care unit admission (OR, 6.615; 95%CI, 2.112–20.718).
POAF was frequently developed in elderly patients following HFS. Age, COPD and elevated E/e’ ratio were found as significant predictors of POAF in HFS patients. Patients with POAF significantly experienced intensive care unit admission and incident congestive heart failure during hospitalization.
Core Tip: This study is a retrospective study to evaluate the predictors and prognosis of post-operative atrial fibrillation (POAF) following hip fracture surgery (HFS) in elderly patients. Atrial fibrillation (AF) was developed in 8.2% following HFS. Patients with older age, COPD, or elevated E/e’ ratio were shown as high risk of suffering POAF following HFS. Moreover, Patients with POAF significantly experienced intensive care unit admission and incident heart failure rather than those without POAF. Therefore, physicians have to carefully observe the occurrence of AF after HFS in elderly patients.
- Citation: Bae SJ, Kwon CH, Kim TY, Chang H, Kim BS, Kim SH, Kim HJ. Predictors and prognostic impact of post-operative atrial fibrillation in patients with hip fracture surgery. World J Clin Cases 2022; 10(11): 3379-3388
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3379.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3379
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is independently associated with increased risks of mortality and morbidity[1,2] Incidence of post-operative AF (POAF) has been reported as 15%–45% of patients after cardiac surgery[3,4], and 0.4%–3% after non-cardiac surgery[5,6]. POAF after cardiac surgery is associated with increased length of hospital stay, early stroke risk, morbidity, and 30-d mortality[7-9]. In addition, a recent study has shown that patients with POAF after non-cardiac surgery have a significantly higher risk of stroke, myocardial infarction, and death at 1 year than patients without developing POAF[10].
Approximately 300000 individuals are hospitalized with hip fractures in the United States per year, and about one-third of these patients go on to receive a hip fracture surgery (HFS)[11,12]. Hip fractures occur frequently with aging in patients older than 65 years[13], and substantially increase the risk of death and major morbidity in elderly patients[14,15]. Moreover, HFS is associated with post-operative cardiovascular complications including AF[16-18]. Therefore, we aimed to investigate the incidence, predictors, and clinical impact of POAF in HFS patients.
This retrospective study involved 435 patients who underwent HFS in the Konkuk University Medical Center between August 2014 and November 2016. We excluded 190 patients who met the following exclusion criteria: (1) Patients with preoperative acute coronary syndrome (ACS) or acute decompensated congestive heart failure (CHF) (n = 18); (2) Patients with AF documented in a preoperative evaluation (n = 35); and (3) Patients with insufficient preoperative clinical or laboratory data (n = 138). Finally, 245 patients were included in this analysis. We evaluated the occurrence of POAF during hospitalization after HFS.
The present study protocol was reviewed and approved by the Institutional Review Board of the Konkuk University Medical Center (protocol No. KUMC 2019-07-053). The requirement for informed consent was waived because de-identified information was retrieved retrospectively.
The primary outcome was the new-onset POAF during hospitalization after HFS. POAF was defined as AF of any duration on 12-lead electrocardiography (ECG) during the post-operative period. We evaluated the incidence of clinical adverse events including ACS, CHF, pulmonary thromboembolism, and death according to the occurrence of POAF. Post-operative ACS was defined as the appearance of appropriate clinical symptoms representing unstable angina or evidence of myocardial infarction defined as creatine kinase-myocardial band levels that increased to > 2 times the upper normal limit in association with at least one of the following ECG findings: New Q wave (≥ 30 ms in 2 continuous leads), persistent significant ST elevation or depression, or a new regional wall motion abnormality. Post-operative CHF was defined as the appearance of appropriate clinical symptoms and signs of CHF that required diuretics or post-operative ventilation regardless of left ventricular ejection fraction. Pulmonary thromboembolism was diagnosed if there was a thrombus in the pulmonary arteries on computed tomographic angiography. We also compared the incidence of transfusion, admission duration, and rate of intensive care unit admission according to the occurrence of POAF.
Statistical analyses were performed using SPSS 17 software (SPSS Inc., Chicago, IL, the United States). The data were expressed as the mean ± SD for continuous variables and as frequencies with percentages for categorical variables. Continuous variables were compared by using a Student’s t-test or Mann–Whitney test, and categorical variables using a chi-square test or Fisher’s exact test. The associations of clinical, echocardiographic, or laboratory variables with the development of POAF were assessed by using univariable and multivariable logistic regression models. All variables with P values < 0.10 in univariable analysis were included in the multivariable analysis. A multivariable logistic regression model with stepwise backward elimination was used to test the independent correlations of these variables with POAF. Significant predictors for incident heart failure and intensive care unit admission were assessed by using univariable and multivariable logistic regression models. All P values were two-tailed, and a P < 0.05 was considered statistically significant.
Figure 1 shows incidence, risk factors, and prognostic impact of POAF following HFS. The mean age of the study patients was 76.4 ± 13.1 years, and 64.9% were female. Among the 245 HFS patients enrolled in this analysis, POAF developed in 20 patients (8.2%) during post-operative hospitalization. POAF occurred on median post-operative day 2 (interquartile range[1-3]). Baseline characteristics of the patients according to occurrence of POAF are shown in Table 1. Patients with POAF were more likely to be older, to have history of previous myocardial infarction, previous CHF, or chronic obstructive pulmonary disease (COPD), and to have higher e/e’ ratio levels significantly.
| Variables | Sinus rhythm (n = 225) | POAF (n = 20) | P value |
| Age (yr) | 75.3 ± 13.3 | 84.3 ± 5.7 | < 0.001 |
| Male | 79 (35.1) | 7 (35.0) | 0.992 |
| Medical history | |||
| Hypertension | 153 (68.0) | 14 (70.0) | 0.854 |
| Diabetes | 68 (30.2) | 5 (25.0) | 0.625 |
| Chronic kidney disease | 30 (13.3) | 5 (25.0) | 0.153 |
| Coronary artery disease | 15 (6.7) | 3 (15.0) | 0.171 |
| Previous MI | 6 (2.7) | 3 (15.0) | 0.005 |
| Previous CHF | 9 (4.0) | 3 (15.0) | 0.029 |
| Previous stroke | 27 (12.0) | 1 (5.0) | 0.346 |
| COPD | 14 (6.2) | 6 (30.0) | < 0.001 |
| Echocardiographic parameter | |||
| LVEF, % | 59.1 ± 6.9 | 56.3 ± 9.2 | 0.096 |
| LVEF < 50% | 18 (8.0) | 2 (10.0) | 0.754 |
| E/e’ | 10.9 ± 3.2 | 12.6 ± 2.8 | 0.047 |
| Laboratory parameter | |||
| Hemoglobin, g/dL | 11.8 ± 2.0 | 11.2 ± 2.1 | 0.227 |
| Creatinine, mg/dL | 1.0 ± 1.1 | 1.2 ± 0.8 | 0.634 |
| CK-MB, ng/mL | 2.9 ± 3.9 | 2.8 ± 4.3 | 0.863 |
| hsTn-I, ng/L | 30.3 ± 155.2 | 24.1 ± 29.1 | 0.858 |
| NT-proBNP, pg/dL | 598.8 ± 1923.4 | 1172.7 ± 1844.5 | 0.251 |
In the univariable logistic regression analysis, age, previous myocardial infarction, previous CHF, and COPD were significantly associated with development of POAF after HFS (Table 2). However, in multivariable logistic regression analysis with stepwise backward elimination, age, COPD, and E/e’ ratio level were left as significant predictors of POAF (Table 2).
| Variables | Unadjusted OR (95%CI) | P value | Adjusted OR (95%CI) | P value |
| Age (yr) | 1.100 (1.031-1.172) | 0.004 | 1.111 (1.022-1.209) | 0.014 |
| Male | 0.995 (0.382-2.596) | 0.992 | ||
| Hypertension | 1.098 (0.405-2.974) | 0.854 | ||
| Diabetes mellitus | 0.770 (0.269-2.202) | 0.625 | ||
| Chronic kidney disease | 2.167 (0.734-6.397) | 0.162 | ||
| Coronary artery disease | 0.405 (0.107-1.537) | 0.184 | ||
| Previous MI | 6.441 (1.479-28.046) | 0.013 | ||
| Previous CHF | 4.235 (1.048-17.120) | 0.043 | ||
| Previous stroke | 0.386 (0.050-3.000) | 0.363 | ||
| COPD | 6.459 (2.153-19.380) | 0.001 | 6.352 (1.561-25.841) | 0.010 |
| LVEF (%) | 0.952 (0.899-1.009) | 0.098 | ||
| E/e’ | 1.151 (0.999-1.327) | 0.051 | 1.174 (1.002-1.376) | 0.047 |
| Hemoglobin (g/dL) | 0.870 (0.694-1.091) | 0.228 | ||
| Creatinine (mg/dL) | 1.083 (0.778-1.506) | 0.636 | ||
| CK-MB (ng/mL) | 0.988 (0.872-1.120) | 0.853 | ||
| hsTn-I (ng/L) | 1.000 (0.996-1.003) | 0.859 | ||
| NT-proBNP (pg/dL) | 1.000 (1.000-1.000) | 0.279 |
Table 3 shows clinical adverse events during hospitalization according to occurrence of POAF. Patients with POAF required more transfusion and longer hospitalization than those without POAF, but the difference was not statistically significant. The incidences of intensive care unit admission and CHF were significantly increased in patients with POAF. Median time of CHF incidence was post-operative day 3 (interquartile range[2-4]). The incidences of pulmonary thromboembolism, ACS, or death during hospitalization were not different significantly between two groups. All death events were developed in patients without POAF. Two patients died from cardiac arrest and one patient died from hypovolemic shock.
| Sinus rhythm (n = 225) | POAF (n = 20) | P value | |
| Transfusion | 190 (84.4) | 19 (95.0) | 0.201 |
| Transfused packed RBC count | 3.4 ± 4.4 | 4.1 ± 2.4 | 0.508 |
| Admission day | 23.0 ± 33.8 | 29.6 ± 18.4 | 0.391 |
| Intensive care unit admission | 33 (14.7) | 11 (55.0) | < 0.001 |
| Congestive heart failure | 24 (10.7) | 9 (45.0) | < 0.001 |
| Pulmonary thromboembolism | 4 (1.8) | 0 (0.0) | 0.548 |
| Acute coronary syndrome | 7 (3.1) | 2 (10.0) | 0.117 |
| Death | 3 (1.3) | 0 (0.0) | 0.548 |
Table 4 shows the results of logistic regression analyses to evaluate independent predictors of incident CHF following HFS. Lower hemoglobin levels and POAF were found as significant predictors of incident CHF following HFS in multivariable analysis. Independent predictors of intensive care unit admission following HFS are shown in Table 5. History of previous stroke, elevated creatinine levels, and POAF were significantly associated with intensive care unit admission following HFS.
| Variables | Unadjusted OR (95%CI) | P value | Adjusted OR (95%CI) | P value |
| Age (yr) | 1.060 (1.015-1.107) | 0.008 | ||
| Male | 1.238 (0.583-2.629) | 0.579 | ||
| Hypertension | 0.924 (0.424-2.015) | 0.843 | ||
| Diabetes mellitus | 0.724 (0.310-1.690) | 0.455 | ||
| Chronic kidney disease | 3.917 (1.692-9.066) | 0.001 | 2.570 (0.946-6.980) | 0.064 |
| Coronary artery disease | 1.951 (0.601-6.333) | 0.266 | ||
| Previous MI | 5.710 (1.450-22.495) | 0.013 | ||
| Previous CHF | 5.230 (1.554-17.602) | 0.008 | ||
| Previous stroke | 1.080 (0.350-3.339) | 0.893 | ||
| COPD | 5.333 (1.989-14.303) | 0.001 | 3.408 (0.898-12.934) | 0.072 |
| LVEF (%) | 0.971 (0.925-1.020) | 0.243 | ||
| E/e’ | 1.088 (0.967-1.224) | 0.159 | ||
| Hemoglobin (g/dL) | 0.688 (0.565-0.838) | < 0.001 | 0.753 (0.597-0.949) | 0.016 |
| Creatinine (mg/dL) | 1.266 (0.997-1.608) | 0.053 | ||
| CK-MB (ng/mL) | 1.042 (0.969-1.121) | 0.268 | ||
| hsTn-I (ng/L) | 1.001 (0.999-1.003) | 0.334 | ||
| NT-proBNP (pg/dL) | 1.000 (1.000-1.000) | 0.050 | ||
| Post-operative AF | 6.852 (2.579-18.209) | < 0.001 | 4.856 (1.437-16.411) | 0.011 |
| Variables | Unadjusted OR (95%CI) | P value | Adjusted OR (95%CI) | P value |
| Age (yr) | 1.017 (0.989-1.046) | 0.242 | ||
| Male | 0.836 (0.417-1.678) | 0.615 | ||
| Hypertension | 0.781 (0.394-1.546) | 0.477 | ||
| Diabetes mellitus | 1.444 (0.727-2.868) | 0.295 | ||
| Chronic kidney disease | 2.458 (1.100-5.496) | 0.028 | ||
| Coronary artery disease | 1.336 (0.418-4.271) | 0.626 | ||
| Previous MI | 2.378 (0.571-9.900) | 0.234 | ||
| Previous CHF | 5.132 (1.571-16.763) | 0.007 | 3.295 (0.860-12.632) | 0.082 |
| Previous stroke | 2.463 (1.030-5.889) | 0.043 | 3.718 (1.326-10.420) | 0.013 |
| COPD | 2.109 (0.762-5.837) | 0.151 | ||
| LVEF (%) | 0.979 (0.937-1.024) | 0.358 | ||
| E/e’ | 1.032 (0.928-1.148) | 0.557 | ||
| Hemoglobin (g/dL) | 0.853 (0.724-1.004) | 0.057 | ||
| Creatinine (mg/dL) | 1.348 (1.054-1.725) | 0.018 | 1.416 (1.085-1.848) | 0.011 |
| CK-MB (ng/mL) | 1.033 (0.963-1.108) | 0.368 | ||
| hsTn-I (ng/L) | 1.002 (1.000-1.004) | 0.112 | ||
| NT-proBNP (pg/dL) | 1.000 (1.000-1.000) | 0.019 | ||
| Post-operative AF | 7.111 (2.736-18.484) | <0.001 | 6.615 (2.112-20.718) | 0.001 |
The major findings of the present study are as follows: (1) The incidence of POAF was 20 (8.2%) among 245 patients with HFS; (2) Age, COPD, and elevated E/e’ ratio were significant predictors of POAF in these patients; (3) Incidences of intensive care unit admission and CHF during hospitalization were significantly higher in patients with POAF; and (4) POAF was significantly associated with intensive care unit admission and incident CHF following HFS.
The 8.2% incidence of POAF was consistent with previous results[5,6,10]. Among various types of non-cardiac surgery, abdominal, thoracic and vascular surgeries have been associated with higher incidence rates of POAF[5,10,19]. Although HFS is orthopedic surgery, most patients with hip fractures are elderly and commonly have impaired functional status and medical comorbidities[15,20]. Moreover, in elderly patients receiving HFS, perioperative atrial arrhythmias were reported to be common (5.6%) and to be associated with greater mortality[21]. Rhythm monitoring might be used only for selected patients after surgery, and patients do not always feel AF symptoms. Therefore, the incidence of POAF in this study might be underestimated. So, we have to monitor rhythm status actively in elderly patients as having high risk of POAF during the perioperative period.
In this analysis, age, COPD, and elevated e/e’ ratio were significant predictors of POAF after HFS. Old age, pre-existing AF, CHF, ischemic heart disease, hypertension, chronic renal failure, sepsis, shock, asthma, and valvular heart disease are associated with increased risk of POAF[5,22-24]. The pathophysiology of the development of POAF after non-cardiac surgery is not fully understood. Potential mechanisms may be explained by a combination of multiple factors including increased sympathetic activity, autonomic stimulation, electrolyte imbalance, anemia, underlying cardiac disease, metabolic alterations, hypothermia, inflammation, hypoxia, and intraoperative adverse events like hypotension[25]. The prevalence and incidence of AF are elevated among patients with COPD[26,27]. Although we did not evaluate lung function in this study, patients with COPD history might have reduced lung function compared to those without COPD. Therefore, these patients are more likely to experience hypoxia in stress situations caused by surgery, which may cause hypoxia-driven POAF. In addition, E/e’ ratio is well known marker for high left ventricular filling pressure[28]. In this analysis, elevated E/e’ ratio was a significant predictor of POAF. Elevated E/e’ ratio has been reported as significant predictor of POAF following non-cardiac surgery[29-31]. Elevated E/e’ ratio presents left ventricular diastolic dysfunction, which is related to increased left atrial filling pressures. With increasing pressure in left atrium, pathological changes including increased atrial afterload, myocyte stretch, and atrial wall stress are developed[32]. This consequent left atrial remodeling is believed as main mechanism of POAF following HFS.
Patients with POAF required a longer hospital stay, had a higher intensive care unit admission, and experienced more development of CHF during hospitalization. Our results are consistent with previous findings that POAF leads to increased length of hospital stay and subsequently elevated health care costs[22,23,33]. Moreover, recent cohort including large number (n = 2922) of patients underwent HFS reported that patients with POAF experienced not only higher length of hospital stay but also higher 1-year mortality in comparison to control group[34]. The present study only showed significant association between POAF and in-hospital complications, but this study revealed poor long-term prognosis of POAF patients. AF and CHF often occur together, and each can precede and follow the other[35]. In this study, POAF occurred on median day 2 after surgery, but CHF developed on median post-operative day 3. Thus, CHF might not result in POAF in these patients. Even then, we cannot conclude that POAF directly causes CHF after surgery. Because patients with POAF had more chronic comorbidities like COPD and CKD, the poor outcomes might be the consequence of their comorbidities rather than the result of POAF. Even we cannot explain complex mechanism between POAF and post-operative CHF development, we need to pay more attention to the development of CHF when patients experience POAF following surgery.
This study had several inherent limitations. First, the study design was retrospective and observational, and thus we could not adjust potential confounding factors. Second, evaluation of 12-lead ECG after HFS was not consistent and uniform because it was left to the discretion of attending physician. Third, diagnosis of POAF was based only on 12-lead ECG. Therefore, the incidence of new-onset POAF might have been underestimated because paroxysmal AF, especially asymptomatic episodes, could not be diagnosed. Fourth, a large population (n = 138) with insufficient laboratory data including high-sensitivity troponin I and/or N-terminal pro-brain natriuretic peptide were excluded from this study because pre-operative biomarker evaluation was not performed in all patients. Therefore, there would be a selection bias associated with this factor. Fifth, frailty is a strong indication for mortality, intensive care unit admission, and AF. But, we could not incorporate frailty score in our analysis. Finally, because study patients and incident POAF patients (n = 20) were relatively small, the statistical power for the predictors of POAF might have been low. Moreover, for this reason, we only evaluated the association between POAF and in-hospital complications, but not long-term prognosis of POAF. Despite these limitations, the present study may have clinical significance because this analysis showed real-world observational results in elderly Korean patients who underwent HFS.
The incidence of POAF was 8.2% in patients with HFS. Age, COPD and elevated E/e’ ratio were potential predictors of POAF in these patients. Patients with POAF significantly experienced intensive care unit admission and incident CHF during hospitalization. POAF was revealed as significant predictor of intensive care unit admission and incident CHF. Therefore, physicians have to observe closely the incidence of POAF in old HFS patients.
Physicians have to carefully observe the occurrence of atrial fibrillation (AF) after hip fracture surgery (HFS) in elderly patients.
Age, chronic obstructive pulmonary disease (COPD) and elevated E/e’ ratio were found as significant predictors of post-operative AF (POAF) in HFS patients. Patients with POAF significantly experienced intensive care unit admission and incident congestive heart failure during hospitalization.
The major findings of the present study are as follows: (1) The incidence of POAF was 20 (8.2%) among 245 patients with HFS; (2) Age, chronic obstructive pulmonary disease, and elevated E/e’ ratio were significant predictors of POAF in these patients; (3) Incidences of intensive care unit admission and congestive heart failure during hospitalization were significantly higher in patients with POAF; and (4) POAF was significantly associated with intensive care unit admission and incident congestive heart failure following HFS.
This retrospective study involved 245 patients who underwent HFS in the Konkuk University Medical Center between August 2014 and November 2016. We evaluated the incidence, risk factors, and prognosis impact during hospitalization following HFS.
We aimed to investigate the incidence, predictors, and hospital prognosis of POAF in HFS patients.
People are getting older, and many elderly patients have been undergoing HFS. Atrial fibrillation is the most common arrhythmia developing in post-operative patients. So, we was wondering if POAF may affect in-hospital outcomes in patients underwent HFS.
Limited data are available regarding pre-operative risk factors and prognostic impact of post-operative atrial fibrillation following hip fracture surgery in Korean population.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sawalha K, United States; Xie M, China S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3186] [Cited by in RCA: 3333] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 2. | Krahn AD, Manfreda J, Tate RB, Mathewson FA, Cuddy TE. The natural history of atrial fibrillation: incidence, risk factors, and prognosis in the Manitoba Follow-Up Study. Am J Med. 1995;98:476-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1032] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 3. | Mathew JP, Fontes ML, Tudor IC, Ramsay J, Duke P, Mazer CD, Barash PG, Hsu PH, Mangano DT; Investigators of the Ischemia Research and Education Foundation; Multicenter Study of Perioperative Ischemia Research Group. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 871] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 4. | Ahlsson AJ, Bodin L, Lundblad OH, Englund AG. Postoperative atrial fibrillation is not correlated to C-reactive protein. Ann Thorac Surg. 2007;83:1332-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Bhave PD, Goldman LE, Vittinghoff E, Maselli J, Auerbach A. Incidence, predictors, and outcomes associated with postoperative atrial fibrillation after major noncardiac surgery. Am Heart J. 2012;164:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 172] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 6. | Butt JH, Olesen JB, Havers-Borgersen E, Gundlund A, Andersson C, Gislason GH, Torp-Pedersen C, Køber L, Fosbøl EL. Risk of Thromboembolism Associated With Atrial Fibrillation Following Noncardiac Surgery. J Am Coll Cardiol. 2018;72:2027-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | LaPar DJ, Speir AM, Crosby IK, Fonner E, Jr. , Brown M, Rich JB, Quader M, Kern JA, Kron IL, Ailawadi G. Postoperative atrial fibrillation significantly increases mortality, hospital readmission, and hospital costs. Ann Thorac Surg. 2014;98:527-533; discussion 533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 205] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 8. | Saxena A, Dinh DT, Smith JA, Shardey GC, Reid CM, Newcomb AE. Usefulness of postoperative atrial fibrillation as an independent predictor for worse early and late outcomes after isolated coronary artery bypass grafting (multicenter Australian study of 19,497 patients). Am J Cardiol. 2012;109:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Steinberg BA, Zhao Y, He X, Hernandez AF, Fullerton DA, Thomas KL, Mills R, Klaskala W, Peterson ED, Piccini JP. Management of postoperative atrial fibrillation and subsequent outcomes in contemporary patients undergoing cardiac surgery: insights from the Society of Thoracic Surgeons CAPS-Care Atrial Fibrillation Registry. Clin Cardiol. 2014;37:7-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Conen D, Alonso-Coello P, Douketis J, Chan MTV, Kurz A, Sigamani A, Parlow JL, Wang CY, Villar JC, Srinathan SK, Tiboni M, Malaga G, Guyatt G, Sivakumaran S, Rodriguez Funes MV, Cruz P, Yang H, Dresser GK, Alvarez-Garcia J, Schricker T, Jones PM, Drummond LW, Balasubramanian K, Yusuf S, Devereaux PJ. Risk of stroke and other adverse outcomes in patients with perioperative atrial fibrillation 1 year after non-cardiac surgery. Eur Heart J. 2020;41:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009;302:1573-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1210] [Article Influence: 75.6] [Reference Citation Analysis (0)] |
| 12. | Dy CJ, McCollister KE, Lubarsky DA, Lane JM. An economic evaluation of a systems-based strategy to expedite surgical treatment of hip fractures. J Bone Joint Surg Am. 2011;93:1326-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Karagas MR, Lu-Yao GL, Barrett JA, Beach ML, Baron JA. Heterogeneity of hip fracture: age, race, sex, and geographic patterns of femoral neck and trochanteric fractures among the US elderly. Am J Epidemiol. 1996;143:677-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 165] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 14. | Wolinsky FD, Fitzgerald JF, Stump TE. The effect of hip fracture on mortality, hospitalization, and functional status: a prospective study. Am J Public Health. 1997;87:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 304] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Bentler SE, Liu L, Obrizan M, Cook EA, Wright KB, Geweke JF, Chrischilles EA, Pavlik CE, Wallace RB, Ohsfeldt RL, Jones MP, Rosenthal GE, Wolinsky FD. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009;170:1290-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 353] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 16. | Chiang CH, Liu CJ, Chen PJ, Huang CC, Hsu CY, Chen ZY, Chan WL, Huang PH, Chen TJ, Chung CM, Lin SJ, Chen JW, Leu HB. Hip fracture and risk of acute myocardial infarction: a nationwide study. J Bone Miner Res. 2013;28:404-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Sathiyakumar V, Avilucea FR, Whiting PS, Jahangir AA, Mir HR, Obremskey WT, Sethi MK. Risk factors for adverse cardiac events in hip fracture patients: an analysis of NSQIP data. Int Orthop. 2016;40:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Kim BS, Kim TH, Oh JH, Kwon CH, Kim SH, Kim HJ, Hwang HK, Chung SM. Association between preoperative high sensitive troponin I levels and cardiovascular events after hip fracture surgery in the elderly. J Geriatr Cardiol. 2018;15:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 19. | Brathwaite D, Weissman C. The new onset of atrial arrhythmias following major noncardiothoracic surgery is associated with increased mortality. Chest. 1998;114:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Sennerby U, Melhus H, Gedeborg R, Byberg L, Garmo H, Ahlbom A, Pedersen NL, Michaëlsson K. Cardiovascular diseases and risk of hip fracture. JAMA. 2009;302:1666-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 21. | Gupta BP, Steckelberg RC, Gullerud RE, Huddleston PM, Kirkland LL, Wright RS, Huddleston JM. Incidence and 1-Year Outcomes of Perioperative Atrial Arrhythmia in Elderly Adults After Hip Fracture Surgery. J Am Geriatr Soc. 2015;63:2269-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Polanczyk CA, Goldman L, Marcantonio ER, Orav EJ, Lee TH. Supraventricular arrhythmia in patients having noncardiac surgery: clinical correlates and effect on length of stay. Ann Intern Med. 1998;129:279-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 187] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Sohn GH, Shin DH, Byun KM, Han HJ, Cho SJ, Song YB, Kim JH, On YK, Kim JS. The incidence and predictors of postoperative atrial fibrillation after noncardiothoracic surgery. Korean Circ J. 2009;39:100-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | La Manna G, Boriani G, Capelli I, Marchetti A, Grandinetti V, Spazzoli A, Dalmastri V, Todeschini P, Rucci P, Stefoni S. Incidence and predictors of postoperative atrial fibrillation in kidney transplant recipients. Transplantation. 2013;96:981-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Danelich IM, Lose JM, Wright SS, Asirvatham SJ, Ballinger BA, Larson DW, Lovely JK. Practical management of postoperative atrial fibrillation after noncardiac surgery. J Am Coll Surg. 2014;219:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Xiao X, Han H, Wu C, He Q, Ruan Y, Zhai Y, Gao Y, Zhao X, He J. Prevalence of Atrial Fibrillation in Hospital Encounters With End-Stage COPD on Home Oxygen: National Trends in the United States. Chest. 2019;155:918-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Buch P, Friberg J, Scharling H, Lange P, Prescott E. Reduced lung function and risk of atrial fibrillation in the Copenhagen City Heart Study. Eur Respir J. 2003;21:1012-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 186] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Andersen OS, Smiseth OA, Dokainish H, Abudiab MM, Schutt RC, Kumar A, Sato K, Harb S, Gude E, Remme EW, Andreassen AK, Ha JW, Xu J, Klein AL, Nagueh SF. Estimating Left Ventricular Filling Pressure by Echocardiography. J Am Coll Cardiol. 2017;69:1937-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 315] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 29. | Nagatsuka Y, Sugimura K, Miyata H, Shinnno N, Asukai K, Hara H, Hasegawa S, Yamada D, Yamamoto K, Haraguchi N, Nishimura J, Motoori M, Wada H, Takahashi H, Yasui M, Omori T, Ohue M, Yano M. Predictive value of preoperative echocardiographic assessment for postoperative atrial fibrillation after esophagectomy for esophageal cancer. Esophagus. 2021;18:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Brecher O, Gulati H, Roistacher N, Zhang H, Shi W, Thaler HT, Amar D. Preoperative Echocardiographic Indices of Diastolic Dysfunction and Brain Natriuretic Peptide in Predicting Postoperative Atrial Fibrillation After Noncardiac Surgery. Anesth Analg. 2017;124:1099-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Nojiri T, Maeda H, Takeuchi Y, Funakoshi Y, Maekura R, Yamamoto K, Okumura M. Predictive value of preoperative tissue Doppler echocardiographic analysis for postoperative atrial fibrillation after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg. 2010;140:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Rosenberg MA, Manning WJ. Diastolic dysfunction and risk of atrial fibrillation: a mechanistic appraisal. Circulation. 2012;126:2353-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 33. | Seguin P, Signouret T, Laviolle B, Branger B, Mallédant Y. Incidence and risk factors of atrial fibrillation in a surgical intensive care unit. Crit Care Med. 2004;32:722-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Rostagno C, Cartei A, Rubbieri G, Ceccofiglio A, Polidori G, Curcio M, Civinini R, Prisco D. Postoperative atrial fibrillation is related to a worse outcome in patients undergoing surgery for hip fracture. Intern Emerg Med. 2021;16:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved Versus Reduced Ejection Fraction. Circulation. 2016;133:484-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 605] [Article Influence: 67.2] [Reference Citation Analysis (0)] |