Published online Apr 16, 2022. doi: 10.12998/wjcc.v10.i11.3369
Peer-review started: September 3, 2021
First decision: October 25, 2021
Revised: December 23, 2021
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: April 16, 2022
Processing time: 217 Days and 0.1 Hours
Shone’s complex is a rare syndrome characterized by congenital left heart defects that can differ among the patients.
To use echocardiography in the diagnosis of Shone’s complex and analyze the causes of missed diagnosis and misdiagnosis.
This was a retrospective study of patients who underwent echocardiography and repair surgery from February 14, 2008, to November 22, 2019. The patients were followed once a year at the outpatient clinic after surgery.
Sixty-six patients were included. The patients were 2.7 (0.8-5.6) years of age, and 54.5% were male. Ten (15.2%) had a history of heart surgery. The most common heart defect was the Annulo-Leaflet mitral ring (ALMR) (50/66, 75.8%), followed by coarctation of the aorta (CoA) (43/66, 65.2%). The patients had a variety of combinations of defects. Only two (3.0%) patients had all four defects. None of the patients had a family history of congenital heart disease. The preoperative echocardiographic findings were examined against the intraoperative findings. Echocardiography missed an ALMR in 31 patients (47.0%), a parachute mitral valve (PMV) in one patient (1.5%), subaortic stenosis in one patient (1.5%), and CoA in two patients (3.0%).
Echocardiography is an effective method to diagnose the Shone’s complex. Due to this disease’s complexity and interindividual variability, Improving the understanding of the disease can reduce misdiagnosis and missed diagnosis.
Core Tip: This was a retrospective study with the largest sample size which aimed to examine the use of echocardiography in the diagnosis of Shone’s complex and to analyze the possible causes of missed diagnosis and misdiagnosis. Sixty-six patients were included. The preoperative echocardiographic findings were examined against the intraoperative findings. Echocardiography missed an Annulo-Leaflet mitral ring in 31 patients, a parachute mitral valve in one patient, subaortic stenosis in one patient, and coarctation of the aorta in two patients. Due to this disease’s complexity and interindividual variability, echocardiography missed diagnosis can occur. Combining the results of echocardiography, computed tomography, magnetic resonance imaging might be helpful.
- Citation: Li YD, Meng H, Pang KJ, Li MZ, Xu N, Wang H, Li SJ, Yan J. Echocardiography in the diagnosis of Shone’s complex and analysis of the causes for missed diagnosis and misdiagnosis. World J Clin Cases 2022; 10(11): 3369-3378
- URL: https://www.wjgnet.com/2307-8960/full/v10/i11/3369.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i11.3369
Shone’s complex is a rare congenital heart disease characterized by multiple left heart obstructive defects, including coarctation of the aorta (CoA), valvular stenosis, and mitral stenosis[1-4]. Those defects interfere with the normal flow of oxygenated blood from the left heart. There are complete and incomplete forms of the syndrome, as well as possible combinations with other heart defects such as patent ductus arteriosus, interrupted aortic arch, bicuspid aortic valve, atrial septal defect, and ventricular septal defect[5]. The spectrum of symptoms, treatments, and outcomes will vary according to the number of defects[6-10]. Shone’s complex represent about 0.7% of the patients with congenital heart disease[11] or 0.03% of echocardiography examinations[12]. The long-term prognosis is poor, and the perioperative mortality rates are 24%-27%[13,14].
Echocardiography is a non-invasive imaging modality that provides hemodynamic information in a short period and at the patient bedside. It can be used to reveal abnormal left ventricular wall motions, right ventricle dilation, an intimal flap in the ascending aorta, pericardial effusion, left ventricular ejection fraction[15-19]. It is a non-invasive, rapid, inexpensive diagnostic modality for a number of heart conditions such as pericardial tamponade, acute coronary syndrome, cardiomyopathy, pulmonary embolism, and Stanford type A aortic dissection[19,20]. It can also be used for the diagnosis, follow-up, and management of congenital heart diseases[21-23].
The studies about the use of echocardiography for the diagnosis of Shone’s complex are mainly limited to case reports[5,24-26] or small case series[12,27,28]. Nevertheless, a study suggested that echocardiography is invaluable in the characterization of the left heart defects found in Shone’s complex, but that diagnosis is complicated by the high variability of the possible combinations of defects[27]. This could result in a missed diagnosis or misdiagnosis. Additional studies are necessary to determine the exact value of echocardiography in the diagnosis of Shone’s complex.
Therefore, this study aims to examine the use of echocardiography in the diagnosis of Shone’s complex and to analyze the possible causes of missed diagnosis and misdiagnosis. The results could support the use of echocardiography for the diagnosis of Shone’s complex.
This was a retrospective study of patients who underwent echocardiography and repair surgery at Fuwai Hospital (Beijing, China) from February 14, 2008, to November 22, 2019. The study was approved by the ethics committee of Fuwai Hospital, Beijing, China (2016YFC1302000). The requirement for informed consent was waived by the committee because of the retrospective study nature.
The inclusion criteria were: (1) Surgically confirmed Shone’s complex; and (2) Underwent echocardiography, and qualified images were available. Patients with incomplete clinical data were excluded.
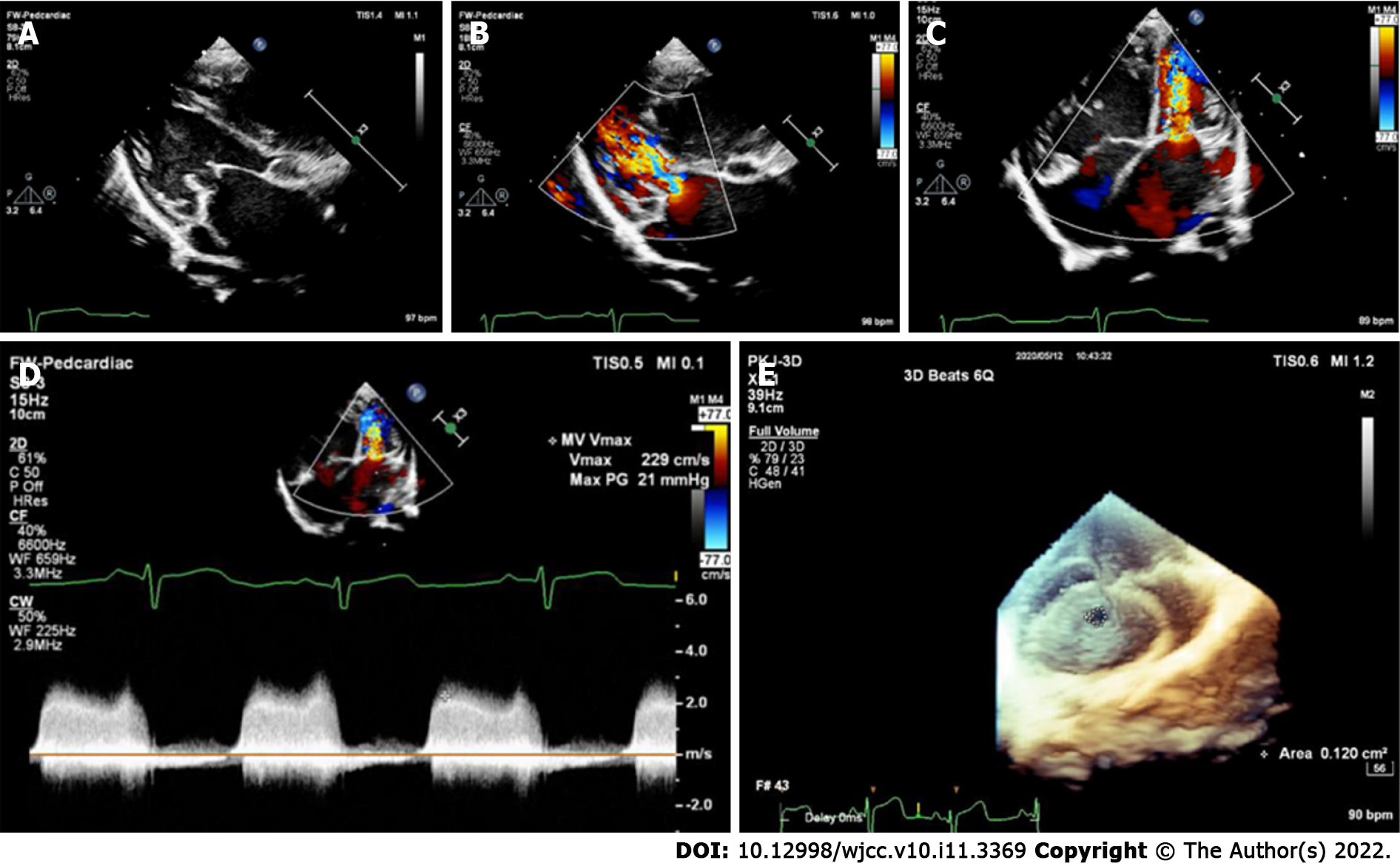
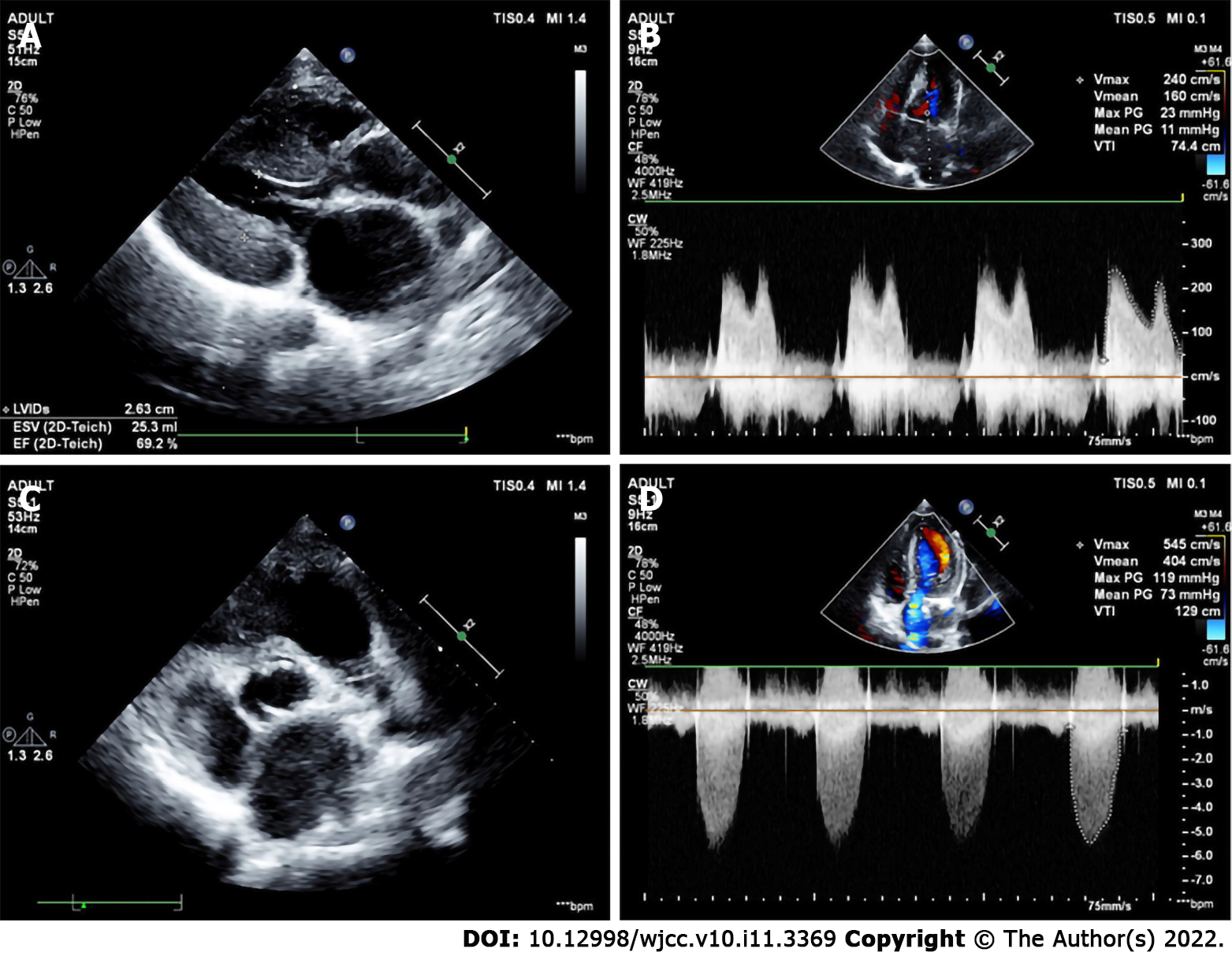
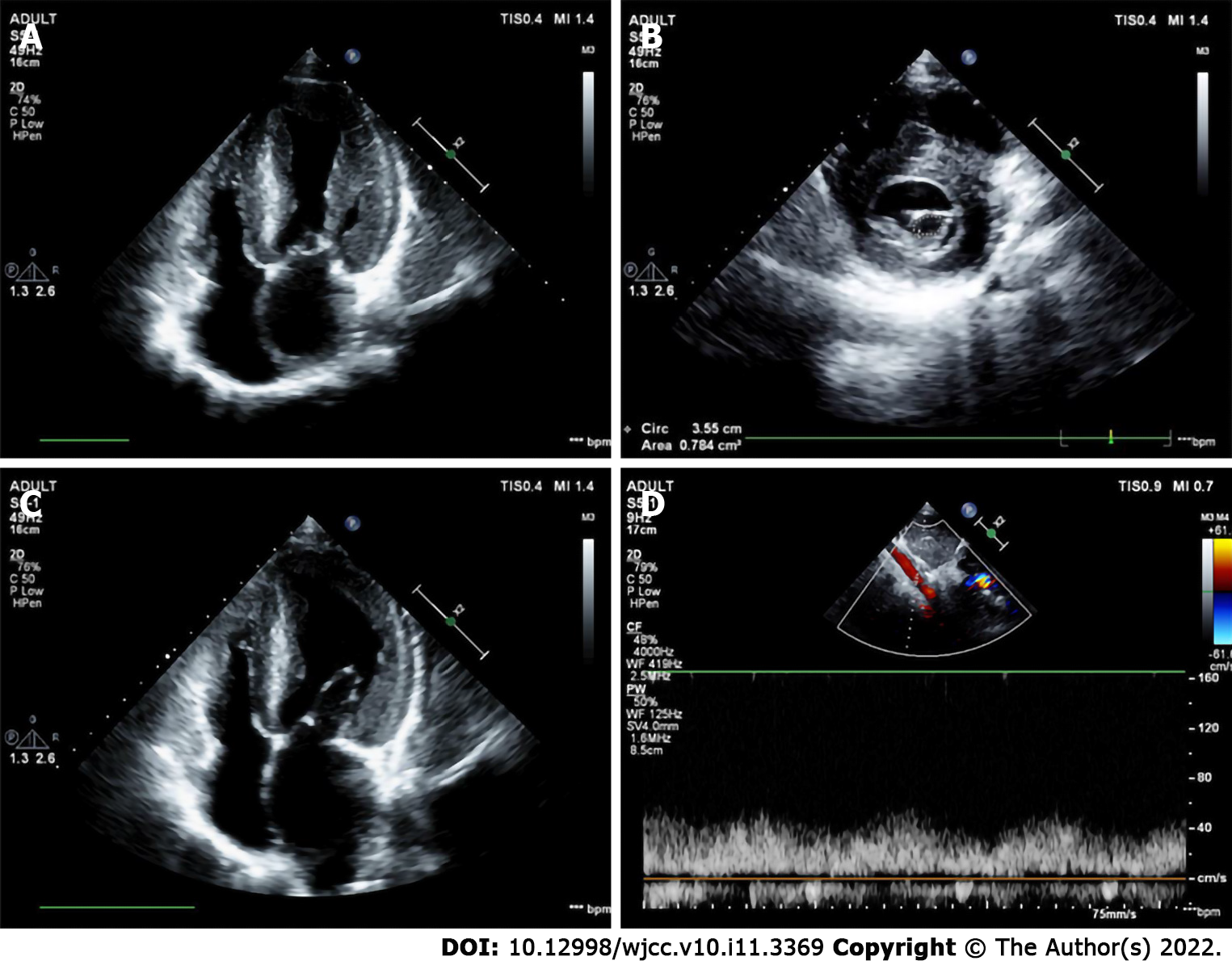
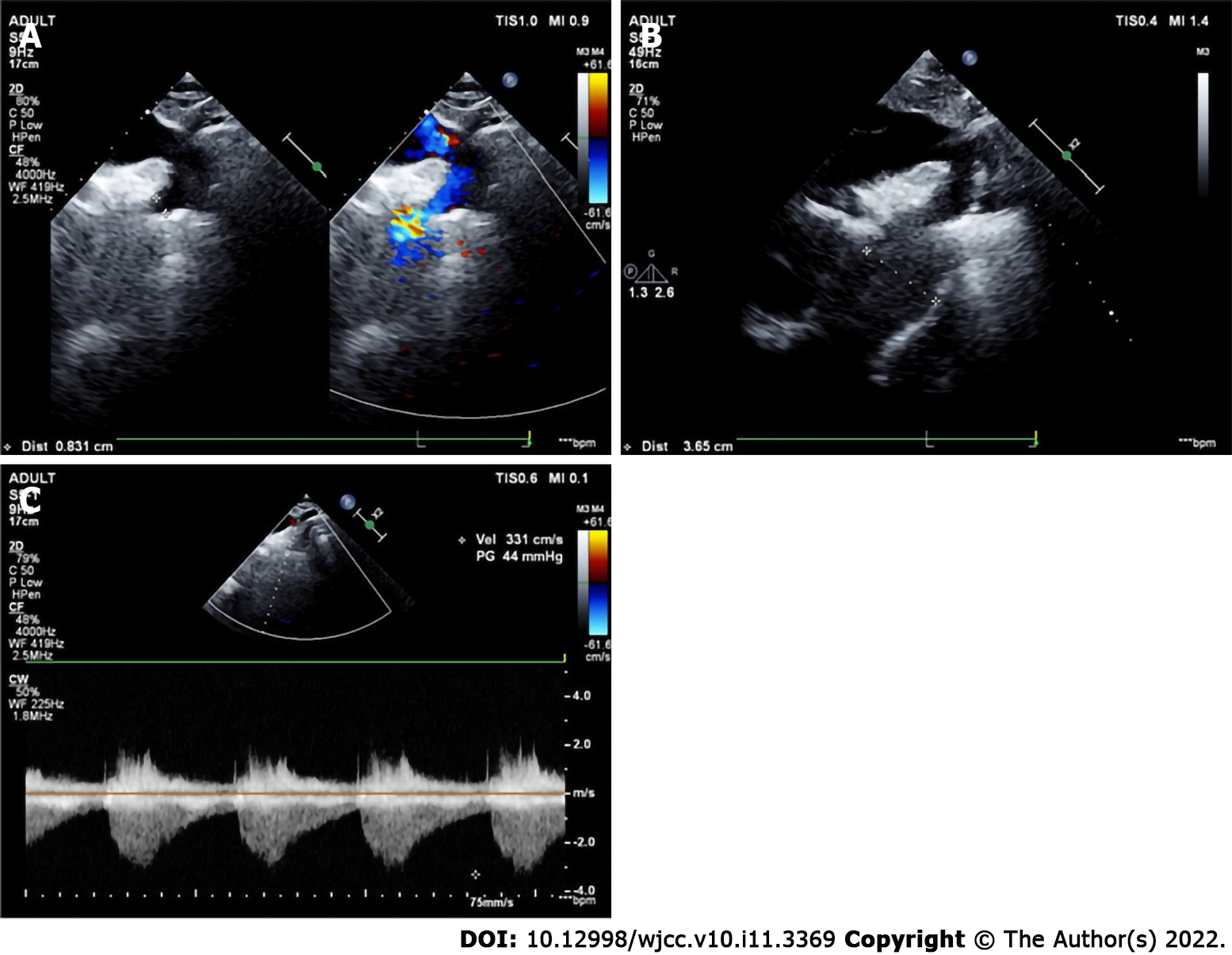
In 1963, Shone et al[29] reported the feature of Shone’s syndrome, which includes Annulo-Leaflet mitral ring (ALMR), parachute mitral valve (PMV), subaortic stenosis (subAS), and CoA. Shone’s syndrome is a rare form of congenital heart disease that consists of several heart defects, including ALMR, PMV, subAS, and CoA. The corresponding pathological changes are as follows: ALMR occurs in the septum of the region above the annulus of the mitral valve. PMV is a form of congenital mitral stenosis where the main pathological change is papillary muscle fusion, which involves mitral chordae tendineae attaching to a single dominant papillary muscle, leading to the inability of the mitral valve to fully open during ventricular diastole. There are two common types of subAS: (1) Limited subaortic stenosis includes fibromuscular septum inferior stenosis and septum inferior aortic stenosis, which is caused by 1.0 - 1.5 cm fibrous septums below the aortic valve; and (2) Diffuse subaortic stenosis is tubular stenosis caused by diffuse thickening of the outflow tract muscle in the left ventricle. Coarctation of the aorta is a local or diffuse narrowing of the aorta that results in reduced blood flow.
When only two or three of the abnormalities are present, Shone’s complex is diagnosed as the incomplete form. Delmo et al[30] believe that a mitral valve abnormality of the inflow tract is the main factor that affected surgical effects. Therefore, when there are outflow abnormalities complicated with ALMR or mitral stenosis, Shone’s complex can be diagnosed as the incomplete form.
Echocardiography was performed within one week before surgery. The ultrasonic examinations of all children were performed by a sonographer with more than 5 years of working experience.
In addition to providing unified technical training and consistent examination conditions, we employed skilled sonographers to minimize bias and strictly controlled objective indicators, thus facilitating diagnoses according to diagnostic criteria. The equipment included Philips IE33 and EPIQ 7C systems, with the S8-3 (3-8 MHz) and S5-1 (1-5 MHz) probes (Philips, Best, The Netherlands). If the children did not cooperate with the examination, a 0.5 mL/kg chloral hydrate solution was orally administrated for sedation. The children were in the horizontal or left lateral position, and echocardiography was performed in the order of subxiphoid, parasternal area, cardiac apex, and suprasternal fossa. The diagnosis was made using the three-segment method, paying special attention to the subxiphoid four-chamber view, parasternal left ventricular long-axis view, parasternal four-chamber view, parasternal left ventricular short-axis view, apical four/five-chamber view, and suprasternal fossa view.
The patients underwent repair surgeries according to different combinations of defects under general anesthesia, including mitral valvuloplasty, resection of supravalvular septum, patch angioplasty for CoA, and CoA resection and end-to-end anastomosis.
The patients were followed once a year at the outpatient clinic after surgery. Echocardiography was performed to observe the forward flow velocity and regurgitation of the mitral valve, forward flow velocity and regurgitation of the aortic valve, and the descending aortic flow velocity, and determine the presence or absence of postoperative re-obstruction (defined as descending aortic flow velocity of < 2 m/s).
Only descriptive statistics were used. Age was presented as median (range), and categorical variables were presented as prequencies and percentages.
The statistical methods of this study were reviewed by Ye-Dan Li, Kun-Jing Pang, Mu-Zi Li, Nan Xu, Hao Wang, Shou-Jun Li and Jun Yan from State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College.
The characteristics of the 66 patients are shown in Table 1. The patients were 2.7 (0.8-5.6) years of age, and 54.5% (36/66) were male. Twenty (30.3%) were born by cesarean section, and 10 (15.2%) had a history of heart surgery. The most common heart defect was an ALMR (50/66, 75.8%), followed by CoA (43/66, 65.2%). None of the patients showed signs of cyanosis, while only one patient displayed symptoms of dyspnea and left heart failure (1/66, 1.5%). The patients had a variety of combinations of defects (Table 1). Only two (3.0%) patients had all four defects. None of the patients had a family history of congenital heart disease.
| Characteristics | Median (range) / n (%) |
| Age (yr) | 2.7 (0.8-5.6) |
| Sex (male) | 36 (54.5%) |
| Cesarean section | 20 (30.3%) |
| History of heart surgery | 10 (15.2%) |
| Shone’s complex | |
| ALMR | 50 (75.8%) |
| CoA | 43 (65.2%) |
| subAS | 25 (37.9%) |
| PMV | 20 (30.3%) |
| Other defects | |
| PDA | 30 (45.5%) |
| VSD | 24 (36.4%) |
| MS | 23 (34.8%) |
| MR | 18 (27.3%) |
| BAV | 18 (27.3%) |
| AS | 13 (19.7%) |
| HAA | 11 (16.7%) |
| supraAS | 8 (12.1%) |
The preoperative echocardiographic findings were examined against the intraoperative findings. Echocardiography missed an ALMR in 31 patients (47.0%), a PMV in one patient (1.5%), subaortic stenosis in one patient (1.5%), and CoA in two patients (3.0%). Figures 1-4 present typical echocardiography images of Shone’s complex.
Shone’s complex is a rare syndrome characterized by congenital left heart defects that can differ among the patients. This retrospective study aims to examine the use of echocardiography in the diagnosis of Shone’s complex and to analyze the possible causes of missed diagnosis and misdiagnosis. The results suggest that echocardiography is an effective, non-invasive, and low-cost method to diagnose the heart defects of Shone’s complex. Due to this disease’s complexity and interindividual variability, missed diagnosis and misdiagnosis can occur. Combining the results of echocardiography, computed tomography, and/or magnetic resonance imaging might be helpful.
Some case reports examined the use of echocardiography in some patients[5,24-26], and small case series are available[12,27,28]. Ma et al[27] reported 38 patients with Shone’s complex that were evaluated by echocardiography. They reported a wide variety of combinations of defects among their patients, as in the present study, and concluded that echocardiography is important in the diagnosis of Shone’s complex, but they did not examine the misdiagnoses. Kumar et al[28] reported five patients with Shone’s complex and transesophageal echocardiographic evaluation and highlighted the usefulness of transesophageal echocardiography. Zucker et al[12] suggested that ultrasound is crucial to discriminate between Shone’s complex and hypoplastic left ventricle, influencing the physician’s management.
An innovation of the present study is the validation of the preoperative echographic findings with the intraoperative findings. A surprising result is that echocardiography missed an ALMR in 47.0% of the patients or 62.0% of the patients with an ALMR, while PMV (1.5% of the patients or 5.0% of the PMVs), subaortic stenosis (1.5% of the patients or 4.0% of the subaortic stenoses), and CoA (3.0% of the patients or 4.7% of the CoAs) were missed in smaller proportions of patients. Various reasons might be involved. The mitral ring is very small. Sometimes, only the ridge adhered to the mitral valve, or only to the anterior and posterior leaflets or annulus of the mitral valve, or did not adhere to the mitral valve but was very close to it. In these cases, it was difficult to identify an ALMR on echocardiography. The mitral valve leaflets can also be thickened and enhancing the echo, which can easily cover the supravalvular ring on the images. If the sonographer is inexperienced, the ALMR might be missed without further careful observation when PMV and mitral stenosis were found. Regarding PMV. If the left ventricular short-axis papillary muscle is not carefully checked, the anomaly of the papillary muscle can be missed. The flow velocity can be increased in the presence of mitral stenosis. If the sonographer considered the increase in flow velocity as a result of mitral stenosis, PMV might be missed due to not paying further attention. Subaortic stenosis is classified as the membranous type and fibromuscular type (isolated and diffuse stenosis). Usually, the manifestation of fibromuscular stenosis is obvious, and it cannot be missed. On the other hand, the membranous type is easy to be missed, because the subvalvular septum is sometimes very small, or the septum is close to the aortic valve. CoA can be classified as two types according to the different positions of the arterial duct: preductual and postductal. If the color Doppler and spectral Doppler images of the descending aortic arch on the suprasternal fossa view are not carefully observed, CoA can be missed. CoA patients are often accompanied by post-stenotic dilation of the descending aorta, which may suggest CoA. When children develop left ventricular wall hypertrophy or decreased left ventricular systolic function, the presence of CoA can be considered. In addition, many patients with anomalies of the bicuspid aortic valve have CoA and should be carefully screened.
Subaortic stenosis was misdiagnosed as aortic stenosis in one case. Because the subaortic septum is often very close to the aortic valve, subaortic stenosis caused by the subaortic septum is commonly mistaken for aortic stenosis. Therefore, it is easy to misdiagnose the condition if the clinician lacks experience or does not make careful observations.
In addition, 10 children in this study had a history of heart surgery, but misdiagnosis or missed diagnosis still occurred because previous surgical procedures were also planned based on the results of echocardiography. For example, if only a mitral valve defect was found and CoA was missed at that time, only the mitral valve was treated during surgery, and the CoA was still missed.
The prognosis of patients after surgery was as follows: one patient developed a third-degree atrioventricular block and had a permanent pacemaker installed. Another case had cyanosis and dyspnea and underwent mitral and tricuspid valve repair. One patient had severe mitral insufficiency in the early stage and received a mechanical mitral valve replacement three days after the operation. Besides, one case underwent ALMR resection nine years after the first operation. There were no instances of in-hospital deaths.
Patients were treated with torasemide tablets and potassium citrate granules after surgery. Surgical methods of inflow tract obstruction mainly included ALMR removal, chordae tendineae release, papillary muscle incision, and mitral valve replacement. Approaches for outflow tract obstruction primarily involved aortic coarctation resection, end-to-end anastomosis, subvalvular septum removal, etc. Among the 66 patients, seven underwent secondary surgery. Besides, there were four cases of complete Shone’s syndrome and 62 cases of incomplete Shone’s syndrome.
This study has limitations. Most previous studies are either case reports or small case series, and the present study is probably the largest series so far, with n = 66, but it is still a small series to draw firm conclusions. All patients were from a single center, and future studies should include multiple hospitals. Indeed, the disease is rare, and collective research efforts should be undertaken. Finally, the available data were limited to those available in the charts.
In conclusion, echocardiography is an effective, non-invasive, and low-cost method to diagnose the heart defects of Shone’s complex. Due to the complexity and interindividual variability of the syndrome, missed diagnosis and misdiagnosis may easily occur. Future studies should examine the combination of multiple imaging modalities, including echocardiography, computed tomography, and magnetic resonance imaging.
Shone’s complex is a rare syndrome characterized by congenital left heart defects that can differ among the patients.
To use echocardiography in the diagnosis of Shone’s complex and analyze the causes of missed diagnosis and misdiagnosis.
Sixty-six patients were included.
This was a retrospective study of patients who underwent echocardiography and repair surgery from February 14, 2008, to November 22, 2019. The patients were followed once a year at the outpatient clinic after surgery.
Sixty-six patients were included. The patients were 2.7 (0.8-5.6) years of age, and 54.5% were male. Ten (15.2%) had a history of heart surgery. The most common heart defect was the Annulo-Leaflet mitral ring (ALMR) (50/66, 75.8%), followed by coarctation of the aorta (CoA) (43/66, 65.2%).
Echocardiography is an effective method to diagnose the Shone’s complex. Due to this disease’s complexity and interindividual variability, Improving the understanding of the disease can reduce misdiagnosis and missed diagnosis.
This was a retrospective study with the largest sample size which aimed to examine the use of echocardiography in the diagnosis of Shone’s complex and to analyze the possible causes of missed diagnosis and misdiagnosis. Sixty-six patients were included. The preoperative echocardiographic findings were examined against the intraoperative findings. Echocardiography missed an ALMR in 31 patients, a parachute mitral valve in one patient, subaortic stenosis in one patient, and CoA in two patients. Due to this disease’s complexity and interindividual variability, echocardiography missed diagnosis can occur. Combining the results of echocardiography, computed tomography, magnetic resonance imaging might be helpful.
The authors thank all the medical workers at the Ultrasound Department of Fuwai Hospital, Chinese Academy of Medical Sciences, for their help in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and Cardiovascular Systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Gulel O, Turkey; Ong LT, Malaysia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Ahmed M, Aziz H, Jiang L. Severe aortic complications in a patient with variant Shone's complex and bicuspid aortic valve. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Bobylev D, Meschenmoser L, Boethig D, Horke A. Surgical repair of Shone's complex with anomalous origin of the left coronary artery arising from the right pulmonary artery. Interact Cardiovasc Thorac Surg. 2015;20:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Elmahrouk AF, Ismail MF, Arafat AA, Dohain AM, Helal AM, Hamouda TE, Galal M, Edrees AM, Al-Radi OO, Jamjoom AA. Outcomes of biventricular repair for shone's complex. J Card Surg. 2021;36:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Escárcega RO, Michelena HI, Bove AA. Bicuspid aortic valve: a neglected feature of Shone’s complex? Pediatr Cardiol. 2014;35:186-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Ganju NK, Kandoria A, Thakur S, Ganju SA. A Constellation of Cardiac Anomalies: Beyond Shone's Complex. Heart Views. 2016;17:23-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Ikemba CM, Eidem BW, Fraley JK, Eapen RS, Pignatelli R, Ayres NA, Bezold LI. Mitral valve morphology and morbidity/mortality in Shone's complex. Am J Cardiol. 2005;95:541-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Narvencar KP, Jaques e Costa AK, Patil VR. Shone's complex. J Assoc Physicians India. 2009;57:415-416. [PubMed] |
| 8. | Nicholson GT, Kelleman MS, De la Uz CM, Pignatelli RH, Ayres NA, Petit CJ. Late outcomes in children with Shone's complex: a single-centre, 20-year experience. Cardiol Young. 2017;27:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 9. | Pizzuto MF, Zampi JD. Left main coronary artery atresia in an infant with Shone's complex. Cardiol Young. 2016;26:991-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Yang LT, Foley TA, Eidem BW, Crestanello JA, Michelena HI. Double-orifice mitral valve associated and bicuspid aortic valve: forme fruste of Shone's complex? Eur Heart J Cardiovasc Imaging. 2020;21:118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Aslam S, Khairy P, Shohoudi A, Mercier LA, Dore A, Marcotte F, Miró J, Avila-Alonso P, Ibrahim R, Asgar A, Poirier N, Mongeon FP. Shone Complex: An Under-recognized Congenital Heart Disease With Substantial Morbidity in Adulthood. Can J Cardiol. 2017;33:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Zucker N, Levitas A, Zalzstein E. Prenatal diagnosis of Shone's syndrome: parental counseling and clinical outcome. Ultrasound Obstet Gynecol. 2004;24:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Bolling SF, Iannettoni MD, Dick M 2nd, Rosenthal A, Bove EL. Shone's anomaly: operative results and late outcome. Ann Thorac Surg. 1990;49:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 68] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Brauner RA, Laks H, Drinkwater DC Jr, Scholl F, McCaffery S. Multiple left heart obstructions (Shone's anomaly) with mitral valve involvement: long-term surgical outcome. Ann Thorac Surg. 1997;64:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Arntfield R, Pace J, Hewak M, Thompson D. Focused Transesophageal Echocardiography by Emergency Physicians is Feasible and Clinically Influential: Observational Results from a Novel Ultrasound Program. J Emerg Med. 2016;50:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Nazerian P, Vanni S, Castelli M, Morello F, Tozzetti C, Zagli G, Giannazzo G, Vergara R, Grifoni S. Diagnostic performance of emergency transthoracic focus cardiac ultrasound in suspected acute type A aortic dissection. Intern Emerg Med. 2014;9:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3897] [Cited by in RCA: 5493] [Article Influence: 549.3] [Reference Citation Analysis (0)] |
| 18. | Kennedy Hall M, Coffey EC, Herbst M, Liu R, Pare JR, Andrew Taylor R, Thomas S, Moore CL. The "5Es" of emergency physician-performed focused cardiac ultrasound: a protocol for rapid identification of effusion, ejection, equality, exit, and entrance. Acad Emerg Med. 2015;22:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Herbst MK, Velasquez J, O'Rourke MC. Cardiac Ultrasound. StatPearls. Treasure Island (FL), 2020. |
| 20. | Arntfield RT, Millington SJ. Point of care cardiac ultrasound applications in the emergency department and intensive care unit--a review. Curr Cardiol Rev. 2012;8:98-108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Simpson JM, van den Bosch A. EDUCATIONAL SERIES IN CONGENITAL HEART DISEASE: Three-dimensional echocardiography in congenital heart disease. Echo Res Pract. 2019;6:R75-R86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Alghamdi MH, Ismail MI, Yelbuz TM, Alhabshan F. Do We Need More Than a Transthoracic Echocardiography When Evaluating Children with Congenital Heart Disease before Cardiac Surgery? Congenit Heart Dis. 2016;11:262-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Moodie DS. Diagnosis and management of congenital heart disease in the adult. Cardiol Rev. 2001;9:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Popescu BA, Jurcut R, Serban M, Parascan L, Ginghina C. Shone's syndrome diagnosed with echocardiography and confirmed at pathology. Eur J Echocardiogr. 2008;9:865-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Shehatha JS, Taha AY, Mizra AJ. Late Shone complex: A case report and literature review. J Egypt Soc Cardio-Thoracic Surg. 2018;26:133-135. |
| 26. | Nkoke C, Lekoubou A, Yonta EW, Dzudie A, Kengne AP. Shone's anomaly: a report of one case in sub-Saharan Africa. Cardiovasc Diagn Ther. 2014;4:495-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Ma XJ, Huang GY, Liang XC, Liu XQ, Jia B. Atypical Shone's complex diagnosed by echocardiography. Pediatr Cardiol. 2011;32:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Kumar A, Bhat IH, Kumar B, T Shyam KS. Role of perioperative echocardiography in repair of incomplete shone complex: A case series. Ann Card Anaesth. 2019;22:444-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (2)] |
| 29. | SHONE JD, SELLERS RD, ANDERSON RC, ADAMS P Jr, LILLEHEI CW, EDWARDS JE. The developmental complex of "parachute mitral valve," supravalvular ring of left atrium, subaortic stenosis, and coarctation of aorta. Am J Cardiol. 1963;11:714-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 346] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 30. | Delmo Walter EM, Komoda T, Siniawski H, Miera O, Van Praagh R, Hetzer R. Long-term surgical outcome of mitral valve repair in infants and children with Shone's anomaly. Eur J Cardiothorac Surg. 2013;43:473-81; discussion 481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |