Published online Apr 6, 2022. doi: 10.12998/wjcc.v10.i10.3297
Peer-review started: November 15, 2021
First decision: January 11, 2022
Revised: January 19, 2022
Accepted: February 20, 2022
Article in press: February 20, 2022
Published online: April 6, 2022
Processing time: 133 Days and 22.7 Hours
Dedifferentiated chondrosarcoma (DDCS) accounts for 10% of all chondro
We report our experience with DDCS in the proximal phalanx of the left middle finger of an 87-year-old woman. She had undergone surgery for enchondroma, with curettage and artificial bone grafting, 11 years ago, in the same location. Several years after the primary surgery, the left middle finger gradually started to enlarge, and the growth speed increased in the past year. Plain radiographs showed an expansive osteolytic lesion with calcifications and residual grafting material. Owing to the suspicion of malignancy, we performed ray amputation. Histological findings revealed an abrupt transition between the low-grade chondrosarcoma and dedifferentiated sarcoma components. The dedifferentiated components showed the features of a high-grade undifferentiated pleomorphic sarcoma. The patient was diagnosed with DDCS arising from a preexisting enchondroma. She had no local recurrence or distant metastasis and died of pneumonia 6 years and 10 months after the second surgery.
The histological findings of a precursor lesion showed a typical enchondroma, suggesting that DDCS can arise from enchondroma.
Core Tip: Dedifferentiated chondrosarcoma (DDCS) is a very rare primary malignant bone tumor. Furthermore, malignant transformation of a single enchondroma of the hand bone into DDCS is considered extremely rare, with no detail of precursor pathological findings being reported previously. There have been no reports showing preexisting enchondromas prior to wide resection. Our report evaluated the histological findings of a precursor lesion, and the specimen showed a typical benign enchondroma, suggesting that DDCS can arise from enchondroma.
- Citation: Yonezawa H, Yamamoto N, Hayashi K, Takeuchi A, Miwa S, Igarashi K, Morinaga S, Asano Y, Saito S, Tome Y, Ikeda H, Nojima T, Tsuchiya H. Dedifferentiated chondrosarcoma of the middle finger arising from a solitary enchondroma: A case report. World J Clin Cases 2022; 10(10): 3297-3305
- URL: https://www.wjgnet.com/2307-8960/full/v10/i10/3297.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i10.3297
Primary chondrosarcoma is the third most common primary malignant tumor of the bone[1]. The most commonly involved sites are the femur and pelvis. In contrast, the small bones of the hands and feet, which mainly harbor enchondromas, are rarely involved in primary chondrosarcoma[2,3]. Dedifferentiated chondrosarcoma (DDCS), which was introduced by Dahlin and Beabout[4], refers to a high-grade sarcoma occurring next to a low-grade malignant cartilage-forming tumor, which generally resembles an enchondroma or low-grade conventional chondrosarcoma. The dedifferentiated portion of the lesion may comprise a wide variety of mesenchymal neoplasms, such as undifferentiated pleomorphic sarcomas, osteosarcomas, fibrosarcomas and rarely rhabdomyosarcomas[4,5]. Typically, histologic findings have a sharp demarcation between the low-grade cartilaginous and high-grade components[4]. It has the poorest outcome, with a 5-year survival rate reported between 7% and 25%[6-9]. Due to the rarity of the diagnosis, only a few extensive studies report the demographic data. In one of these large series, Grimer et al[6] reported that 233 (69.1%) out of 337 tumors involved long bones of the peripheral skeleton whilst 104 (30.9%) involved the axial skeleton (pelvis, rib, etc.).
DDCS arising in the small tubular bones of the hands is extremely rare. Furthermore, the histological findings of preexisting solitary enchondroma samples are important and valuable for diagnosing malignant transformations. Herein, we present a rare case of DDCS developing in the proximal phalanx of the left middle finger. In addition, this report shows a histological specimen of the preexisting enchondroma. To our knowledge, this is the first case report of the DDCS of the finger, with detailed histological findings of both enchondroma and DDCS.
An 87-year-old woman presented with pain and enlarged mass of left middle finger.
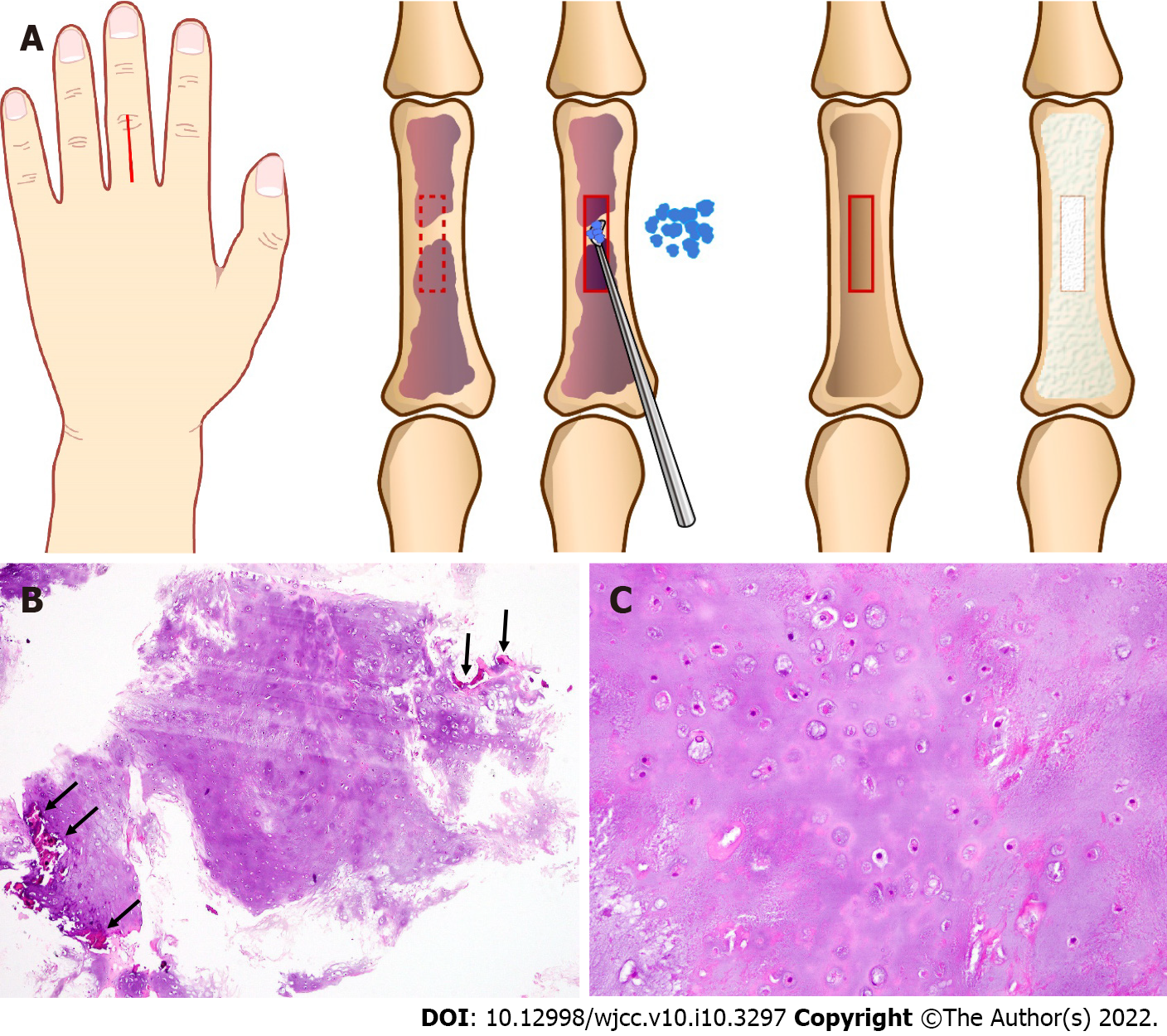
At 76 year old, the patient complained of finger pain due to a mallet finger. The radiographs incidentally revealed a typical enchondroma of the proximal phalanx of the left middle finger. To prevent pathological fracture, curettage was performed under axillary nerve block. The cortex adjacent to the lesion was approached dorsally, splitting the extensor tendon. After fenestration of the bone cortex, curettage and packing of the granular artificial bone graft made of hydroxyapatite were performed (Figure 1A). The histological examination revealed a hypocellular area, with an abundance of hyaline cartilage matrix, and the lesion was diagnosed as an enchondroma (Figure 1B and C). Several years postoperatively, the left middle finger gradually started to enlarge, and the growth speed increased in the past year.
She underwent an excision of uterine leiomyoma 30 years previously.
She had no significant personal or family history.
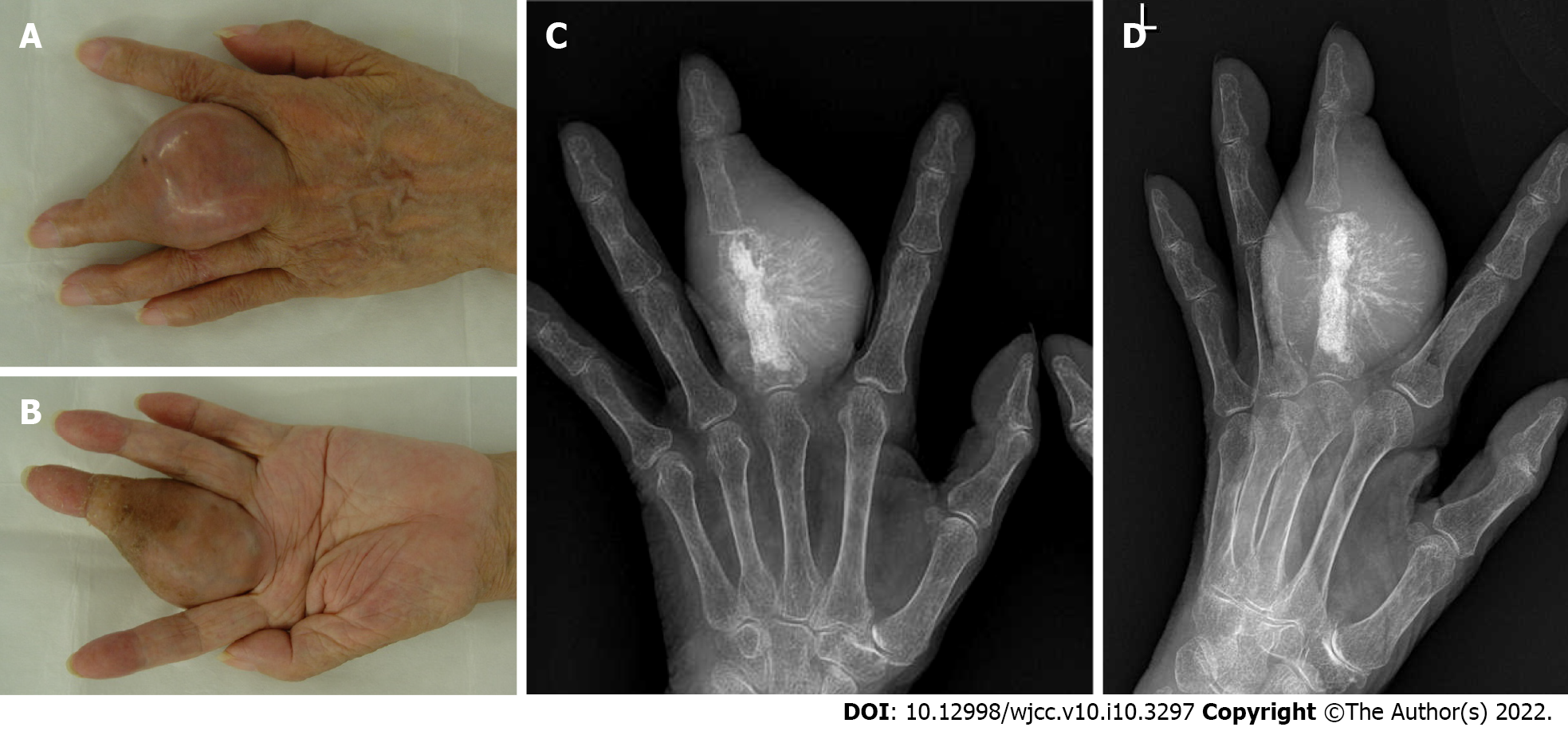
Her physical findings at our clinic were as follows: Height, 145 cm; weight, 42 kg; and body mass index, 20.0 kg/m2. The left middle finger appeared swollen and tense (Figure 2A and B). The size of the tumor was 5.5 cm × 5.5 cm × 4.5 cm, and it was elastic and hard.
Her laboratory test results were as follows: hemoglobin, 12.1 g/dL (normal range, 11.2-14.5); total leukocyte count, 6040/μL (normal range, 3300–8800); platelet count, 240000/μL (normal range, 130000-350000); erythrocyte sedimentation rate, 49.0 mm/hour (normal range, 0.0-15.0); C-reactive protein, 0.1 mg/dL (normal range, 0.0-0.14); lactate dehydrogenase, 152 IU/L (normal range, 119-229); and alkaline phosphatase (Japan Society of Clinical Chemistry; JSCC), 207 IU/L (normal range, 115-359).
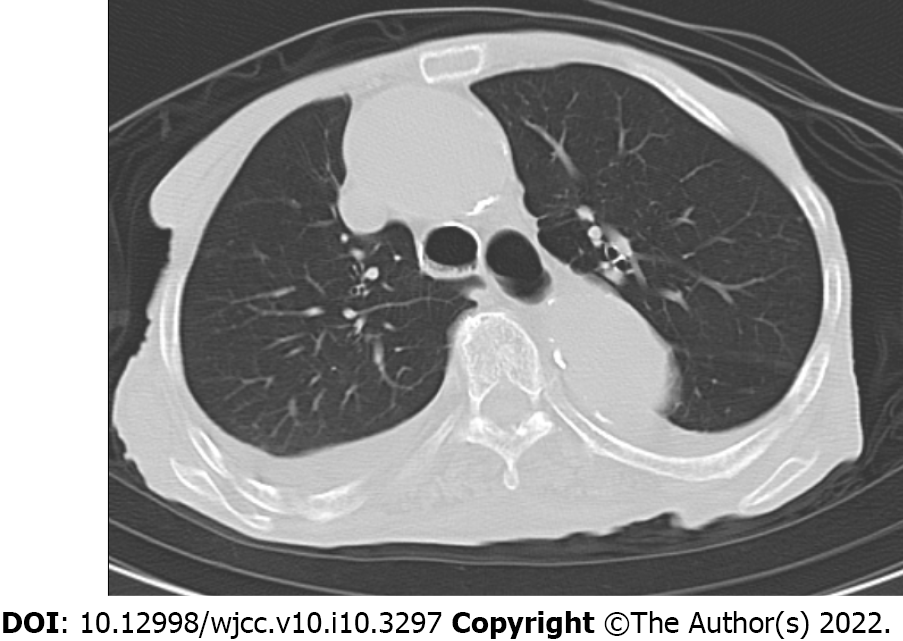
Chest computed tomography showed no lung metastases.
The diagnosis prior to operation was chondrosarcoma of middle finger arising from a preexisting enchondroma.
DDCS of middle finger arising from a preexisting enchondroma.
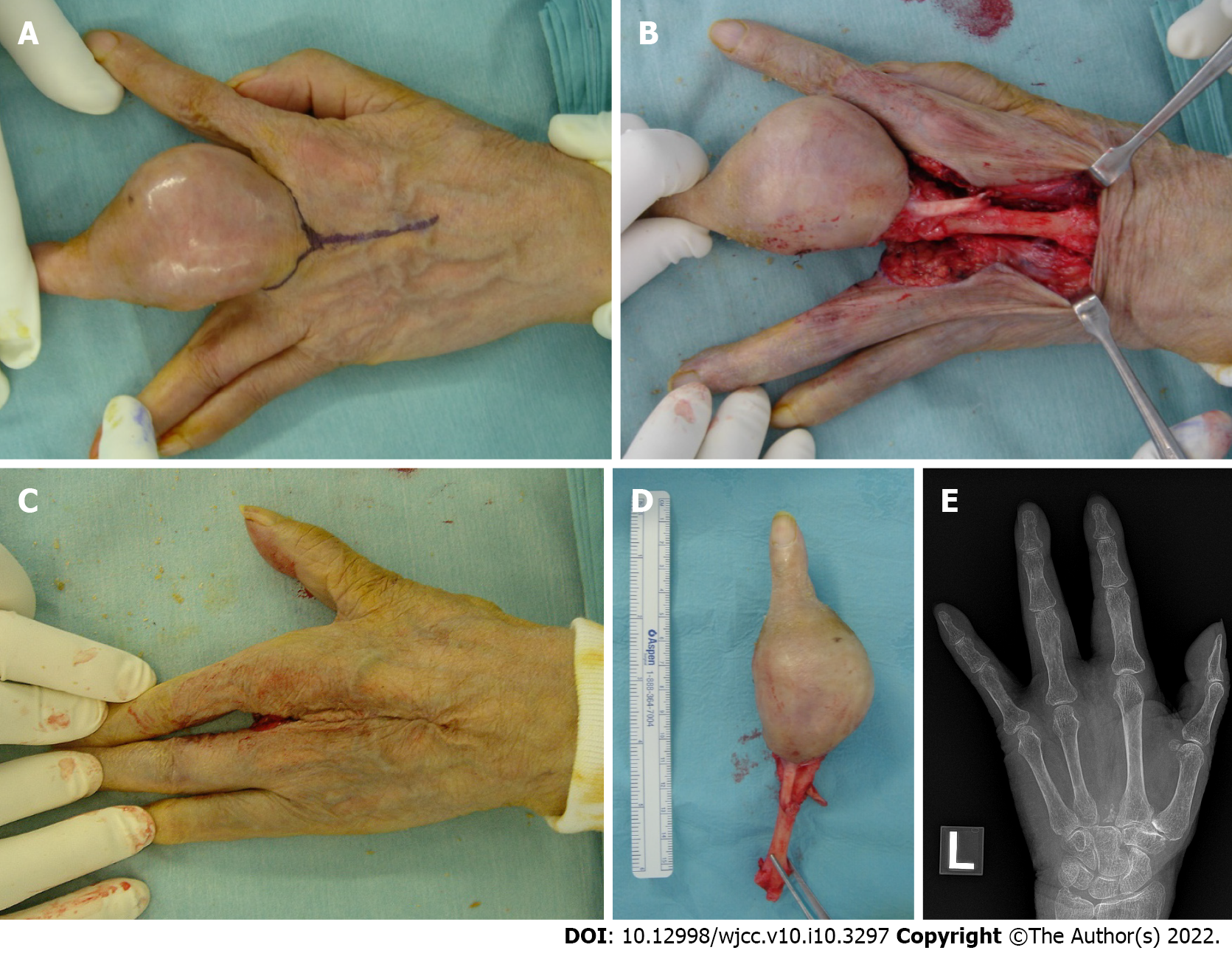
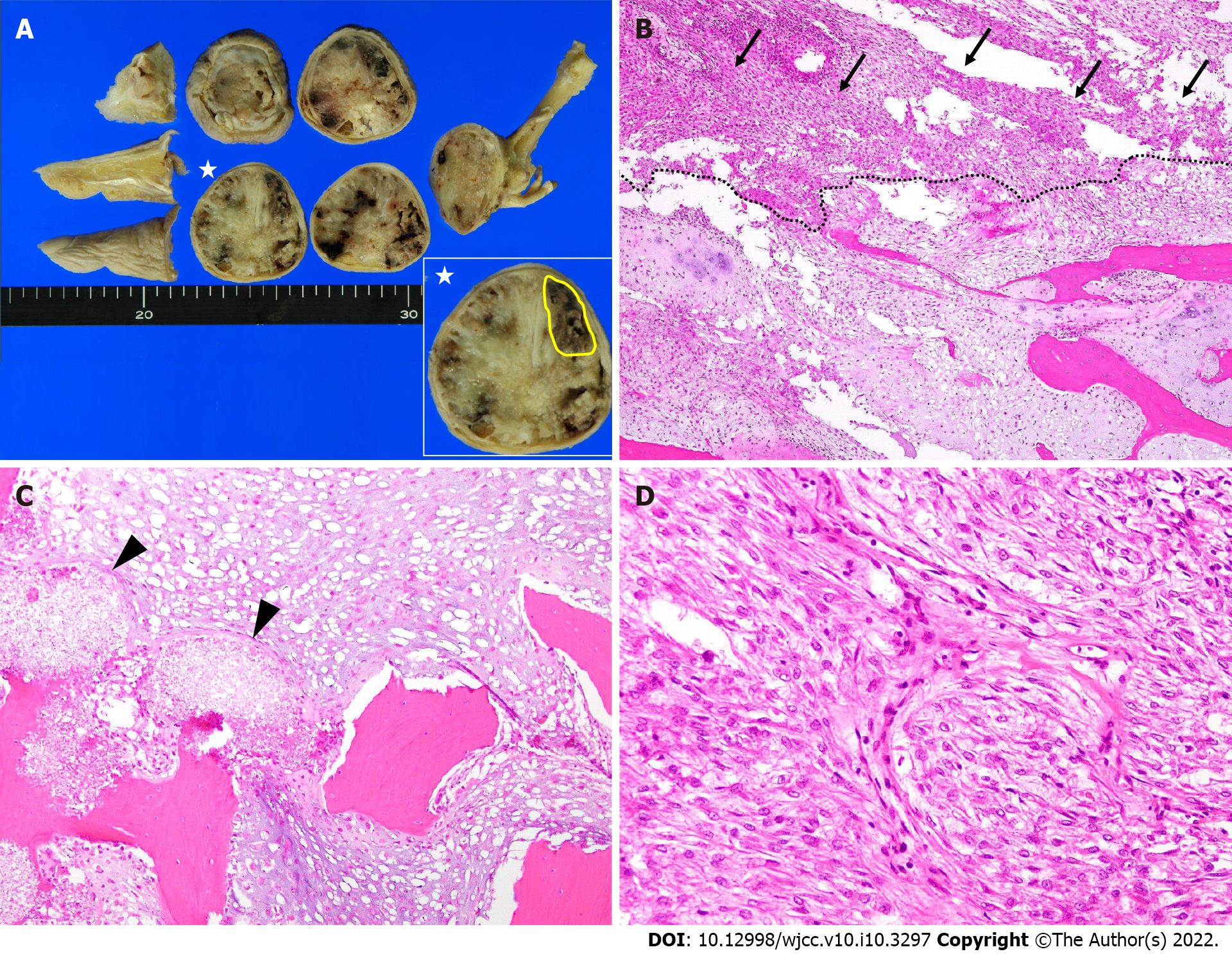
Conventional chondrosarcoma developing from a preexisting enchondroma was suspected, and ray amputation was performed, which involved excision of the entire middle metacarpal and careful soft tissue repair (Figure 3A-E). Removal of the metacarpal base created a good four-finger hand from both a functional and cosmetic viewpoint. The gross examination of the amputation specimen revealed a 4.5 cm × 4.3 cm × 5.5 cm tumor located mainly in the proximal phalanx and the tumor extended to the soft tissue below the skin. The cut surface of the intraosseous tumor had a chondroid-looking matrix with central calcified areas, consistent with the plain radiographic findings, while the other component was fleshy and tan-white (Figure 4A). The microscopic examination revealed that the tumor consisted of two morphologically distinct parts. A considerable part of the tumor showed chondroid areas with low-grade chondrosarcomatous morphology, and a small part of it had fibrous tumor cells showing nuclear pleomorphism, hyperchromatism and abnormal mitoses, consistent with those of high-grade undifferentiated pleomorphic sarcomas (Figure 4B). The cartilaginous portion of the tumor showed grade 1 or grade 2 chondrosarcoma with bone destruction. We also observed a residual hydroxyapatite artificial bone graft used 11 years ago (Figure 4C). The high-grade dedifferentiated area was composed of spindle cells arranged in a fascicular growth pattern. Cellularity was prominent and the tumor showed necrosis; however, no osteoid or bone matrix production was noted (Figure 4D). Immunohistochemically, the dedifferentiated portion was negative for the α-smooth muscle actin, desmin, CD34, S-100 protein, and epithelial membrane antigen. This high-grade dedifferentiated component accounted for 10.2% of the tumor in the maximum cross-section (Figure 4A). The surgical margins were negative. Postoperative pathology results and immunohistochemical results indicated DDCS of the middle finger arising from a preexisting enchondroma.
There was no local recurrence or distant metastasis. She did not undergo any additional chemotherapy or radiotherapy and died of pneumonia 6 years and 10 mo after the ray amputation (Figure 5).
DDCS is defined as a nonchondrogenic, high-grade sarcoma associated with a well-differentiated chondrosarcoma, with an abrupt transition[4]. Grimer et al[6] reported that the median age of these cases was 59 years (range: 15-89 years), and there was a slight predominance of men[6]. The average size of the tumor at diagnosis was 12.6 cm[6].
The most common location of DDCS is in the femur[6,10]. Grimer et al[6] reported that the tumor involved the femur in 154 patients (45.7%), pelvis in 95 (28.2%), humerus in 37 (11.0%), scapula in 18 (5.3%), and other locations in 33 (9.8%). In total, 233 tumors involved the long bones of the peripheral skeleton, while 104 involved the axial skeleton (pelvis, rib, etc.)[6]. Johnson et al[10] reported that the tumors were located in the femur in eight cases (30.8%), ilium in four (15.4%), humerus in three (11.6%), ischium in two (7.7%), acetabulum in two (7.7%), pubic ramus in two (7.7%), rib in two (7.7%), scapula in one (3.8%), fibula in one (3.8%), and tibia in one (3.8%). DDCS of the finger is extremely rare with only two case reports being available till date (Table 1). Doganavsargil et al[11] reported a case of DDCS developing in the proximal phalanx of the left thumb of a 66-year-old man. The high-grade component showed fibrosarcoma-like findings. The patient was treated with amputation and died of the disease within 9 months after the surgery. Liu et al[12] reported a case of DDCS of the right finger in a 32-year-old man. The high-grade component of this case was spindle cell sarcoma, and the patient was treated with amputation and alive 18 mo after the surgery[12]. In our case, radical amputation was performed with an adequate surgical margin, and there was no local recurrence or distant metastasis for over 6 years postoperatively.
| Author, yr | Sex | Age | Location | Symptoms | Treatment | Recurrence | Lung metastasis | Oncological outcome | Follow-up |
| Doganavsargil et al[11] 2009 | M | 66 | L thumb | Pain, swelling | Amputation | No | Yes | DOD | 9 mo |
| Liu et al[12] 2017 | M | 32 | R finger | Pathological fracture | Amputation | No | No | AWD | 18 mo |
| Present case | F | 87 | L middle finger | Pain, swelling | Amputation | No | No | DOC | 6 yr 10 mo |
Malignant transformation of a single enchondroma of the hand bone into a chondrosarcoma is considered extremely rare and unusual[2,3]. Müller et al[2] reported a case of malignant transformation of a benign enchondroma of the hand into a secondary chondrosarcoma. Culver et al[3] reported a chondrosarcoma of the hand arising from a preexisting solitary enchondroma. However, they only evaluated the amputated specimen and histological findings showed a secondary chondrosarcoma and enchondroma in the same specimen. No reports have shown preexisting enchondromas prior to wide resection. Our report evaluated the histological findings of a precursor lesion, and the specimen showed a typical benign enchondroma, suggesting that DDCS can arise from an enchondroma. Some authors have reported a specific mutation that can promote the development of DDCS. Grote et al[13] reported evidence that the p53 mutation may be regarded as at least a co-factor that “switched” the preexisting low-grade conventional chondrosarcoma into a highly malignant dedifferentiated tumor. The expression of membrane-type 1 matrix metalloproteinase also plays an important role in the morphological degradation of the cartilaginous matrix with an increasing histological grade[14]. Franchi et al[15] suggested that alterations of genes implicated in regulating the G1–S phase cell cycle checkpoint contribute to the process of dedifferentiation in peripheral secondary chondrosarcoma. Yang et al[16] reported that combining clonality analysis with isocitrate dehydrogenase 1 (IDH1) and IDH2 mutation detection revealed that cartilaginous and non-cartilaginous components of DDCS originate from the same primitive cells, which must have the potential to differentiate into cartilage.
An abrupt transition occurs between the conventional hyaline cartilage and high-grade sarcoma components of DDCS[4]. The cartilaginous portion can range from an enchondroma-like appearance to grade 1 or grade 2 chondrosarcoma[17]. The high-grade dedifferentiated component generally has features of mesenchymal neoplasms, such as undifferentiated pleomorphic sarcoma, osteosarcoma, fibrosarcoma and rarely rhabdomyosarcoma[4,5]. In our case, the high-grade component showed undifferentiated pleomorphic sarcoma-like findings.
Patients with DDCS have a dismal prognosis, most often as a result of widespread lung metastases[17]. Overall 5-year survival rates of 7%-25% have been reported[6-9]. DDCS appears to be resistant to the currently available chemotherapy. The best possibility of cure is linked to the excision of the tumor with clear surgical margins[6]. Poor prognostic factors include a tumor size > 8 cm, the presence of a pathological fracture, presence of a metastatic disease at diagnosis, pelvic location, inadequate surgical margin, and non-surgical treatment[6,9,17,18]. In our case, the tumor was resected widely, and the patient had a good prognosis. The patients reported by Sissons et al[19] and Levin et al[20] also had a good prognosis and survived for 8.5 and 4.5 years respectively, after the initial surgery. This suggests the possibility of a more favorable prognosis for this variant of tumor compared to conventional DDCS[19,21].
Our report evaluated the histological findings of a precursor lesion, and the specimen showed a typical enchondroma, suggesting that DDCS can arise from enchondroma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Orthopedics
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang L, Yao W S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Murphey MD, Walker EA, Wilson AJ, Kransdorf MJ, Temple HT, Gannon FH. From the archives of the AFIP: imaging of primary chondrosarcoma: radiologic-pathologic correlation. Radiographics. 2003;23:1245-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 310] [Article Influence: 14.1] [Reference Citation Analysis (1)] |
| 2. | Müller PE, Dürr HR, Nerlich A, Pellengahr C, Maier M, Jansson V. Malignant transformation of a benign enchondroma of the hand to secondary chondrosarcoma with isolated pulmonary metastasis. Acta Chir Belg. 2004;104:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Culver JE Jr, Sweet DE, McCue FC. Chondrosarcoma of the hand arising from a pre-existent benign solitary enchondroma. Clin Orthop Relat Res. 1975;128-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Dahlin DC, Beabout JW. Dedifferentiation of low-grade chondrosarcomas. Cancer. 1971;28:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Bovée JV, Cleton-Jansen AM, Rosenberg C, Taminiau AH, Cornelisse CJ, Hogendoorn PC. Molecular genetic characterization of both components of a dedifferentiated chondrosarcoma, with implications for its histogenesis. J Pathol. 1999;189:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Grimer RJ, Gosheger G, Taminiau A, Biau D, Matejovsky Z, Kollender Y, San-Julian M, Gherlinzoni F, Ferrari C. Dedifferentiated chondrosarcoma: prognostic factors and outcome from a European group. Eur J Cancer. 2007;43:2060-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Frassica FJ, Unni KK, Beabout JW, Sim FH. Dedifferentiated chondrosarcoma. A report of the clinicopathological features and treatment of seventy-eight cases. J Bone Joint Surg Am. 1986;68:1197-1205. [PubMed] |
| 8. | Amer KM, Munn M, Congiusta D, Abraham JA, Basu Mallick A. Survival and Prognosis of Chondrosarcoma Subtypes: SEER Database Analysis. J Orthop Res. 2020;38:311-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Lex JR, Evans S, Stevenson JD, Parry M, Jeys LM, Grimer RJ. Dedifferentiated chondrosarcoma of the pelvis: clinical outcomes and current treatment. Clin Sarcoma Res. 2018;8:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Johnson S, Têtu B, Ayala AG, Chawla SP. Chondrosarcoma with additional mesenchymal component (dedifferentiated chondrosarcoma). I. A clinicopathologic study of 26 cases. Cancer. 1986;58:278-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Doganavsargil B, Argin M, Sezak M, Sabah D, Sarsik B, Omur O, Oztop F. Dedifferentiated chondrosarcoma of the thumb: a case report. Arch Orthop Trauma Surg. 2009;129:161-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Liu C, Xi Y, Li M, Jiao Q, Zhang H, Yang Q, Yao W. Dedifferentiated chondrosarcoma: Radiological features, prognostic factors and survival statistics in 23 patients. PLoS One. 2017;12:e0173665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Grote HJ, Schneider-Stock R, Neumann W, Roessner A. Mutation of p53 with loss of heterozygosity in the osteosarcomatous component of a dedifferentiated chondrosarcoma. Virchows Arch. 2000;436:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Sakamoto A, Oda Y, Iwamoto Y, Tsuneyoshi M. Expression of membrane type 1 matrix metalloproteinase, matrix metalloproteinase 2 and tissue inhibitor of metalloproteinase 2 in human cartilaginous tumors with special emphasis on mesenchymal and dedifferentiated chondrosarcoma. J Cancer Res Clin Oncol. 1999;125:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Franchi A, Baroni G, Sardi I, Giunti L, Capanna R, Campanacci D. Dedifferentiated peripheral chondrosarcoma: a clinicopathologic, immunohistochemical, and molecular analysis of four cases. Virchows Arch. 2012;460:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Yang T, Bai Y, Chen J, Sun K, Luo Y, Huang W, Zhang H. Clonality analysis and IDH1 and IDH2 mutation detection in both components of dedifferentiated chondrosarcoma, implicated its monoclonal origin. J Bone Oncol. 2020;22:100293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Inwards CY, Bloem JL, Hogendoorn PCW. Dedifferentiated chondrosarcoma. In: WHO Classification of Tumors Editorial Board. WHO classification of tumors: Soft tissue and bone tumors. 5th ed. Lyon: IARC Press, 2020: 112–113. [DOI] [Full Text] |
| 18. | Strotman PK, Reif TJ, Kliethermes SA, Sandhu JK, Nystrom LM. Dedifferentiated chondrosarcoma: A survival analysis of 159 cases from the SEER database (2001-2011). J Surg Oncol. 2017;116:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 19. | Sissons HA, Matlen JA, Lewis MM. Dedifferentiated chondrosarcoma. Report of an unusual case. J Bone Joint Surg Am. 1991;73:294-300. [PubMed] |
| 20. | Levin A, Morris CD. Acral Dedifferentiated Chondrosarcoma of a Toe Phalanx: A Case Report. JBJS Case Connect. 2014;4:e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Ishida T, Dorfman HD, Habermann ET. Dedifferentiated chondrosarcoma of humerus with giant cell tumor-like features. Skeletal Radiol. 1995;24:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |