Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.79
Peer-review started: April 21, 2021
First decision: June 13, 2021
Revised: June 25, 2021
Accepted: November 23, 2021
Article in press: November 23, 2021
Published online: January 7, 2022
Processing time: 252 Days and 21.9 Hours
Transient receptor potential vanilloid-1 (TRPV1), a nonselective cation channel, is activated by capsaicin, a pungent ingredient of hot pepper. Previous studies have suggested a link between obesity and capsaicin-associated pathways, and activation of TRPV1 may provide an alternative approach for obesity treatment. However, data on the TRPV1 distribution in human gastric mucosa are limited, and the degree of TRPV1 distribution in the gastric and duodenal mucosal cells of obese people in comparison with normal-weight individuals is unknown.
To clarify gastric and duodenal mucosal expression of TRPV1 in humans and compare TRPV1 expression in obese and healthy individuals.
Forty-six patients with a body mass index (BMI) of > 40 kg/m2 and 20 patients with a BMI between 18-25 kg/m2 were included. Simultaneous biopsies from the fundus, antrum, and duodenum tissues were obtained from subjects between the ages of 18 and 65 who underwent esophagogastroduodenoscopy. Age, sex, history of alcohol and cigarette consumption, and past medical history regarding chronic diseases and medications were accessed from patient charts and were analyzed accordingly. Evaluation with anti-TRPV1 antibody was performed separately according to cell types in the fundus, antrum, and duodenum tissues using an immunoreactivity score. Data were analyzed using SPSS 17.0.
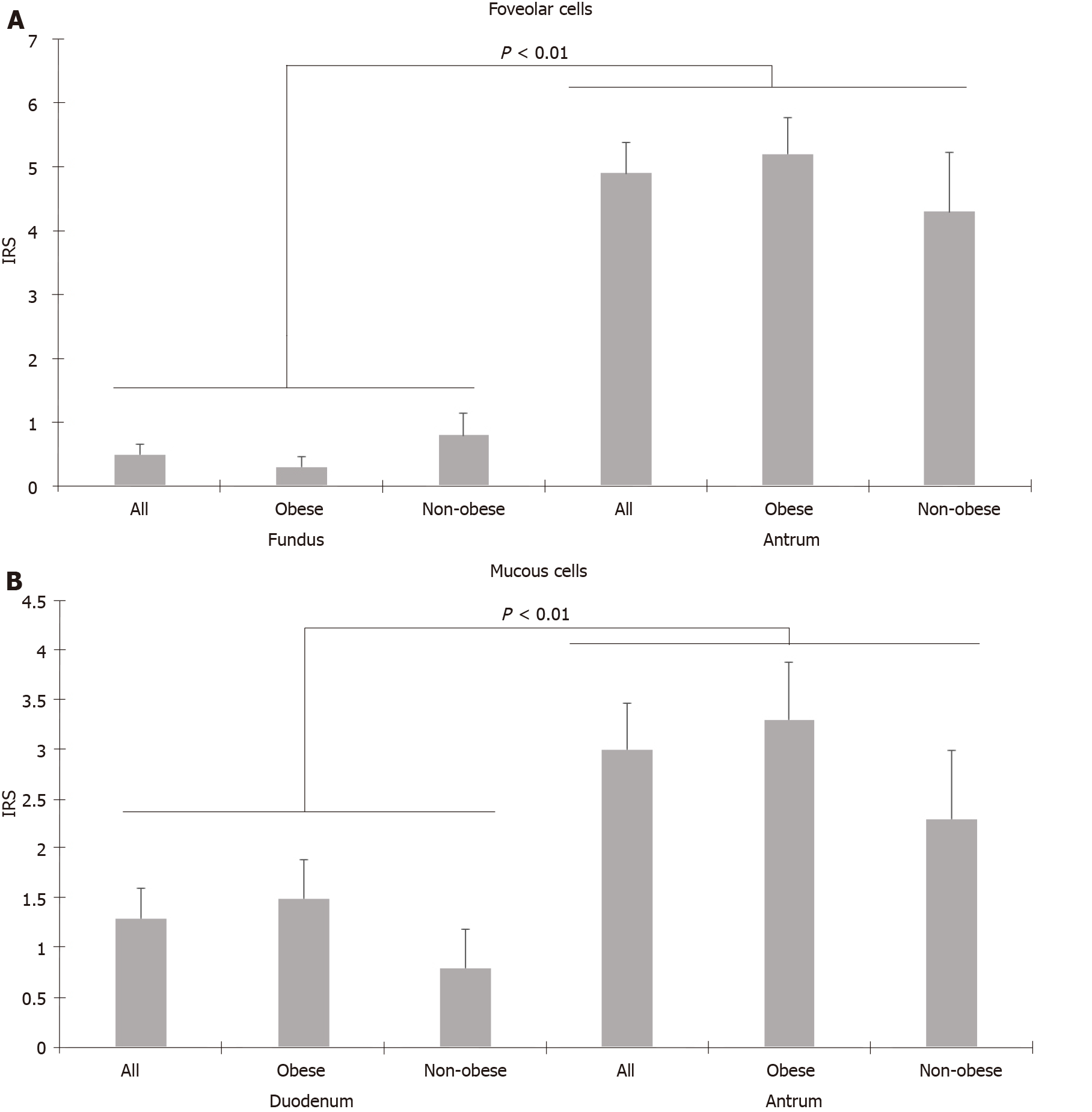
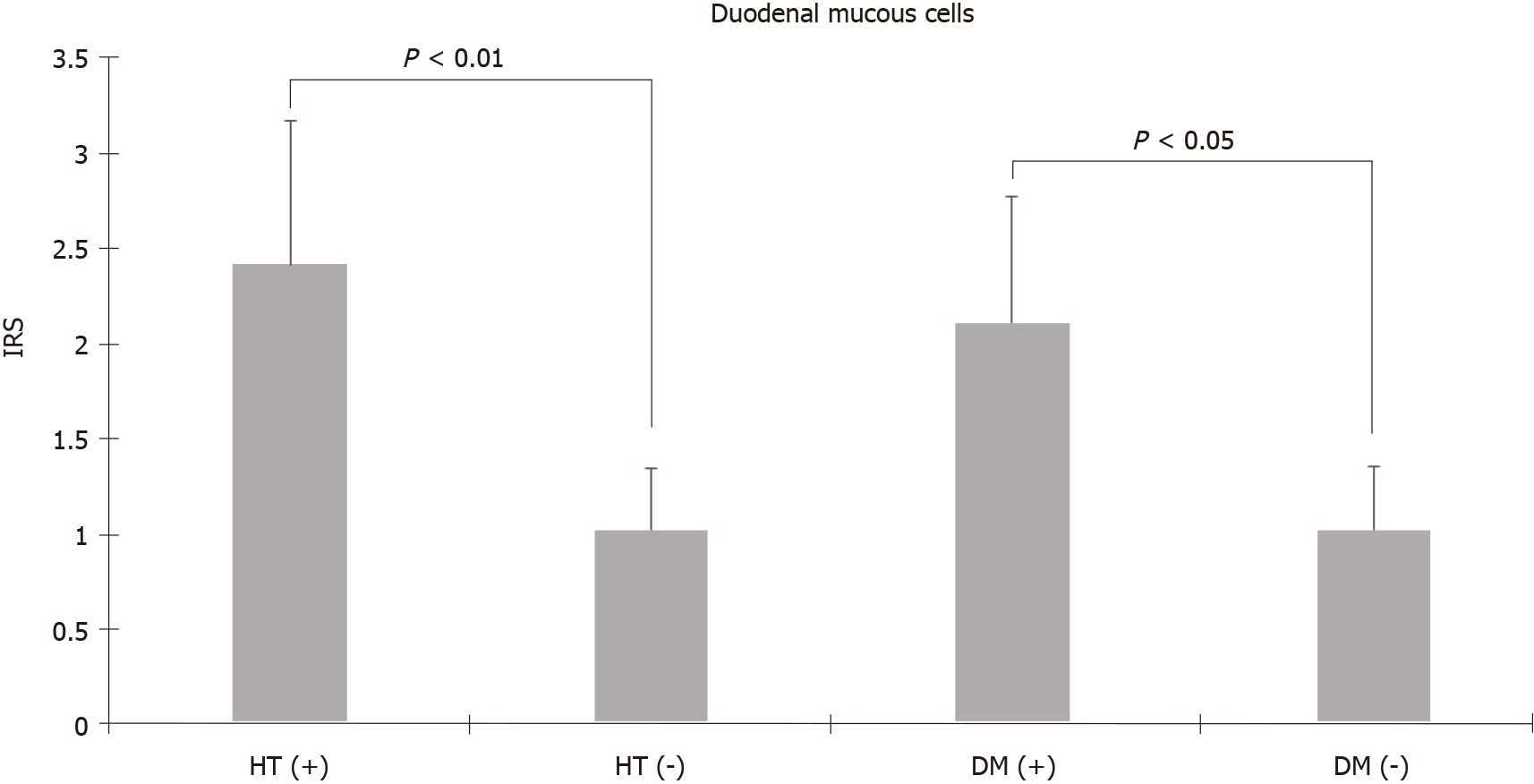
TRPV1 expression was higher in the stomach than in the duodenum and was predominantly found in parietal and chief cells of the fundus and mucous and foveolar cells of the antrum. Unlike foveolar cells in the antrum, TRPV1 was relatively low in foveolar cells in the fundus (4.92 ± 0.49 vs 0.48 ± 0.16, P < 0.01, Mann-Whitney U test). Additionally, the mucous cells in the duodenum also had low levels of TRPV1 compared to mucous cells in the antrum (1.33 ± 0.31 vs 2.95 ± 0.46, P < 0.01, Mann-Whitney U test). TRPV1 expression levels of different cell types in the fundus, antrum, and duodenum tissues of the morbidly obese group were similar to those of the control group. Staining with TRPV1 in fundus chief cells and antrum and duodenum mucous cells was higher in patients aged ≥ 45 years than in patients < 45 years (3.03 ± 0.42, 4.37 ± 0.76, 2.28 ± 0.55 vs 1.9 ± 0.46, 1.58 ± 0.44, 0.37 ± 0.18, P = 0.03, P < 0.01, P < 0.01, respectively, Mann-Whitney U test). The mean staining levels of TRPV1 in duodenal mucous cells in patients with diabetes and hypertension were higher than those in patients without diabetes and hypertension (diabetes: 2.11 ± 0.67 vs 1.02 ± 0.34, P = 0.04; hypertension: 2.42 ± 0.75 vs 1.02 ± 0.33, P < 0.01 Mann-Whitney U test).
The expression of TRPV1 is unchanged in the gastroduodenal mucosa of morbidly obese patients demonstrating that drugs targeting TRPV1 may be effective in these patients.
Core Tip: Capsaicin-associated pathways may provide an alternative approach for obesity treatment. Our results suggest that transient receptor potential vanilloid (TRPV1) activation can provide anti-obesity and gastroprotective activity. TRPV1 distribution in the gastric and duodenal mucosal cells of morbidly obese people is similar to that in patients with normal weight. Hence, drugs targeting TRPV1 may be an effective approach in these patients. Further studies are needed in this area.
- Citation: Atas U, Erin N, Tazegul G, Elpek GO, Yıldırım B. Distribution of transient receptor potential vanilloid-1 channels in gastrointestinal tract of patients with morbid obesity. World J Clin Cases 2022; 10(1): 79-90
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/79.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.79
Obesity is an important public health problem that leads to chronic diseases, increasing mortality and morbidity[1]. Along with the increase in obesity, which is associated with metabolic dysfunctions and chronic inflammation, there has also been an increase in obesity-related diseases such as type 2 diabetes, hypertension, cardiovascular and cerebrovascular diseases, asthma, some cancers, and psychological problems[2,3]. Preventing obesity or ensuring weight loss in patients will reduce these health problems[4,5]. However, the efficacy of behavioral, pharmacological, and surgical approaches in morbidly obese individuals is limited due to problems with implementation and severe side effects of drugs[6-8]. Surgical approaches are invasive and expensive, and long-term effects are unknown[9,11]. Due to the inadequacy of existing treatment methods in most cases and the fact that many surgical methods can cause serious complications, an effective, safe, widely available, and easily usable alternative anti-obesity treatment option is necessary for the prevention and treatment of obesity.
Capsaicin, which is found in pepper and causes a bitter taste, has recently been shown to be an anti-obese agent in animal studies, with few studies conducted in humans, and data on obese and morbidly obese patients are limited[12]. Transient receptor potential vanilloid-1 (TRPV1), a nonselective cation channel, is mainly found in neuronal tissue and has also been shown to be present in adipose tissue and skeletal muscles and mediates the effect of capsaicin on adipose tissue[13-16]. Previous rodent studies have shown that approximately 80% of capsaicin is passively absorbed in the gastrointestinal and upper small intestinal segments, and dietary capsaicin con
In mice, TRPV1 expression was shown in the nerve endings of the gastric mucosa, submucosa, and muscle layer[25-27]. Data on the TRPV1 distribution in human gastric mucosa are limited: TRPV1 has been previously shown in gastric parietal cells and antral glandular cells, and it was shown to affect gastrin levels[28,29]. However, there are no data on the TRPV1 distribution in the gastric and duodenal mucosal cells of obese people in comparison with normal-weight individuals. Previous studies have suggested a link between obesity and capsaicin-associated pathways, and activation of TRPV1 may provide an alternative approach for obesity treatment. It is also unknown whether the expression of TRPV1 changes in the upper GIT of obese and nonobese individuals and which cells express TRPV1 in the gastric and duodenal mucosal cells of humans. Determination of these factors can guide the creation of treatment approaches targeting TRPV1. Therefore, in this study, we aimed to clarify the gastric and duodenal mucosal expression of TRPV1 in humans and compare the TRPV1 expression profiles of obese and healthy individuals.
This retrospective case-control study was conducted per the Declaration of Helsinki. Approval for this study was obtained from the Akdeniz University Faculty of Medicine Clinical Research Ethics Committee (Date: 07/09/2016; Approval number: 2016/486). All patients and healthy volunteers provided written informed consent for participation in the study.
Simultaneous biopsies from the fundus, antrum, and duodenum tissues were obtained from subjects between the ages of 18 and 65 who underwent esophagogastroduodenoscopy. For the study group, the inclusion criteria were a body mass index (BMI) of > 40 kg/m2, and for the control group, patients with a BMI between 18-25 kg/m2 were eligible to be included. The exclusion criteria were as follows: being treated for an indication of upper GIT bleeding, active peptic ulcer, active inflammatory/infectious condition during the procedure, a history of malignancy, and a history of upper GIT surgery. Forty-six patients with a BMI of > 40 kg/m2 and 20 patients with a BMI between 18-25 kg/m2 referred for any indication of esophagogastroduodenoscopy were included in our study.
Age, sex, history of alcohol and cigarette consumption, and past medical history regarding chronic diseases and medications were accessed from patient charts and were analyzed accordingly.
Samples from fundus, antrum, and duodenum tissues of each case were prepared on a 4-micron-thick positively charged single slide for immunohistochemical staining. These tissue preparations were partially deparaffinized and placed in the device. Per the protocol, rabbit polyclonal anti-VR1 antibody (Abcam Cat No: AB111973) was used to stain the samples.
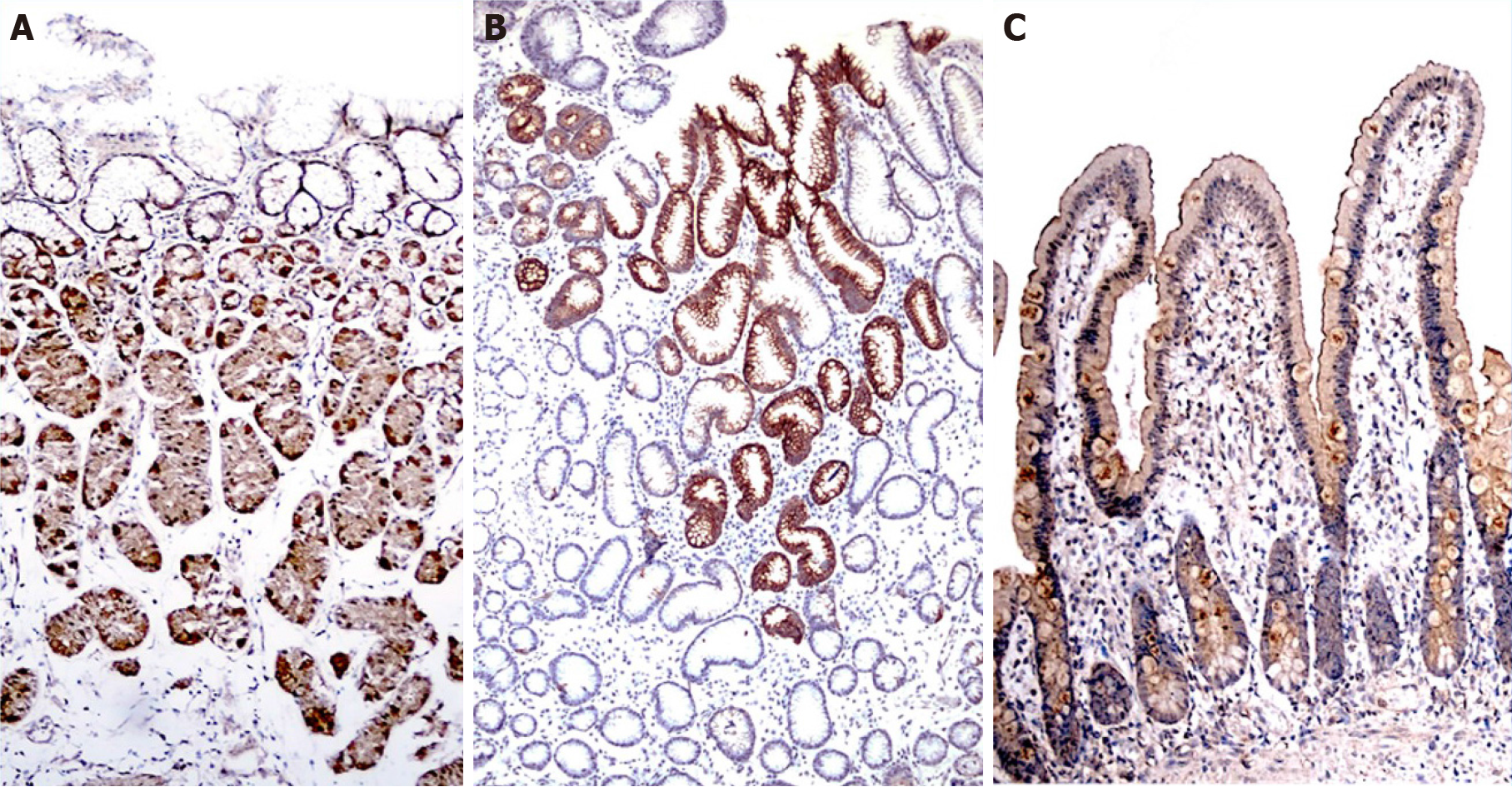
Evaluation with anti-TRPV1 antibody was performed separately according to cell types in the fundus, antrum, and duodenum tissues. The parietal, chief, and foveolar cells in the fundus; mucous cells in the antrum; and foveolar, mucous, goblet and absorbent cells in the duodenum of the cases were evaluated separately in terms of staining intensity and staining percentages with TRPV1. Staining density was evaluated on a 4-stage (0: negative, 1: weak, 2: moderate, and 3: strong staining intensity) immunoreactivity scale. The staining area was also graded as a percentage scale (0: no stained area; 1: below 25%; 2: between 25%-49%; 3: between 50%-74%; and 4: above 75%). Median staining was approximately 80% in the whole study group; therefore, 80% and below was considered limited staining, and 80% and above was considered widespread staining. The immunoreactivity score (IRS) was calculated as the staining scale (0-3) times the percentage scale (0-4) and was included in the analysis.
The data were analyzed in the SPSS 17.0 package program. Continuous variables were evaluated by Shapiro-Wilk tests for normal distribution. Nominal and ordinal data are expressed as frequencies (percentages), and continuous variables are expressed as the mean ± SE. Significant differences between the data were analyzed using the Mann-Whitney U test for independent variables and the Wilcoxon signed rank test for dependent variables. The threshold P value was accepted as 0.05 for statistical significance.
In this study, we included a total of 66 cases, specifically 46 cases of morbidly obese patients and 20 control cases. The mean age of the morbidly obese group was 45.2 ± 11.9, and that of the control group was 40.4 ± 13.0. Thirty-eight cases (82.6%) were female and 8 (17.4%) were male in the morbidly obese group, whereas 14 (70%) cases were female and 6 (30%) were male in the control group. The age and sex distributions in both groups were similar. Nineteen patients in the morbidly obese group had diabetes mellitus, 15 patients had hypertension, eight patients had hyperlipidemia, and three patients had coronary artery disease. There were no cases with these comorbidities in the control group. The proportions of patients using proton pump inhibitors and consuming alcohol and cigarettes were similar in both groups.
The TRPV1 staining levels of each cell type were determined using the IRS. TRPV1 Levels in fundus, antrum, and duodenum tissues of the morbidly obese patients were similar to those of the control group (Tables 1-3, Figure 1). However, when the staining of the cells was compared with other tissue sites in terms of staining properties, the levels of TRPV1 in antrum and fundus cells in the stomach were higher than those in duodenal cells. Both the intensity and prevalence of TRPV1 were higher in parietal and chief cells in the fundus, foveolar and mucous cells in the antrum, and absorbent cells in the duodenum. Compared to other cells in the stomach, the type of cell with the least TRPV1 staining was the foveolar cells in the fundus.
| Fundus | Obese | Control | All cases | P value | ||
| Parietal | No staining | 6 (13.0) | 7 (35) | 13 (19.6) | ||
| Distribution | Limited | 8 (17.3) | 3 (15) | 11 (16.6) | ||
| Widespread | 32 (69.5) | 10 (50) | 42 (63.6) | |||
| Intensity | Poor | 33 (71.7) | 11 (55) | 44 (66.6) | ||
| Strong | 7 (15.2) | 2 (10) | 9 (13.6) | |||
| IRS ± SE | 5.42 ± 0.49a,c | 4.84 ± 0.93a,c | 5.25 ± 0.44a,c | 0.60 | ||
| Chief | No staining | 15 (32.6) | 11 (55) | 26 (39.3) | ||
| Distribution | Limited | 9 (19.5) | 6 (30) | 15 (22.7) | ||
| Widespread | 22 (47.8) | 5 (25) | 27 (40.9) | |||
| Intensity | Poor | 30 (65.2) | 11 (55) | 41 (62.1) | ||
| Strong | 1 (2.1) | 0 (0) | 1 (1.5) | |||
| IRS ± SE | 2.78 ± 0.40a,e | 1.74 ± 0.50a | 2.47 ± 0.32a,e | 0.13 | ||
| Foveolar | No staining | 42 (91.3) | 16 (80) | 58 (87.8) | ||
| Distribution | Limited | 1 (2.1) | 0 (0) | 1 (1.5) | ||
| Widespread | 3 (6.5) | 4 (20) | 7 (10.6) | |||
| Intensity | Poor | 4 (8.6) | 4 (20) | 8 (12.1) | ||
| Strong | 0 (0) | 0 (0) | 0 (0) | |||
| IRS ± SEM | 0.33 ± 0.16c,e | 0.84 ± 0.38c | 0.48 ± 0.16c,e | 0.16 | ||
| Antrum | Obese | Control | All cases | P value | ||
| Foveolar | No staining | 14 (30.4) | 7 (35) | 21 (31.8) | ||
| Distribution | Limited | 10 (21.7) | 1 (5) | 11 (16.6) | ||
| Widespread | 22 (47.8) | 12 (60) | 34 (51.5) | |||
| Intensity | Poor | 27 (58.6) | 10 (50) | 37 (56.0) | ||
| Strong | 5 (10.8) | 3 (15) | 8 (12.1%) | |||
| IRS ± SE | 5.18 ± 0.59a | 4.35 ± 0.91a | 4.92 ± 0.49a | 0.44 | ||
| Mucous | No staining | 20 (43.4) | 9 (45) | 29 (43.9) | ||
| Distribution | Limited | 21 (45.6) | 7 (35) | 28 (42.4) | ||
| Widespread | 5 (10.8) | 4 (20) | 9 (13.6) | |||
| Intensity | Poor | 19 (41.3) | 8 (40) | 27 (40.9) | ||
| Strong | 7 (15.2) | 3 (15) | 10 (15.1) | |||
| IRS ± SE | 3.24 ± 0.60a | 2.30 ± 0.70a | 2.95 ± 0.46a | 0.56 | ||
| Duodenal | Obese | Control | All cases | P value | ||
| Goblet | No staining | 30 (65.2) | 15 (75) | 45 (68.1) | ||
| Distribution | Limited | 12 (26.0) | 2 (10) | 14 (21.2) | ||
| Widespread | 4 (8.6) | 3 (15) | 7 (10.6) | |||
| Intensity | Poor | 16 (3.7) | 5 (25) | 21 (31.8) | ||
| Strong | 0 (0) | 0 (0) | 0 (0%) | |||
| IRS (± SEM) | 1.14 ± 0.25b | 0.8 ± 0.34a | 1.03 ± 0.2b | 0.40 | ||
| Absorptive | No staining | 24 (52.1) | 9 (45) | 33 (50) | ||
| Distribution | Limited | 11 (23.9) | 8 (40) | 19 (28.7) | ||
| Widespread | 11 (23.9) | 3 (15) | 24 (36.3) | |||
| Intensity | Poor | 19 (41.3) | 6 (30) | 25 (37.8) | ||
| Strong | 3 (6.5) | 5 (25) | 8 (12.1) | |||
| IRS (± SEM) | 2.14 ± 0.41b | 2.25 ± 0.56a,c | 2.17 ± 0.33b,d | 0.90 | ||
| Mucous | No staining | 31 (67.3) | 15 (75) | 46 (69.6) | ||
| Distribution | Limited | 12 (26.0) | 3 (15) | 15 (22.7) | ||
| Widespread | 3 (6.5) | 2 (10) | 5 (7.5) | |||
| Intensity | Poor | 9 (19.5) | 5 (25) | 14 (21.2) | ||
| Strong | 6 (13.0) | 0 (0) | 6 (9.0) | |||
| IRS (± SEM) | 1.55 ± 0.41 | 0.85 ± 0.39c | 1.33 ± 0.31d | 0.41 | ||
The IRS of the cells were also compared in their respective localizations. In the fundus, the highest IRS was seen in parietal cells, while the lowest occurred in foveolar cells among all three cell groups. Higher levels of TRPV1 expression were observed in parietal cells than in chief and foveolar cells, as demonstrated in Table 1. TRPV1 expression was lowest in foveolar cells in all cases (Table 1). In the antrum, foveolar cells stained more intensely than mucous cells (Table 2). In the duodenum, TRPV1 staining of absorptive cells was significantly higher than that of goblet cells, while TRPV1 staining of mucous and goblet cells was similar in both groups (Table 3). When the staining levels of the same type of cells in different tissues were compared, cells of the antrum had the highest staining intensity, followed by the fundus and duodenum. Specifically, foveolar cells in the antrum had higher IRS than foveolar cells in the fundus. Similarly, mucous cells in the antrum had higher IRS than mucous cells in the duodenum (Figure 2A and B).
The cases were evaluated in terms of staining intensity according to age (< 45 years and ≥ 45 years), sex (male and female), diabetes mellitus and hypertension, smoking, and proton pump inhibitor use. TRPV1 staining of all cells were similar in men and women. In addition, there was no difference in terms of TRPV1 staining of all cells between smokers and non-smokers, proton pump inhibitor users and non-users. Staining with TRPV1 in fundus chief cells and antrum and duodenum mucous cells was higher in patients aged 45 years and older than in patients under 45 years (3.03 ± 0.42, 4.37 ± 0.76, 2.28 ± 0.55 vs 1.9 ± 0.46, 1.58 ± 0.44, 0.37 ± 0.18, P = 0.03, P < 0.01, P < 0.01, respectively, Mann-Whitney U test). The mean staining levels of TRPV1 in duodenal mucous cells in patients with diabetes and hypertension were higher than those in patients without diabetes and hypertension (diabetes: 2.11 ± 0.67 vs 1.02 ± 0.34, P = 0.04; hypertension: 2.42 ± 0.75 vs 1.02 ± 0.33, P < 0.01 Mann-Whitney U test) (Figure 3).
Herein, we aimed to investigate the distribution of TRPV1 in human gastroduodenal cells in morbidly obese patients and controls. TRPV1 expression was similar in the morbidly obese and control groups at all tissue sites. We found that TRPV1 expression was higher in the stomach than in the duodenum and was predominantly found in parietal and chief cells of the fundus and mucous and foveolar cells of the antrum. Interestingly, unlike foveolar cells in the antrum, TRPV1 was relatively low in foveolar cells in the fundus. In the duodenum, there was more intense staining in absorbent cells than in goblet and mucous cells. The mucous cells here also had low levels of TRPV1 compared to mucous cells in the antrum. Therefore, capsaicin possibly exerts its mucosal effects via TRPV1 in the stomach rather than the duodenum. We have also demonstrated that TRPV1 expression changes in a given cell type depending on the localization, demonstrating the phenotypic and possibly functional heterogeneity of a given cell type throughout the GIT. These findings also suggest that the sensitivity of the cells to TRPV1 activation differs depending on the localization.
TRPV1 has been previously demonstrated by immunohistochemistry to be localized at nerve endings along the rat stomach wall (mucosa, submucosa, and muscle layer), and its functions have been investigated thoroughly; however, studies on human gastric tissue are scarce[26,27]. Previously, the presence of TRPV1 in neurons and parietal cells of gastric tissue in human samples was shown and suggested that TRPV1 regulates acid secretion and enhances local gastroprotection[28]. These findings are also supported by the present study in which we found high expression of TRPV1 in fundus parietal cells. Activation of TRPV1 increases intracellular calcium. The increase in intracellular calcium in parietal cells stimulates acid secretion. The acid secretion-enhancing property of TRPV1 activation can be used for therapeutic purposes, especially in cases where basal acid secretion is reduced because of neurodegeneration, such as advanced age and diabetes[30]. TRPV1 activation in chief cells is also likely to enhance digestion since chief cells secrete pepsinogen, which is converted to pepsin in acidic environments and helps protein digestion. Our study found that TRPV1 expression on chief cells in the fundus was significantly higher in patients aged 45 years and older, suggesting that TRPV1 activation may benefit elderly patients with lower pepsin levels[30,31]
Animal studies have reported that TRPV1 is expressed in gastric mucosa and can participate in mucosal protection by increasing cell proliferation and blood flow[26,27]. In addition, an animal study suggested that capsaicin has gastroprotective effects against gastric antral ulcers through capsaicin-sensitive afferent nerves[25,27]. Our study showed the presence of TRPV1 on mucous and foveolar cells that have mucosal protective effects. In particular, TRPV1 Levels in mucous and foveolar cells in the antrum were higher than those in foveolar cells in the fundus and mucous cells in the duodenum. The high incidence of TRPV1 in cells in the antrum where ulcers are mostly localized supports that capsaicin shows a protective effect against ulcers through both neuronal and nonneuronal cells. It has been shown that the risk of peptic ulcers and bleeding as well as mortality increases when comorbidities such as diabetes and hypertension, which are prevalent in advanced ages, are present[32]. The increased levels of TRPV1 in antrum and duodenum mucous cells in patients aged 45 years and older and in patients with diabetes and hypertension suggest that TRPV1 activation in gastroduodenal mucosa may be gastroprotective, especially in these groups.
The relationship between the gastric emptying rate and obesity has been discussed in many studies. Several peptides and hormones that regulate GIT motility and digestion provide the balance of hunger and satiety in the hypothalamus with neuronal feedback[33,34]. In general, it has been shown that the rate of gastric emptying is high in obese people, leading to inadequate negative feedback[35,36]. Previous studies have shown that activation of neuronal TRPV1 with capsaicin decreases motility and delays gastric emptying, especially in the upper GIT[37,38]. We postulate that capsaicin-induced TRPV1 can suppress appetite in this way, and this is one of the possible mechanisms of action against obesity. Another possible mechanism may be the faster release of hormones and peptides that reduce gastric motility and cause anorexigenic effects due to faster digestion of nutrients, especially after the activation of TRPV1 in cells involved in digestion, such as fundus chief cells and parietal cells. In one study, TRPV1 was shown to increase somatostatin and gastrin levels, which help digestion in antral glands and reduce gastroduodenal motility[29]. Finally, it has been shown that leptin, which is produced by adipocytes and produces a feeling of satiety in general, is also synthesized from mucosal chief cells in the gastric fundus, where we have shown intense TRPV1 staining[39]. Therefore, TRPV1 activation may increase leptin release from mucosal chief cells; this hypothesis needs to be investigated further in different experimental setups.
It is evident that the effect of TRPV1 on gastroduodenal mucosal tissues is multifaceted. Therefore, it is not easy to pinpoint the exact underlying mechanism of capsaicin’s anorexigenic effect via TRPV1. Several animal studies and epidemiological studies have been conducted to explain the potential mechanisms of capsaicin’s anorexigenic effect. TRPV1, mainly found in neurons, has also been shown to be present in rodent adipocytes, skeletal muscle, and the hypothalamus[14-16,19,40,41]. Capsaicin has multisystemic effects via widely distributed TRPV1 expression: it promotes lipolysis, inhibits adipogenesis, and increases the transition from white adipose tissue to thermogenic brown adipose tissue in adipose tissue; in the hypothalamus, it reduces appetite and creates a feeling of satiety; it also increases intestinal blood flow and has close relations with glucose homeostasis by increasing insulin secretion in the pancreas and by increasing glucagon like peptide-1 levels[14,17,18,20,42-44]. In light of this information, capsaicin can positively affect energy balance and obesity via TRPV1 in neuronal and nonneuronal cells in the GIT, hypothalamus, and adipose tissue. Although the results support that the effect is mostly TRPV1-mediated, the physiological process and mechanism of action are not yet fully understood. Although some human studies on the dietary intake of capsaicin show negative energy balance and anti-obesity effects[21,22,45,46], there are no studies in the literature in which the mucosal cells in the upper GIT are discussed together, especially in obese individuals.
In conclusion, we have demonstrated that the expression of TRPV1 is similar in the gastroduodenal mucosa of morbidly obese patients and control subjects. TRPV1 expression was significantly higher in parietal and chief cells of the fundus and mucous and foveolar cells in the antrum, which affects digestion, mucosal protection, and motility. It is difficult to say that capsaicin, which is thought to act on the GIT, central nervous system, and adipocyte tissue triangle, exerts its anti-obesity effect through a single pathway. In addition, the unchanged expression of gastric and duodenal TRPV1 in the obese patient group suggests that drugs targeting TRPV1 may be effective in these patients. Further studies are needed in this area.
New agents and methods are sought to prevent and treat obesity, which is a serious public health problem.
It has been reported that capsaicin acting on transient receptor potential vanilloid-1 (TRPV1) increases thermogenesis, lipid oxidation and energy expenditure and prevents diet-induced obesity.
This study aimed to compare obese individuals with normal individuals in terms of TRPV1 staining in the gastroduodenal mucosa and to determine the distribution of TRPV1 in gastroduodenal mucosa cells.
Total 46 morbidly obese patients and 20 control patients were included. Simultaneous biopsies from the fundus, antrum, and duodenum tissues were obtained from subjects. Evaluation with anti-TRPV1 antibody was performed separately according to cell types in the fundus, antrum, and duodenum tissues using an immunoreactivity score (IRS).
TRPV1 Levels in fundus, antrum, and duodenum tissues of the morbidly obese patients were similar to those of the control group. When the staining of the cells was compared with other tissue sites in terms of staining properties, the levels of TRPV1 in antrum and fundus cells in the stomach were higher than those in duodenal cells. The highest IRS was observed in fundus parietal cells, foveolar cells in the antrum, and absorptive cells in the duodenum. When the staining levels of the same type of cells in different tissues were compared, cells of the antrum had the highest staining intensity, followed by the fundus and duodenum. In addition, Staining with TRPV1 in fundus chief cells and antrum and duodenum mucous cells was higher in patients aged 45 years and older than in patients under 45 years. The mean staining levels of TRPV1 in duodenal mucous cells in patients with diabetes and hypertension were higher than those in patients without diabetes and hypertension.
We have demonstrated that the expression of TRPV1 is similar in the gastroduodenal mucosa of morbidly obese patients and control subjects. TRPV1 expression was significantly higher in parietal and chief cells of the fundus and mucous and foveolar cells in the antrum, which affects digestion, mucosal protection, and motility. In addition, the unchanged expression of gastric and duodenal TRPV1 in the obese patient group suggests that drugs targeting TRPV1 may be effective in these patients.
There is a need for more comprehensive long-term studies that can reveal the pathophysiology of obesity for the prevention and treatment of obesity.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Trujillo X S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Caballero B. Humans against Obesity: Who Will Win? Adv Nutr. 2019;10:S4-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 378] [Article Influence: 63.0] [Reference Citation Analysis (0)] |
| 2. | Zeller J, Strack C, Fenk S, Mohr M, Loew T, Schmitz G, Maier L, Fischer M, Baessler A. Relation Between Obesity, Metabolic Syndrome, Successful Long-Term Weight Reduction, and Right Ventricular Function. Int Heart J. 2016;57:441-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Sharma N, Lee J, Youssef I, Salifu MO, McFarlane SI. Obesity, Cardiovascular Disease and Sleep Disorders: Insights into the Rising Epidemic. J Sleep Disord Ther. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Kornet-van der Aa DA, Altenburg TM, van Randeraad-van der Zee CH, Chinapaw MJ. The effectiveness and promising strategies of obesity prevention and treatment programmes among adolescents from disadvantaged backgrounds: a systematic review. Obes Rev. 2017;18:581-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 5. | Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 6. | Dietrich MO, Horvath TL. Limitations in anti-obesity drug development: the critical role of hunger-promoting neurons. Nat Rev Drug Discov. 2012;11:675-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 7. | Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008;31:53-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 266] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 8. | Prasad-Reddy L, Isaacs D. A clinical review of GLP-1 receptor agonists: efficacy and safety in diabetes and beyond. Drugs Context. 2015;4:212283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 253] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 9. | Yavuz Y, Kumral ZN, Memi G, Çevik ÖD, Yeğen C, Yeğen BÇ. Serum Leptin, Obestatin, and Ghrelin Levels and Gastric Emptying Rates of Liquid and Solid Meals in Non-obese Rats with Roux-en-Y Bypass Surgery or Prosthesis Placement: Implications for the Role of Vagal Afferents. Obes Surg. 2017;27:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Bužga M, Zavadilová V, Holéczy P, Švagera Z, Švorc P, Foltys A, Zonča P. Dietary intake and ghrelin and leptin changes after sleeve gastrectomy. Wideochir Inne Tech Maloinwazyjne. 2014;9:554-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Hanipah ZN, Schauer PR. Surgical Treatment of Obesity and Diabetes. Gastrointest Endosc Clin N Am. 2017;27:191-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Li R, Lan Y, Chen C, Cao Y, Huang Q, Ho CT, Lu M. Anti-obesity effects of capsaicin and the underlying mechanisms: a review. Food Funct. 2020;11:7356-7370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Hsu CL, Yen GC. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 cells. J Agric Food Chem. 2007;55:1730-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Baskaran P, Krishnan V, Ren J, Thyagarajan B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br J Pharmacol. 2016;173:2369-2389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 15. | Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 560] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 16. | Xin H, Tanaka H, Yamaguchi M, Takemori S, Nakamura A, Kohama K. Vanilloid receptor expressed in the sarcoplasmic reticulum of rat skeletal muscle. Biochem Biophys Res Commun. 2005;332:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Leung FW. Capsaicin-sensitive intestinal mucosal afferent mechanism and body fat distribution. Life Sci. 2008;83:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Kawada T, Suzuki T, Takahashi M, Iwai K. Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats. Toxicol Appl Pharmacol. 1984;72:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Zhang LL, Yan Liu D, Ma LQ, Luo ZD, Cao TB, Zhong J, Yan ZC, Wang LJ, Zhao ZG, Zhu SJ, Schrader M, Thilo F, Zhu ZM, Tepel M. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ Res. 2007;100:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 316] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 20. | Yu Q, Wang Y, Yu Y, Li Y, Zhao S, Chen Y, Waqar AB, Fan J, Liu E. Expression of TRPV1 in rabbits and consuming hot pepper affects its body weight. Mol Biol Rep. 2012;39:7583-7589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Yoshioka M, St-Pierre S, Suzuki M, Tremblay A. Effects of red pepper added to high-fat and high-carbohydrate meals on energy metabolism and substrate utilization in Japanese women. Br J Nutr. 1998;80:503-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 127] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Yoshioka M, Lim K, Kikuzato S, Kiyonaga A, Tanaka H, Shindo M, Suzuki M. Effects of red-pepper diet on the energy metabolism in men. J Nutr Sci Vitaminol (Tokyo). 1995;41:647-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Taghizadeh M, Farzin N, Taheri S, Mahlouji M, Akbari H, Karamali F, Asemi Z. The Effect of Dietary Supplements Containing Green Tea, Capsaicin and Ginger Extracts on Weight Loss and Metabolic Profiles in Overweight Women: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Ann Nutr Metab. 2017;70:277-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Rigamonti AE, Casnici C, Marelli O, De Col A, Tamini S, Lucchetti E, Tringali G, De Micheli R, Abbruzzese L, Bortolotti M, Cella SG, Sartorio A. Acute administration of capsaicin increases resting energy expenditure in young obese subjects without affecting energy intake, appetite, and circulating levels of orexigenic/anorexigenic peptides. Nutr Res. 2018;52:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Yamamoto H, Horie S, Uchida M, Tsuchiya S, Murayama T, Watanabe K. Effects of vanilloid receptor agonists and antagonists on gastric antral ulcers in rats. Eur J Pharmacol. 2001;432:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 26. | Nozawa Y, Nishihara K, Yamamoto A, Nakano M, Ajioka H, Matsuura N. Distribution and characterization of vanilloid receptors in the rat stomach. Neurosci Lett. 2001;309:33-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Horie S, Yamamoto H, Michael GJ, Uchida M, Belai A, Watanabe K, Priestley JV, Murayama T. Protective role of vanilloid receptor type 1 in HCl-induced gastric mucosal lesions in rats. Scand J Gastroenterol. 2004;39:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Faussone-Pellegrini MS, Taddei A, Bizzoco E, Lazzeri M, Vannucchi MG, Bechi P. Distribution of the vanilloid (capsaicin) receptor type 1 in the human stomach. Histochem Cell Biol. 2005;124:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Ericson A, Nur EM, Petersson F, Kechagias S. The effects of capsaicin on gastrin secretion in isolated human antral glands: before and after ingestion of red chilli. Dig Dis Sci. 2009;54:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Feldman M, Cryer B, McArthur KE, Huet BA, Lee E. Effects of aging and gastritis on gastric acid and pepsin secretion in humans: a prospective study. Gastroenterology. 1996;110:1043-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 180] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Gritti I, Banfi G, Roi GS. Pepsinogens: physiology, pharmacology pathophysiology and exercise. Pharmacol Res. 2000;41:265-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Leontiadis GI, Molloy-Bland M, Moayyedi P, Howden CW. Effect of comorbidity on mortality in patients with peptic ulcer bleeding: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:331-45; quiz 346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (37)] |
| 33. | Camilleri M, Grudell AB. Appetite and obesity: a gastroenterologist's perspective. Neurogastroenterol Motil. 2007;19:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Cork SC. The role of the vagus nerve in appetite control: Implications for the pathogenesis of obesity. J Neuroendocrinol. 2018;30:e12643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Gallagher TK, Geoghegan JG, Baird AW, Winter DC. Implications of altered gastrointestinal motility in obesity. Obes Surg. 2007;17:1399-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Duggan JP, Booth DA. Obesity, overeating, and rapid gastric emptying in rats with ventromedial hypothalamic lesions. Science. 1986;231:609-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Gonzalez R, Dunkel R, Koletzko B, Schusdziarra V, Allescher HD. Effect of capsaicin-containing red pepper sauce suspension on upper gastrointestinal motility in healthy volunteers. Dig Dis Sci. 1998;43:1165-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Shibata C, Jin XL, Naito H, Matsuno S, Sasaki I. Intraileal capsaicin inhibits gastrointestinal contractions via a neural reflex in conscious dogs. Gastroenterology. 2002;123:1904-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Cammisotto P, Bendayan M. A review on gastric leptin: the exocrine secretion of a gastric hormone. Anat Cell Biol. 2012;45:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 40. | Luo Z, Ma L, Zhao Z, He H, Yang D, Feng X, Ma S, Chen X, Zhu T, Cao T, Liu D, Nilius B, Huang Y, Yan Z, Zhu Z. TRPV1 activation improves exercise endurance and energy metabolism through PGC-1α upregulation in mice. Cell Res. 2012;22:551-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 375] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 41. | Molinas AJR, Desmoulins LD, Hamling BV, Butcher SM, Anwar IJ, Miyata K, Enix CL, Dugas CM, Satou R, Derbenev AV, Zsombok A. Interaction between TRPV1-expressing neurons in the hypothalamus. J Neurophysiol. 2019;121:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Zheng J, Zheng S, Feng Q, Zhang Q, Xiao X. Dietary capsaicin and its anti-obesity potency: from mechanism to clinical implications. Biosci Rep. 2017;37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 43. | Janssens PL, Hursel R, Westerterp-Plantenga MS. Capsaicin increases sensation of fullness in energy balance, and decreases desire to eat after dinner in negative energy balance. Appetite. 2014;77:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Gram DX, Ahrén B, Nagy I, Olsen UB, Brand CL, Sundler F, Tabanera R, Svendsen O, Carr RD, Santha P, Wierup N, Hansen AJ. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. Eur J Neurosci. 2007;25:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 45. | Janssens PL, Hursel R, Martens EA, Westerterp-Plantenga MS. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS One. 2013;8:e67786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Snitker S, Fujishima Y, Shen H, Ott S, Pi-Sunyer X, Furuhata Y, Sato H, Takahashi M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: possible pharmacogenetic implications. Am J Clin Nutr. 2009;89:45-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |