Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.62
Peer-review started: May 3, 2021
First decision: October 16, 2021
Revised: October 30, 2021
Accepted: November 19, 2021
Article in press: November 19, 2021
Published online: January 7, 2022
Processing time: 241 Days and 5.5 Hours
The anatomical features of the atlantoaxial spine increase the difficulty of complete and safe removal of atlantoaxial intradural extramedullary (IDEM) tumors. Studies concerning surgical interventions via a posterior approach are limited.
To investigate the safety and efficacy of atlantoaxial IDEM tumor resection using a one-stage posterior approach.
We retrospectively analyzed clinical databases for one-stage atlantoaxial IDEM tumor resection via a posterior approach between January 2008 and January 2018. The analyzed data included tumor position, histopathological type, pre- and post-operative Japanese Orthopedic Association (JOA) scores and Nurick grades, postoperative complication and recurrence status.
A total of 13 patients who underwent C1-C2 Laminectomy and/or unilateral facetectomy via the posterior approach were enrolled in the study. In all cases reviewed, total tumor resection and concomitant C1-C2 fusion were achieved. The average follow-up was 35.3 ± 6.9 mo (range, 26-49 mo). A statistically significant difference was noted between the preoperative JOA score (11.2 ± 1.1) and the score at the last final follow-up (15.6 ± 1.0) (P < 0.05). A statistically significant difference was noted between the preoperative Nurick grade (2.3 ± 0.9) and that at the last follow-up (1.2 ± 0.4) (P < 0.05). However, no statistically significant difference was noted between the preoperative and last follow-up C1-2 Cobb angle and C2-7 Cobb angle (P > 0.05). No mortalities, severe complications or tumor recurrence were observed during the follow-up period.
Total resection of atlantoaxial IDEM tumors is feasible and effective via a posterior approach. Surgical reconstruction should be considered to avoid iatrogenic kyphosis and improve spinal stability and overall clinical outcomes.
Core Tip: This retrospective study investigated the safety and efficacy of atlantoaxial intradural extramedullary (IDEM) tumor resection using a one-stage posterior approach. Statistically significant differences were noted between the preoperative Japanese Orthopedic Association (JOA) score and Nurick grade and those at the last follow-up (JOA: 11.2 ± 1.1 vs 15.6 ± 1.0; Nurick grade: 2.3 ± 0.9 vs 1.2 ± 0.4; all P < 0.05). No mortalities, severe complications or tumor recurrence were observed during the follow-up period. Total resection of atlantoaxial IDEM tumors is feasible and effective via a posterior approach.
- Citation: Meng DH, Wang JQ, Yang KX, Chen WY, Pan C, Jiang H. Surgical resection of intradural extramedullary tumors in the atlantoaxial spine via a posterior approach. World J Clin Cases 2022; 10(1): 62-70
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/62.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.62
Intradural extramedullary (IDEM) tumors have an incidence rate of approximately 5 to 10 per 100,000 people[1]. IDEM tumors in the atlantoaxial spine are uncommon and present with progressive pain and neurological deficits. With advances in surgical technology, the management of these tumors has shifted toward radical resection with decompression of surrounding neural structures[2]. The odontoid process and lack of bony foramen make the anatomical characteristics of the atlantoaxial spine uniquely complex, thus making surgical approach for the treatment of an atlantoaxial IDEM tumor both difficult and controversial. Commonly used surgical strategies include the anterior or anterolateral approach, posterior approach, or combined anterior-posterior approach. The anterior or anterolateral approach has been reported to facilitate easy access to lesions located anterior to the atlantoaxial spine[3]. However, the operation fields of these approaches are deep and narrow and require the resection of vital components of the atlantoaxial spine. The prolonged surgical time of the combined anterior-posterior approach has been linked to iatrogenic traumatism[4]. The posterior approach is the most widely used. However, there is no general consensus regarding the surgical outcome. The objective of this study was to evaluate the safety and efficacy of atlantoaxial IDEM tumor resection via a one-stage posterior approach.
We retrospectively searched the clinical databases of the First Affiliated Hospital of Guangxi Medical University over a period of ten years (January 2008 to January 2018) for patients who underwent atlantoaxial IDEM tumor resection via a one-stage posterior approach. The study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (No. 2020-KY-NSFC-025), and all participants provided written informed consent. Patients were included in the study if (1) age > 18 years; (2) atlantoaxial IDEM tumor was confirmed by magnetic resonance imaging; (3) underwent standard open IDEM tumor resection via a one-stage posterior approach; and (4) postoperative histopathological studies of surgical samples confirmed IDEM tumor diagnosis. The exclusion criteria were as follows: (1) preoperative, intraoperative or postoperative diagnosis of non-IDEM tumor; (2) patient with history of cervical spine surgery; (3) patient with congenital atlantoaxial anomalies; and (4) patients with incomplete pre- and postoperative data.
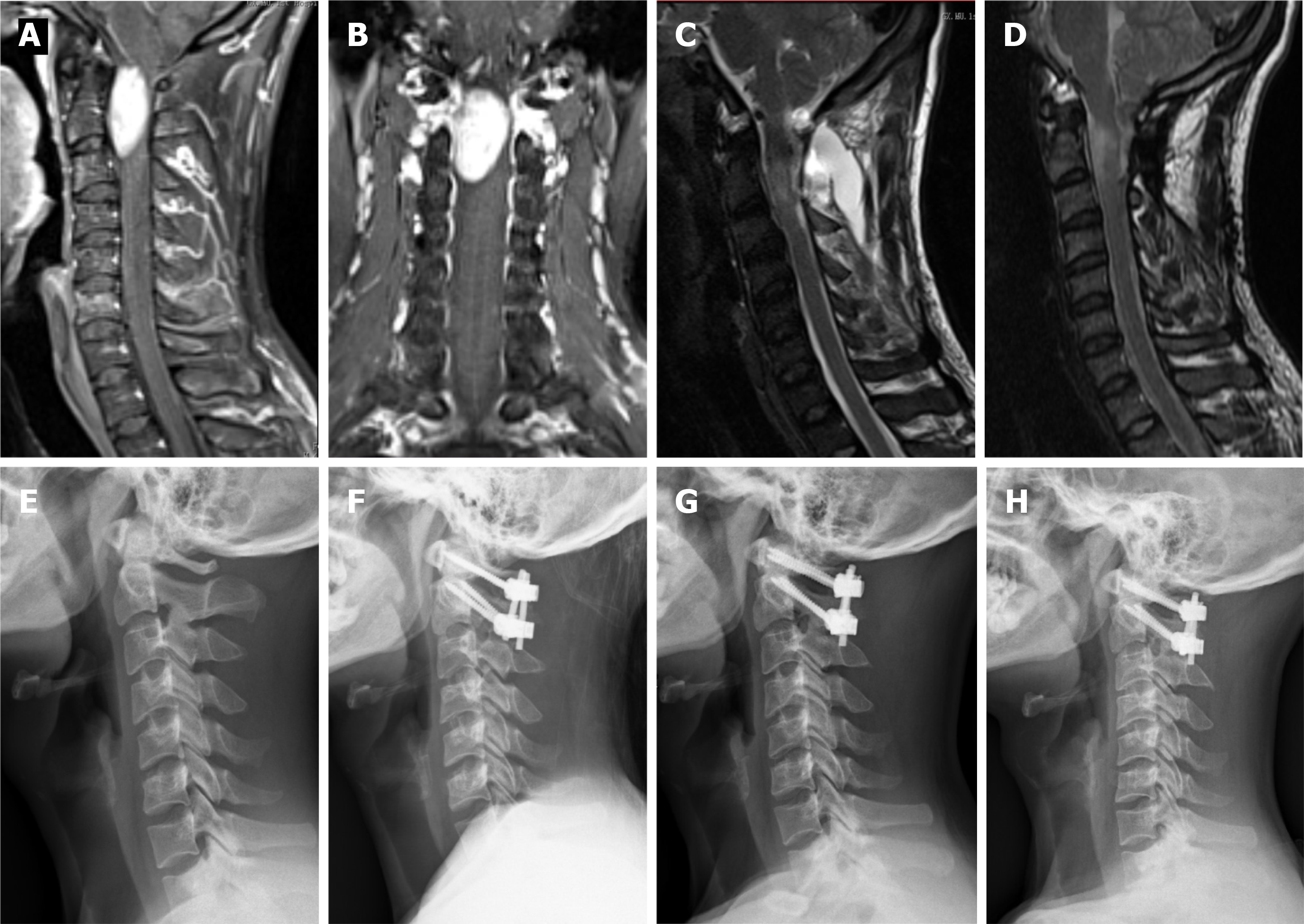
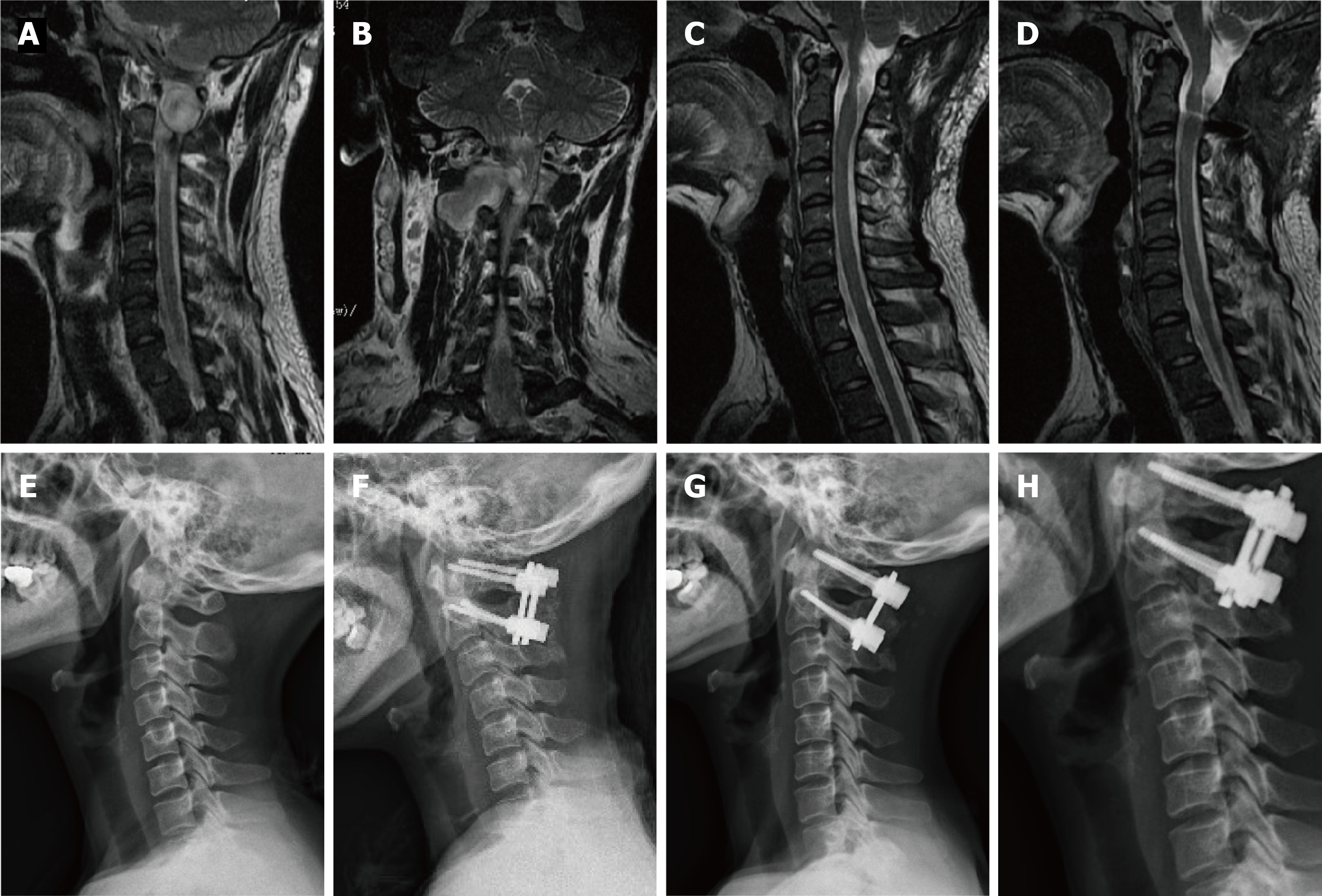
Following the induction of general anesthesia, a patient was placed in a prone position, and a longitudinal midline incision was made. Paravertebral soft tissues were then dissected to expose the occiput, posterior arch of C1, and spinous process and lamina of C2. The inferior part of the C1 posterior arch and the superior part of the C2 Lamina were then drilled off according to the location of the lesion. In some cases, the facet joint may be unilaterally removed to expose the tumor and protect the spinal cord. In the case of dumbbell tumors, the extradural components were first removed, and the intradural procedure was performed following the exposure of the dura mater. All tumor resections were performed using standard microsurgical techniques, and the dura mater was closed using running sutures with 4-0 Nurolon. This procedure is followed by spinal instrumentation and fusion. Under fluoroscopic guidance, C1 Lateral mass screws and C2 pedicle screws were fixed followed by grafting with cancellous bone granules between the C1 and C2 vertebrae.
Nurick grade and Japanese Orthopaedic Association (JOA) score were used to evaluated preoperative and follow-up symptoms[5,6]. The intra- and postoperative reviewed data included lesion level, operation time, surgery-related complications, histopathological type and tumor recurrence.
The following preoperative, postoperative and last follow-up parameters were measured on radiographs: (1) C1-2 Cobb angle: the angle between the line connecting the superior margins of the anterior and posterior arches of C1 and another line along the inferior endplate of C2[7], and (2) C2-7 angle: the angle between the lines along the inferior endplate of C2 and C7 vertebrae[8]. The C1-C2 and C2-C7 cervical lordosis were expressed as positive values.
Data collected for the assessment of pre- and postoperative spinal alignment and surgical outcomes, including the Nurick grade, JOA score, C1-2 angle and C2-7 angle, were analyzed using paired t tests for statistical significance. All statistical analyses were performed using SPSS version 13.0 (SPSS, Inc., Chicago, IL, United States), with a P value < 0.05 considered statistically significant.
A total of 13 patients (6 males and 7 females) with an average age of 49.3 years (range 29-66 years) were enrolled for this study (Table 1). The mean follow-up period was 35.3 mo and ranged between 26 and 49 mo. The tumor location was at the C1-C2 Level in 8 patients, the occiput-C1 Level in 3 patients, and the occiput-C2 Level in 2 patients. The maximum tumor diameter within the spinal canal ranged between 5 and 11 mm with a mean of 8.7 mm. All patients reviewed were symptomatic: 2 patients presented with occipital neuralgia, 7 patients presented with extremity pain and/or numbness, and 4 patients presented with clumsiness of the upper extremities. The average duration from onset of initial symptoms to surgery was 10 mo (range 2-18 mo). The histopathological studies confirmed 5 cases as meningiomas, 6 as schwannomas, and 2 as neurofibromas.
| Patient | Gender | Age (yrs) | Affected level | Location | Tumor type | Tumor size (mm) | Complication | Follow up (mo) |
| 1 | F | 54 | Occiput-C1 | Ventral | Meningioma | 7 | 41 | |
| 2 | M | 29 | Occiput-C2 | Ventrolateral | Schwannoma | 11 | CSF leaks | 31 |
| 3 | M | 57 | C1-C2 | Ventral | Meningioma | 8 | 28 | |
| 4 | F | 37 | C1-C2 | Ventral | Schwannoma | 7 | 34 | |
| 5 | F | 51 | C1-C2 | Ventrolateral | Schwannoma | 7 | 42 | |
| 6 | M | 39 | Occiput-C1 | Ventral | Meningioma | 6 | 32 | |
| 7 | M | 61 | C1-C2 | Ventrolateral | Neurofibroma | 8 | 26 | |
| 8 | F | 48 | Occiput-C2 | Ventrolateral | Schwannoma | 10 | 38 | |
| 9 | F | 45 | C1-C2 | Ventral | Neurofibroma | 6 | 42 | |
| 10 | M | 59 | Occiput-C1 | Ventral | Meningioma | 7 | CSF leaks | 36 |
| 11 | F | 43 | C1-C2 | Ventrolateral | Schwannoma | 5 | 49 | |
| 12 | M | 66 | C1-C2 | Ventrolateral | Schwannoma | 6 | 26 | |
| 13 | M | 52 | C1-C2 | Ventral | Meningioma | 5 | 34 |
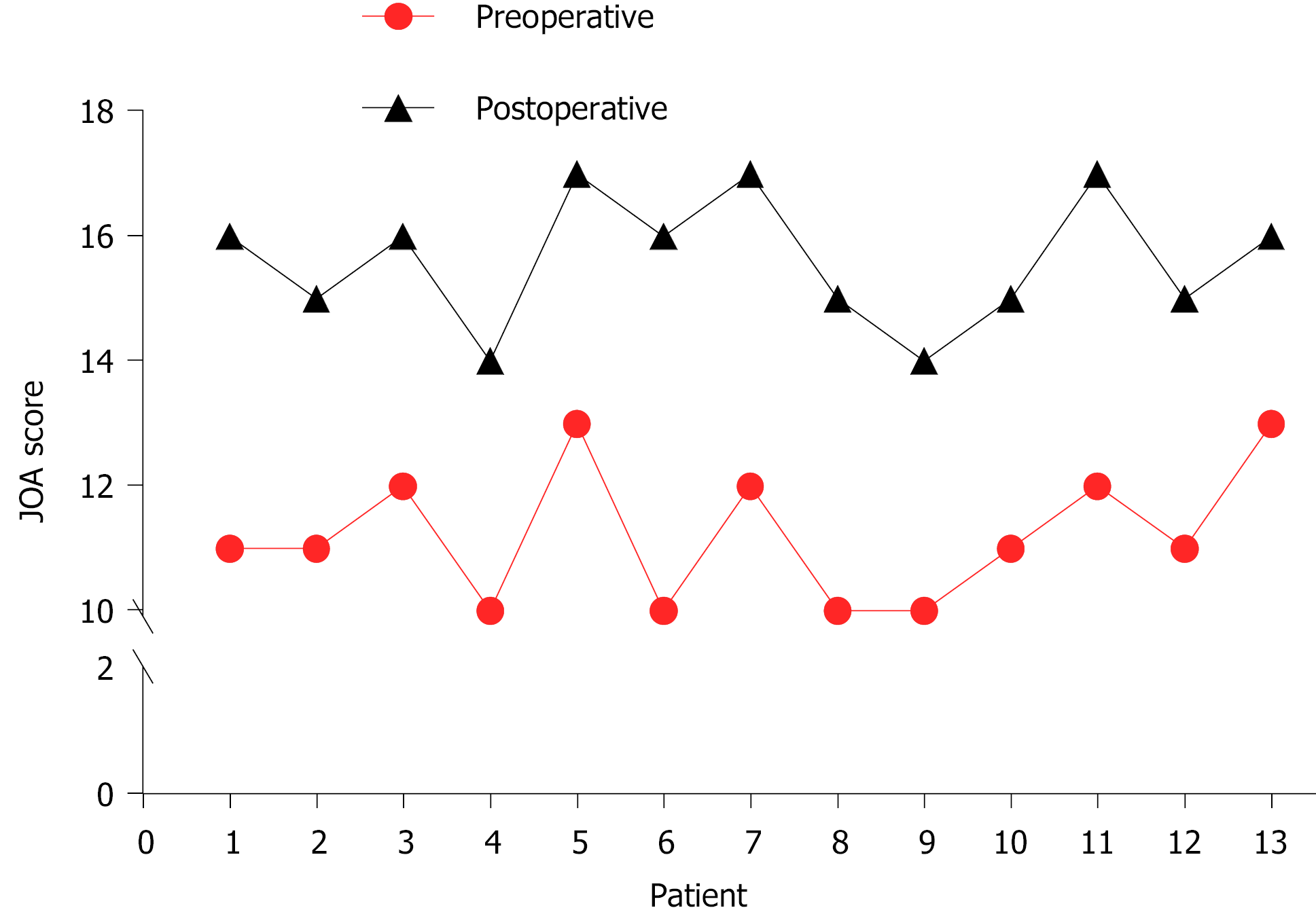
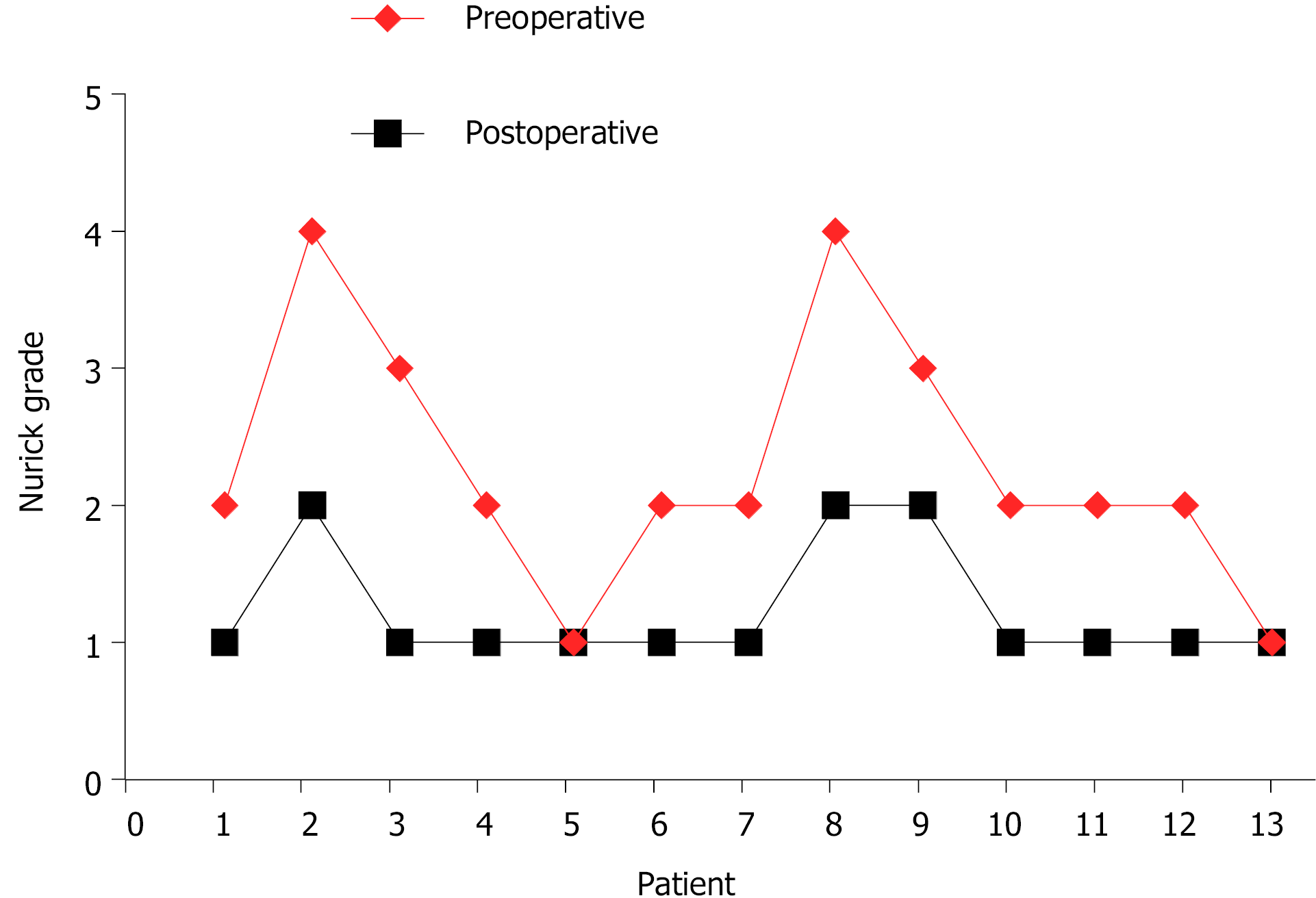
In all cases, the atlantoaxial IDEM tumors were successfully removed via a one-stage posterior approach (Figures 1 and 2). The surgical time ranged from 121-368 min with an average of 205 ± 85 min and an average intraoperative blood loss of 263 ± 155 mL (range 150-650 mL). Total resection and C1-C2 fusion were successfully performed in all the reviewed cases, among which 8 were treated with C1-C2 Laminectomy and 5 with C1-C2 Laminectomy and unilateral facetectomy. With the exception of 2 patients who developed cerebrospinal fluid (CSF) leakage, no major surgery-related complications or deaths were observed. CSF leakage was successfully treated using neck wrapping and strict bed rest. No tumor recurrence or surgical revision was observed during the follow-up period. Significant improvements in the JOA score and Nurick grade were noted (Figures 3 and 4). The JOA score increased from 11.2 ± 1.1 to 15.6 ± 1.0, while the Nurick grade improved from 2.3 ± 0.9 to 1.2 ± 0.4. There were statistically significant differences between the preoperative and postoperative JOA scores and Nurick grade (P < 0.05). This suggests significant postoperative benefits of the one-stage posterior approach resection of IDEM tumors. The mean preoperative C1-2 Cobb angle and C2-7 Cobb angle were 25.7 ± 8.1° and 14.8 ± 15.3°, respectively. At the last follow-up, the mean C1-2 Cobb angle increased to 27.2 ± 6.8°, whereas the C2-7 Cobb angle decreased to 16.5 ± 12.7°. However, the difference was not statistically significant (P > 0.05).
Although the atlantoaxial spine accounts for approximately 14% of all spinal cord tumors[9], there are still limited published reports about its clinical features and overall postoperative outcome. The atlantoaxial spine has specific anatomical complexities not found at the other spinal levels. Thus, a detailed understanding of this condition is paramount for safe and efficient surgical intervention of tumors located at this level. In this study, all patients underwent a one-stage posterior approach, and our results demonstrate that this approach provided the adequate exposure required for safe and complete tumor resection. The results also revealed an improved overall postoperative neurological function at the last follow-up.
Currently, the various surgical approaches employed for the resection of atlantoaxial IDEM tumors include anterior, anterolateral, posterior and combined anterior and posterior approaches. The anterior approach provides the most direct access to intradural lesions ventral to the craniocervical junction. Thus, the procedure is advantageous for the exposure of ventral IDEM tumors without the need for extensive manipulation of the cervical spinal cord. However, the available surgical corridor is narrow and shallow, and access to lateral masses is limited[10]. Therefore, this approach is infrequently used for intradural tumor resections and has been used for the removal of small lesions ventral to the mid-portion of the atlantoaxial spine without spinal cord compression[11,12]. However, there is an increased difficulty in achieving watertight closure of the dura mater. Posterior approach atlantoaxial IDEM tumor resection is a familiar, low-risk procedure used by spine surgeons. In most instances, adequate exposure can be attained using the standard posterior approach with laminectomy. In addition, the standard posterior approach allows for direct ventral canal access via the removal of lateral bone structures, such as facet joints, or part of the pedicle. The cervical spinal cord is more susceptible to intraoperative damage; therefore, there is the need to secure a wide operative field to allow for gentle maneuvering of the spinal cord. Successful cervical tumor resection includes successful gross total tumor resection, and gentle manipulation of the spinal cord can help minimize postoperative neurological deficits and maximize postoperative neurological symptom recovery[13]. In this study, the surgical technique used in all patients included C1-C2 Laminectomy, which was occasionally combined with unilateral facetectomy on the tumor side. This process provides sufficient extraspinal and intraspinal access for tumor excision and produces satisfactory postoperative outcomes. Our results showed that atlantoaxial IDEM tumors smaller than 10 mm, even those located ventral to the spinal canal, can be safely and completely removed via posterior approach laminectomy and/or unilateral facetectomy.
The development of postlaminectomy kyphosis following cervical IDEM tumors resection is a relatively common problem with a reported incidence rate between 24% and 75%[14,15]. However, the incidence of craniocervical instability or deformity in relation to C1-C2 Laminectomy has not been reported[16-19]. Increased risk of postoperative deformity have been reported to correlate with C2 Laminectomy[15], facet joint damage[20], and preoperative loss of cervical lordosis[21]. Additionally, there are reported cases of intraspinal tumors causing preoperative spinal deformities[22]. Thus, an inevitable risk of postoperative atlantoaxial instability and/or deformity is noted following C1-C2 Laminectomy.
Several studies have reported postoperative atlantoaxial instability and postlaminectomy kyphosis following posterior decompression of atlantoaxial spinal pathologies[23-25]. Currently available studies of cadavers of animals and humans point to the C2 Lamina and facet joints being of prominent importance in the maintenance of cervical stability, especially of the atlantoaxial spine[26-28]. However, it remains controversial whether internal fixation and fusion of the atlantoaxial spine should be performed after C2 Laminectomy or facetectomy. McCormick et al[29] and Jiang et al[30] reported upper cervical instability following resection of greater than 50% of the unilateral facet joint. These researchers suggested spinal reconstruction following the resection of greater than 50% of the unilateral facet joint. To obtain sufficient space for tumor removal, the posterior arch of C1 and lamina of C2 were resected and occasionally combined with unilateral facet joint resection followed by overturning of the spinal cord in the present study. Adequate stabilization was achieved by posterior fixation and fusion using an autologous bone graft. During the long follow-up periods, no cervical spine deformity was observed in any of the cases. Prevention of postlaminectomy deformity is an important consideration in preoperative surgical planning. Therefore, in cases where C1-C2 Laminectomy was performed or the facet joint was destroyed, we recommend the concomitant application of surgical fusion after resection of atlantoaxial IDEM tumors. It is our understanding that this is an effective surgical option for improving the functional status, quality of life, and preservation of sagittal alignment in postoperative IDEM tumor patients.
There are several limitations in this study. First, this is a single-center study with a relatively small study population. Future studies are warranted to confirm our results. Second, this was a retrospective study, which may introduce the potential for information bias. Finally, the average follow-up period of 35 mo was too short to completely understand postoperative cervical stability and sagittal balance.
Total resection of atlantoaxial IDEM tumors can be safely and effectively resected via one-stage posterior approach laminectomy and/or unilateral facetectomy. For longer stability and better clinical outcomes, spinal reconstruction should be considered to prevent iatrogenic kyphosis.
The goal of new surgical treatments is to reduce the incidence of cerebrospinal fluid leakage and relieve injury to patients.
Total resection of atlantoaxial intradural extramedullary (IDEM) tumors is feasible and effective via a posterior approach.
A statistically significant difference was noted between the preoperative Japanese Orthopedic Association score (11.2 ± 1.1) and the score at the last final follow-up (15.6 ± 1.0) (P < 0.05). A statistically significant difference was also noted between the preoperative Nurick grade (2.3 ± 0.9) and that at the last follow-up (1.2 ± 0.4) (P < 0.05). However, no statistically significant difference was noted between the preoperative and last follow-up C1-2 Cobb angle and C2-7 Cobb angle (P > 0.05). No mortalities, severe complications or tumor recurrence were observed during the follow-up period.
This was a retrospective study of 13 patients who underwent atlantoaxial IDEM tumor resection via a posterior approach.
To investigate the efficacy of surgical resection for atlantoaxial IDEM tumors and its influencing factors.
To explore the safety and feasibility of atlantoaxial IDEM tumor resection.
IDEM tumors in the atlantoaxial spine are uncommon and present with progressive pain and neurological deficits.
The authors thank all the participants in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koumantakis GA S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Traul DE, Shaffrey ME, Schiff D. Part I: spinal-cord neoplasms-intradural neoplasms. Lancet Oncol. 2007;8:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Jiang H, He J, Zhan X, He M, Zong S, Xiao Z. Occipito-cervical fusion following gross total resection for the treatment of spinal extramedullary tumors in craniocervical junction: a retrospective case series. World J Surg Oncol. 2015;13:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Ohnishi Y, Iwatsuki K, Taketsuna S, Ninomiya K, Yoshimine T. Retro-odontoid synovial cyst resected via an anterolateral approach without fusion. Eur Spine J. 2015;24:S508-S513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Chotai S, Zuckerman SL, Parker SL, Wick JB, Stonko DP, Hale AT, McGirt MJ, Cheng JS, Devin CJ. Healthcare Resource Utilization and Patient-Reported Outcomes Following Elective Surgery for Intradural Extramedullary Spinal Tumors. Neurosurgery. 2017;81:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 610] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Yonenobu K, Abumi K, Nagata K, Taketomi E, Ueyama K. Interobserver and intraobserver reliability of the japanese orthopaedic association scoring system for evaluation of cervical compression myelopathy. Spine (Phila Pa 1976). 2001;26:1890-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 370] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Kato Y, Itoh T, Kanaya K, Kubota M, Ito S. Relation between atlantoaxial (C1/2) and cervical alignment (C2-C7) angles with Magerl and Brooks techniques for atlantoaxial subluxation in rheumatoid arthritis. J Orthop Sci. 2006;11:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Tang X, Dong L, Tan M, Yi P, Yang F, Hao Q. Long-Term Influence of C1-C2 Pedicle Screw Fixation on Occipitoatlantal Angle and Subaxial Cervical Spine in the Pediatric Population. Pediatr Neurosurg. 2018;53:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Zozulya YP, Slynko YI, Al-Qashqish II. Surgical treatment of ventral and ventrolateral intradural extramedullary tumors of craniovertebral and upper cervical localization. Asian J Neurosurg. 2011;6:18-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Angevine PD, Kellner C, Haque RM, McCormick PC. Surgical management of ventral intradural spinal lesions. J Neurosurg Spine. 2011;15:28-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Slin'ko EI, Al-Qashqish II. Intradural ventral and ventrolateral tumors of the spinal cord: surgical treatment and results. Neurosurg Focus. 2004;17:ECP2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Naito K, Yamagata T, Kawahara S, Ohata K, Takami T. High Cervical Lateral Approach to Safely Remove the Cystic Retro-odontoid Pseudotumor: Technical Note. Neurol Med Chir (Tokyo). 2019;59:392-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Altas M, Cerci A, Silav G, Sari R, Coskun K, Balak N, Isik N, Elmaci I. Microsurgical management of non-neurofibromatosis spinal schwannoma. Neurocirugia (Astur). 2013;24:244-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Tatter C, Fletcher-Sandersjöö A, Persson O, Burström G, Grane P, Edström E, Elmi-Terander A. Incidence and predictors of kyphotic deformity following resection of cervical intradural tumors in adults: a population-based cohort study. Acta Neurochir (Wien). 2020;162:2905-2913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Abduljabbar FH, Teles AR, Bokhari R, Weber M, Santaguida C. Laminectomy with or Without Fusion to Manage Degenerative Cervical Myelopathy. Neurosurg Clin N Am. 2018;29:91-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Fassett DR, Clark R, Brockmeyer DL, Schmidt MH. Cervical spine deformity associated with resection of spinal cord tumors. Neurosurg Focus. 2006;20:E2. [PubMed] |
| 17. | McGirt MJ, Chaichana KL, Atiba A, Bydon A, Witham TF, Yao KC, Jallo GI. Incidence of spinal deformity after resection of intramedullary spinal cord tumors in children who underwent laminectomy compared with laminoplasty. J Neurosurg Pediatr. 2008;1:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Nakamura M, Iwanami A, Tsuji O, Hosogane N, Watanabe K, Tsuji T, Ishii K, Toyama Y, Chiba K, Matsumoto M. Long-term surgical outcomes of cervical dumbbell neurinomas. J Orthop Sci. 2013;18:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Yu Y, Hu F, Zhang X, Gu Y, Xie T, Ge J. Application of the hemi-semi-laminectomy approach in the microsurgical treatment of C2 schwannomas. J Spinal Disord Tech. 2014;27:E199-E204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Ahmed R, Menezes AH, Awe OO, Mahaney KB, Torner JC, Weinstein SL. Long-term incidence and risk factors for development of spinal deformity following resection of pediatric intramedullary spinal cord tumors. J Neurosurg Pediatr. 2014;13:613-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 21. | Kaptain GJ, Simmons NE, Replogle RE, Pobereskin L. Incidence and outcome of kyphotic deformity following laminectomy for cervical spondylotic myelopathy. J Neurosurg. 2000;93:199-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 114] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Bansal S, Suri A, Borkar SA, Kale SS, Singh M, Mahapatra AK. Management of intramedullary tumors in children: analysis of 82 operated cases. Childs Nerv Syst. 2012;28:2063-2069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Mukai Y, Hosono N, Sakaura H, Fujii R, Iwasaki M, Fuchiya T, Fujiwara K, Fuji T, Yoshikawa H. Sagittal alignment of the subaxial cervical spine after C1-C2 transarticular screw fixation in rheumatoid arthritis. J Spinal Disord Tech. 2007;20:436-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Guo Q, Ni B, Yang J, Liu K, Sun Z, Zhou F, Zhang J. Relation between alignments of upper and subaxial cervical spine: a radiological study. Arch Orthop Trauma Surg. 2011;131:857-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Huang JC, Qian BP, Qiu Y, Yu Y, Ni HB. Surgical overreduction and hyperlordotic fusion of C1-C2 joint are associated with cervical sagittal malalignment. Arch Orthop Trauma Surg. 2017;137:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Claybrooks R, Kayanja M, Milks R, Benzel E. Atlantoaxial fusion: a biomechanical analysis of two C1-C2 fusion techniques. Spine J. 2007;7:682-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Cadena G, Duong HT, Liu JJ, Kim KD. Atlantoaxial fixation using C1 posterior arch screws: feasibility study, morphometric data, and biomechanical analysis. J Neurosurg Spine. 2018;30:314-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Liu S, Song Z, Liu L, Yin X, Hu X, Yang M, Wu Q, Song Y, Hao D. Biomechanical evaluation of C1 lateral mass and C2 translaminar bicortical screws in atlantoaxial fixation: an in vitro human cadaveric study. Spine J. 2018;18:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | McCormick PC. Surgical management of dumbbell tumors of the cervical spine. Neurosurgery. 1996;38:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 99] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Jiang L, Lv Y, Liu XG, Ma QJ, Wei F, Dang GT, Liu ZJ. Results of surgical treatment of cervical dumbbell tumors: surgical approach and development of an anatomic classification system. Spine (Phila Pa 1976). 2009;34:1307-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |