Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.345
Peer-review started: August 13, 2021
First decision: September 5, 2021
Revised: September 16, 2021
Accepted: November 25, 2021
Article in press: November 25, 2021
Published online: January 7, 2022
Processing time: 139 Days and 5.2 Hours
Breast cancer patients have a high skin metastasis rate. However, reports on treatment of cutaneous metastases of breast cancer are scarce.
We report the treatment process for one breast cancer case with bone, lung, and skin metastases. The patient was a 43-year-old woman with advanced breast cancer and skin metastasis. She underwent pathological diagnosis by needle biopsy to guide the treatment. When the disease progressed, a new pathological diagnosis was determined by needle biopsy to guide the treatment. The patient received chemotherapy, endocrine therapy, and photodynamic dynamic therapy, followed by sonodynamic therapy.
Repeated puncture should be performed for advanced breast cancer with skin metastasis, in order to obtain the pathology and directly determine diagnosis when the disease progresses. The treatment should focus on controlling the systemic metastasis, rather than the local disease.
Core Tip: Breast cancer as the most prevalent neoplasm among female individuals, is also the main source of cutaneous metastasis other than melanoma. Breast cancer skin metastasis presents at the terminal stage of advanced cancer, and local treatment can be used to control locally metastatic lesions. As for cases involving a huge breast mass with ulceration or local lesion, radiation therapy is a useful tool for symptomatic control. Advanced breast cancer with skin metastasis can be repeatedly punctured to obtain pathology to directly inform diagnosis when the disease progresses.
- Citation: Li ZH, Wang F, Zhang P, Xue P, Zhu SJ. Diagnosis and guidance of treatment of breast cancer cutaneous metastases by multiple needle biopsy: A case report. World J Clin Cases 2022; 10(1): 345-352
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/345.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.345
There were more than two million newly diagnosed female breast cancer cases in 2018. Furthermore, breast cancer alone is expected to account for 25% of all new cancers in women[1]. Cutaneous metastases of primary internal malignancy are relatively uncommon, with an overall incidence of 0.7%-10.4%[2,3]. A total of 4020 patients with metastatic skin cancer, including 212 cases of skin metastasis of breast cancer, have been recorded. Breast cancer has the highest incidence of cutaneous metastasis, when compared to other solid malignancies, accounting for 51% of the total cases and 73% of the cases in women. The incidence of cutaneous metastases in patients with breast carcinoma is 30%[4]. However, the prognosis of breast cancer cutaneous metastases remains poor[5]. Furthermore, the specific treatment for cutaneous metastases of breast cancer remains unestablished. Therefore, we present a case diagnosed with cutaneous metastases of breast cancer, who received several different treatments through the guidance of needle biopsy, and eventually acquired a relatively long survival time.
The patient was a 43-year-old woman who had breast cancer with lymph node, bone, and cutaneous metastases.
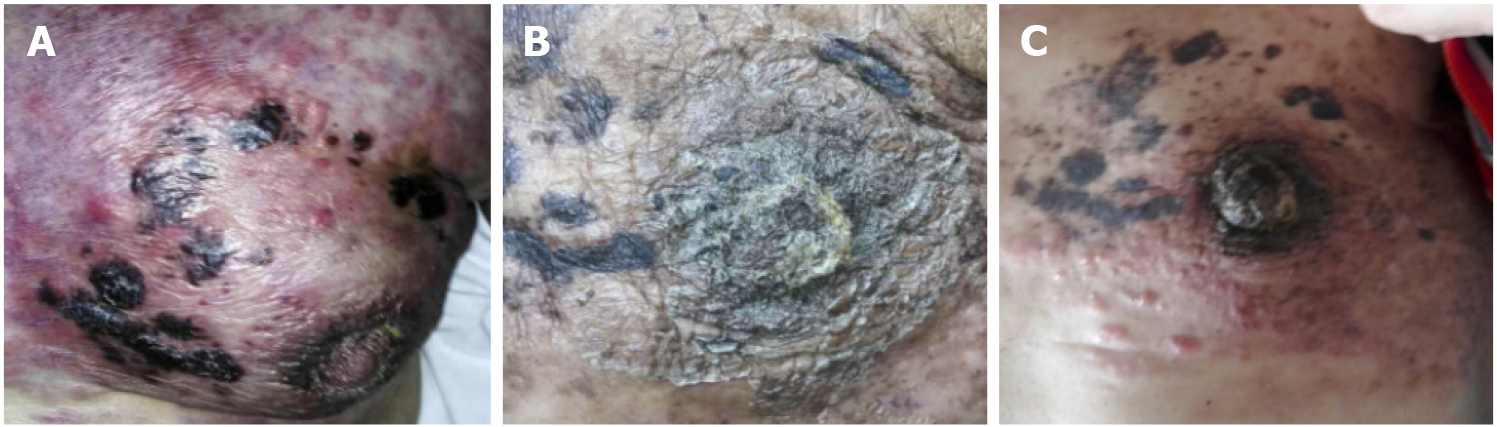
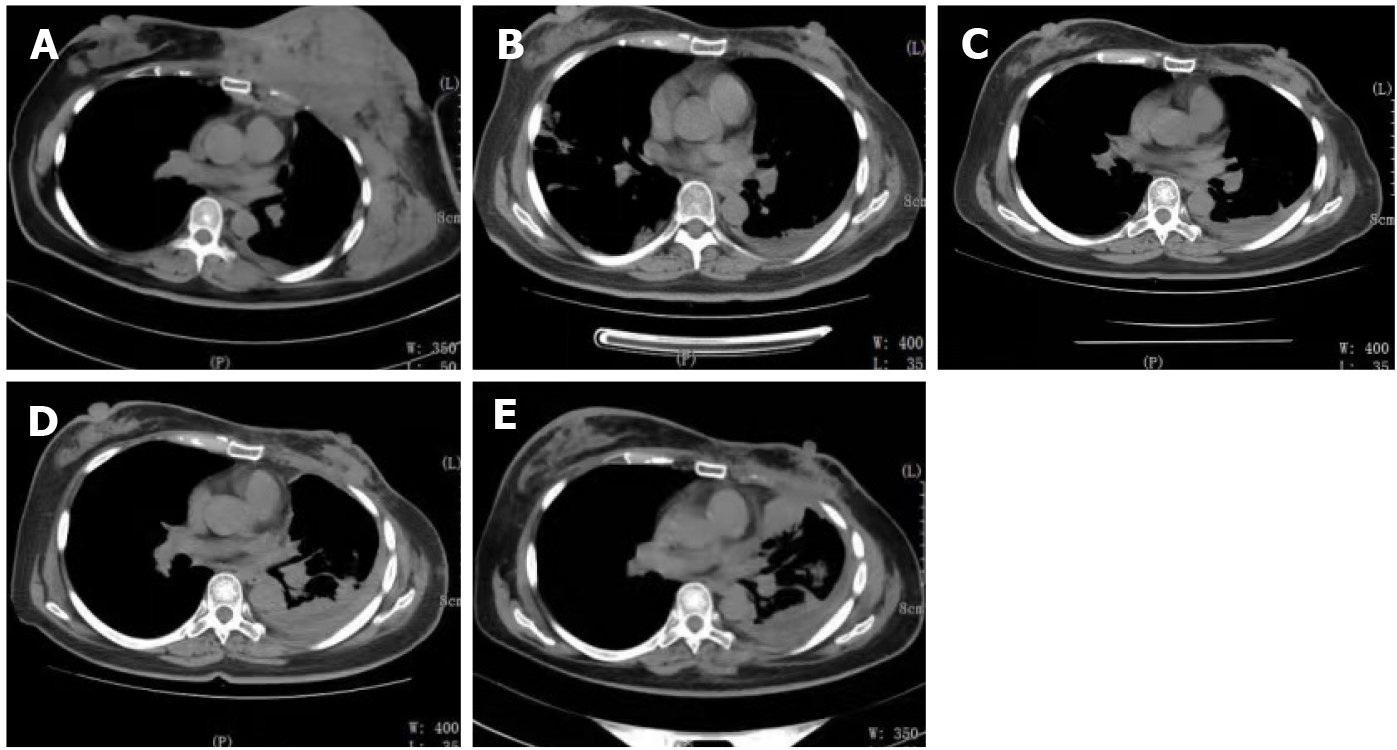
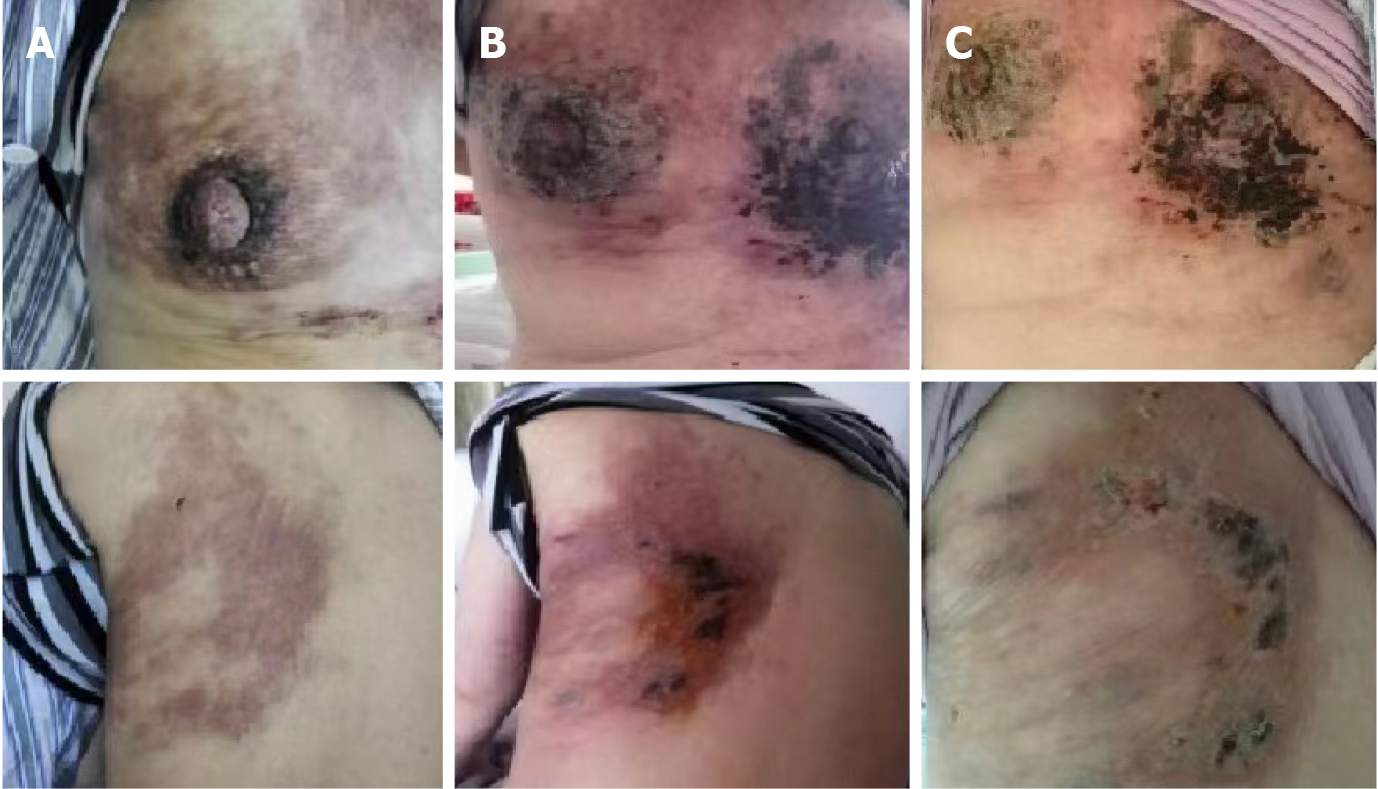
The patient visited Wangjing Hospital of CACMS (Beijing, China) after 5 mo of left breast pain. During this period, the patient received alternative therapy. The patient presented with a diffuse red subcutaneous rigid nodule over the left breast and chest wall (Figure 1A). In addition, the subcutaneous nodules were found to be necrotic, and the patient suffered from breathing difficulties due to malignant pleural effusion (Figure 2A).
The patient had a history of type 2 diabetes for more than half a year.
The patient had no history of contact with an epidemic area, smoking, or drinking, and no history of familial tumor disease.
The left axillary lymph nodes were enlarged and hard, and had poor activity. The left breast shrank, and the skin tissue hardened.
The serum CA15-3 concentration continued to be above the upper limit of the reference value.
Thoracic computed tomography (CT) showed a mass in the left breast and chest wall muscles before treatment.
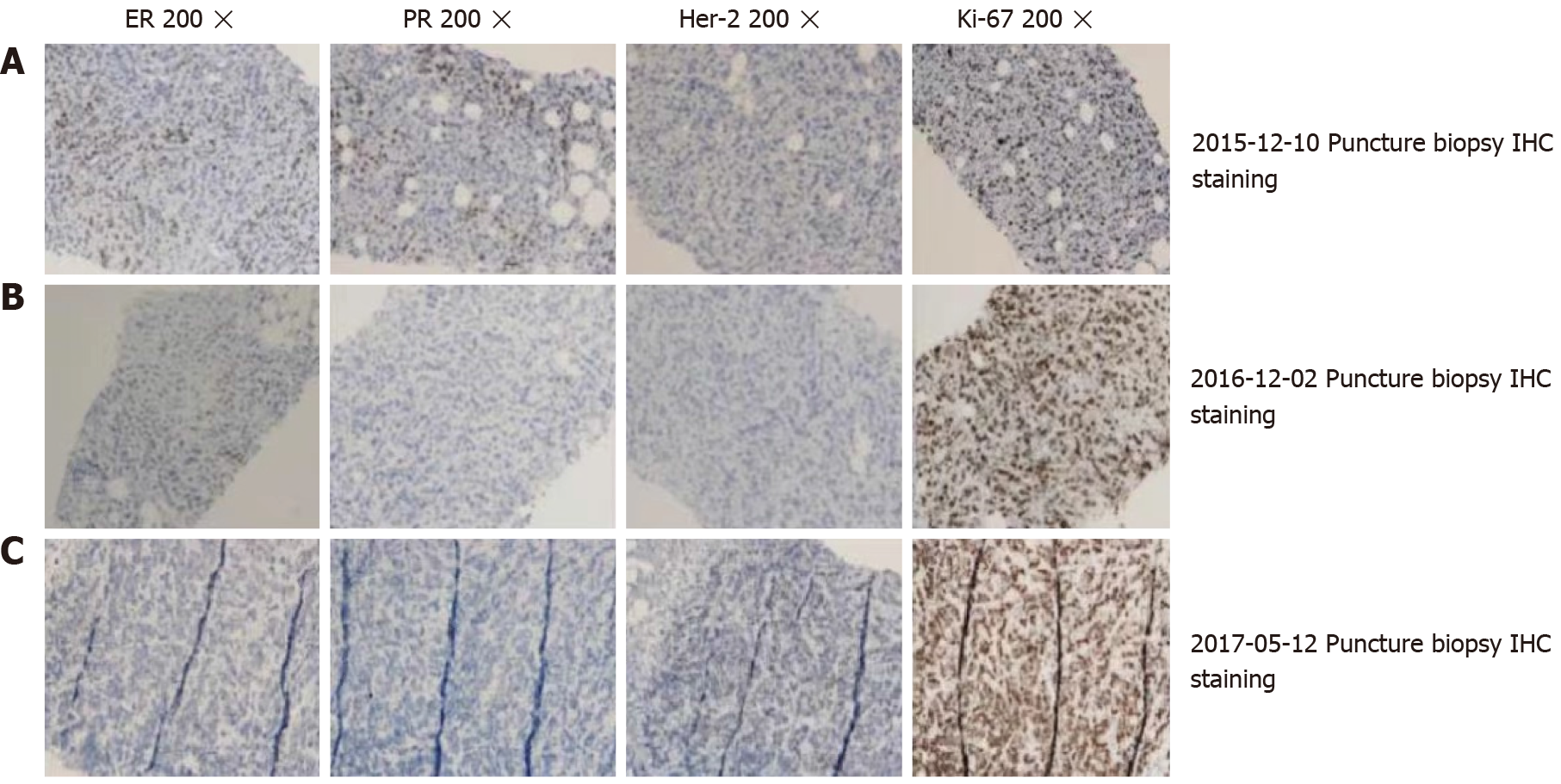
The patient was diagnosed with invasive ductal carcinoma of the left breast (stage IV) in December 2015. The pathological examination of the needle biopsy sample was performed. Immunohistochemical (IHC) analysis revealed that the tumor cells were positive for estrogen receptors and progesterone receptors, and that human epidermal growth factor receptor 2 (HER2) was not amplified (Figure 3A).
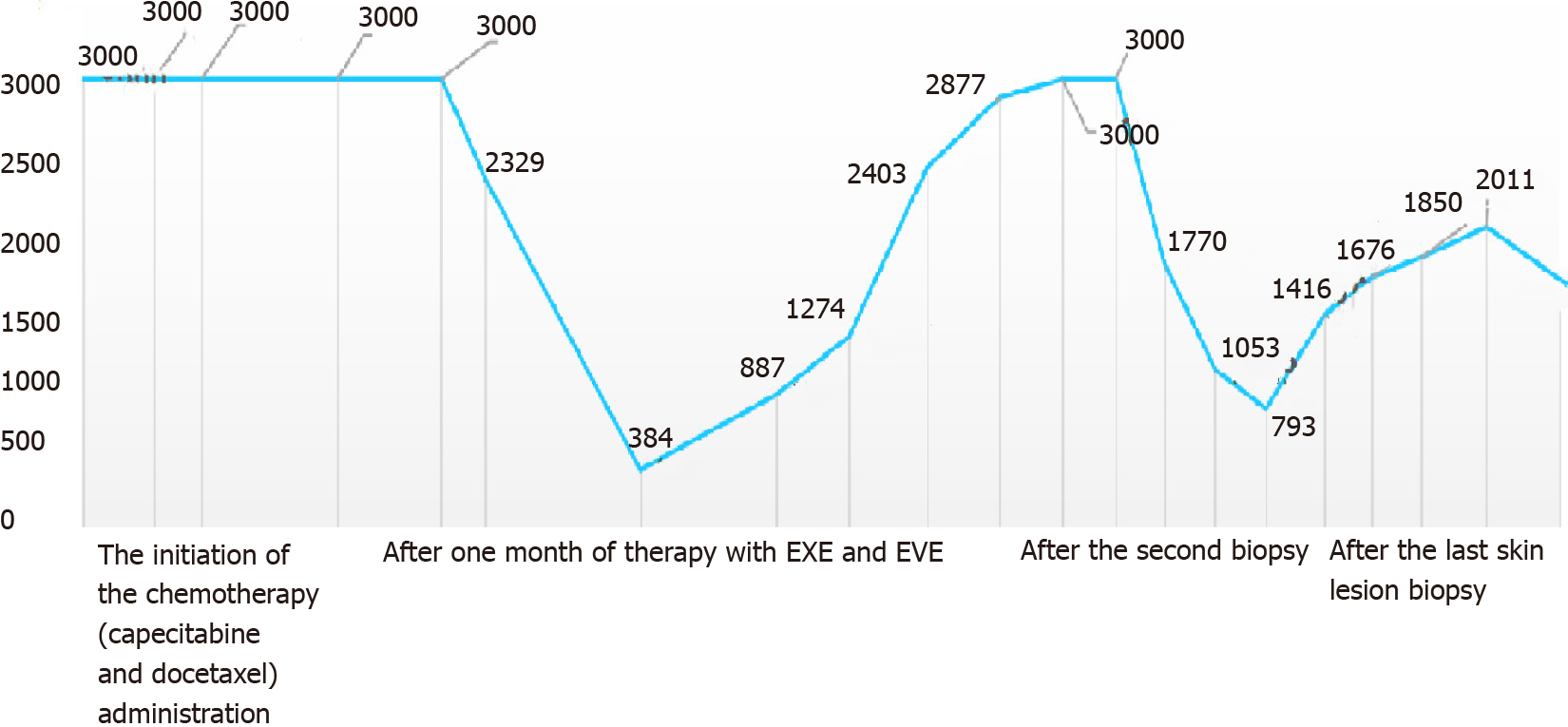
The patient received chemotherapy, which consisted of capecitabine (1500 mg/m2, twice a day on days 1-14) and docetaxel (120 mg, intravenous infusion on day one). The subcutaneous rigid nodules over the left breast and chest wall exhibited a partial response after two cycles. However, the pleural effusion increased (Figure 2B). Since the estrogen receptors and progesterone receptors were positive, the chemotherapy was changed to endocrine therapy with 25 mg of exemestane (EXE) and 10 mg of everolimus (EVE), and this commenced on April 2016. At the same time, ovarian suppression with leuprorelin was performed every 4 wk. A significant response was clinically observed after 3 mo (Figure 1B), and the cancer antigen (CA15-3) level significantly decreased (Figure 4).
The patient remained without progression for 8 mo, after which progression was noted (Figure 1C). In November 2016, progression of the metastatic skin lesions was detected (Figures 1C and 2D). Then, the serum CA15-3 concentration returned to the upper limit of the reference value. The patient received a skin punch biopsy again, and the pathology indicated triple-negative breast cancer by IHC (Figure 3B). Then, the patient received chemotherapy with adriamycin plus cyclophosphamide from December 2016 to April 2017. For the first two cycles of chemotherapy, the efficacy was evaluated as stable (Figure 2E). However, after six cycles of chemotherapy, a new red subcutaneous nodule appeared again on the left breast and chest wall skin, accompanied by stabbing pains. Hence, the patient underwent third subcutaneous nodule puncture biopsy, and the following immunostaining results were obtained: ER (10%), PR (-), HER-2 (-), and Ki-67 (90%) (Figure 3C). Although ER was positive, the ratio was low, and this could not benefit from the hormonal therapy. Hence, the patient continued to receive chemotherapy with paclitaxel liposome and carboplatin. Unfortunately, the same skin lesion progressed again after two cycles of chemotherapy. Furthermore, the metastatic lesion in the lung progressed, and the pleural effusion significantly increased. The gemcitabine chemotherapy combined with bevacizumab antiangiogenic therapy was attempted again. After one cycle of combined treatment, the skin lesion improved again, and the main signs of skin alleviation and nodule atrophy were observed. However, the patient had dyspnea due to the malignant pleural effusion. At the same time, edema began to appear on the face and neck, and the patient no longer tolerated the chemotherapy. Local treatment was chosen due to the extensive metastasis of the skin, and failure to tolerate the che
Regardless of the relief in skin lesion after the PDT and SDT treatments, the patient’s condition did not show a significant improvement.
Because the patient used large amounts of glucocorticoids during the treatment period, the patient had lung metastasis. The patient had serious lung infection, and finally died of severe pneumonia. The patient gained a 21-mo overall survival time. Comprehensive therapy and the several attempts to obtain the pathology to guide the therapy improved the overall survival and quality of life of the patient.
Breast cancer as the most prevalent neoplasm among female individuals, is also the main source of cutaneous metastasis other than melanoma. Generally, skin metastasis of breast cancer presents at the terminal stage of advanced cancer, but there are exceptions. For example, a recently reported case of skin metastasis of breast cancer in an old women belongs to this rare group since cutaneous metastasis was identified before the primary cancer. And the case also showed the interest of biopsy and imaging in the confirmation of the diagnosis[6]. The lesions of cutaneous metastasis can clinically present in various patterns. Inflammation and nodules are the most common presentations, while ulcerated nodules are relatively less common, which account for 10% of cases. Other rare clinical manifestations include zosteriform and lymphatic blockage, and carcinoma en cuirasse. One patient can have multiple types at the same time[7]. The lesions can be painless, or associated with pain and sensitivity, with fast initial growth and subsequent stabilization. The most common site of metastasis is the chest wall, as well as the site of the contralateral breast, the scars at the surgical incisional site, the arms, and the head and neck region[8]. For the present case, this was first detected in the chest and breast skin, and the main presentation was diffuse red rigid nodules.
The systematical analysis of patient survival after the occurrence of skin metastasis revealed that half of the patients with cutaneous metastasis die within the first 6 mo after the diagnosis, and that the median survival of patients with breast carcinoma with cutaneous metastasis is 13.8 mo[5]. Some breast cancer patients with local skin recurrence but without clinical evidence of disseminated disease, may have a good prognosis and prolonged survival[9]. However, the prognosis of advanced breast cancer patients with skin metastasis is generally poor.
There is no specific treatment for cutaneous metastases of breast cancer. For patients with advanced skin metastasis of breast cancer, systemic treatment such as chemotherapy, hormonal therapy, and anti-HER2 are main methods of therapy. The treatment of skin metastases from breast cancer mainly relies on puncture biopsy to identify the molecular type of breast cancer and then determine the specific treatment plan according to the molecular type, so puncture biopsy is indispensable for treatment.
The subtype of hormone receptor-positive breast cancer cutaneous metastases can be treated with hormonal therapy (aromatase inhibitors). For breast cancer endocrine therapy, intrinsic and acquired resistance remains a common feature that limits the success of this therapeutic strategy. The mammalian target of rapamycin (mTOR) signaling pathway plays a central role in cancer cell proliferation and resistance to endocrine therapies. Therefore, mTOR inhibitors, such as EVE, in combination with nonsteroidal aromatase inhibitors (NSAIs), might reverse the endocrine resistance and improve the clinical outcomes of patients. EVE in combination with different endocrine agents in the treatment of hormone receptor-positive breast cancer enhances the efficacy of the endocrine therapy in endocrine-naive patients, and in patients exposed to prior NSAIs. In a phase II randomized study, the use of EVE plus letrozole, when compared to placebo plus letrozole neoadjuvant therapy, for patients with estrogen receptor-positive breast cancer revealed that EVE can significantly increase the letrozole efficacy in the neoadjuvant therapy of patients with ER-positive breast cancer[10]. In the GINECO study, EVE combined with tamoxifen vs tamoxifen alone were used to treat patients with advanced disease pre-exposed to aromatase inhibitors, and the results suggested that tamoxifen plus EVE increased the time to progress-free survival (PFS) and overall survival, when compared to tamoxifen alone, in postmenopausal women with aromatase inhibitor-resistant metastatic breast cancer[11]. The BOLERO-2 trial revealed that EVE plus EXE is well-tolerated by patients, and provides a clinically significant PFS benefit vs EXE plus placebo, in the overall population of patients with hormone receptor-positive and HER-2 negative advanced breast cancer progressing during/after NSAI therapy[12]. The median PFS nearly tripled with EVE plus EXE vs EXE plus placebo (11.5 vs 4.1 mo, respectively) in patients whose disease recurred during or after the (neo)-adjuvant therapy[13]. These trials support the use of EVE combined with different endocrine agents in enhancing the efficacy of endocrine therapy for endocrine-naive patients, and for patients exposed to prior NSAIs. For the present case, EVE plus EXE was used to treat the skin metastasis of hormone receptor-positive breast cancer, and the patient received 8 mo of PFS. Therefore, EVE plus EXE for hormone receptor-positive breast cancer skin metastasis can better control the skin metastatic lesions.
In addition, local treatment can be used to control locally metastatic lesions. For cases that involve a huge breast mass with ulceration or local lesion, radiation therapy is a useful tool for symptomatic control. PDT[14,15] and SDT[16,17] have also been used to treat cutaneous metastases of breast cancer. Electrochemotherapy (ECT) is another new promising method for cutaneous metastasis, and represents a valuable skin-directed therapy for selected patients with breast cancer. A European study analyzed 125 patients with BC skin metastases who underwent ECT. It was revealed that the overall response rate at 2 mo was 90.2%, and the complete response rate was 58.4%. And in the first 48 h, 10.4% of the patients reported severe skin pain. Derma
The present study suggests that skin metastasis of some breast cancers is an intrinsic feature, and acquired drug resistance increases the complexity of the treatment. In addition, the molecular subtypes of breast cancer may be constantly changed from the treatment process. Therefore, evaluating the immunohistochemical assay of the lesion biopsies can directly provide a diagnosis and treatment strategy. The present patient who received EVE plus EXE therapy exhibited a significant response with the resolution of the skin lesion, which lasted for over 8 mo. But after PDT and ECT treatment, the skin will experience a temporary inflammatory reaction, sometimes with erosions or ulcers and eventually crusting. Also, in the case of tumor regression, the skin may show slight hyperpigmentation. For small local skin metastasis lesions, radiotherapy, PDT, and ECT can be used to control the local lesion and provide better quality of life for patients. However, treatment strategies for cutaneous metastases of breast cancer should focus on controlling the systemic disease, rather than the local lesion, especially in patients with extensive skin infiltration.
Provenance and peer review: Unsolicited manuscript; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alvarez-Bañuelos MT S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Wang JJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55833] [Article Influence: 7976.1] [Reference Citation Analysis (132)] |
| 2. | Hu SC, Chen GS, Wu CS, Chai CY, Chen WT, Lan CC. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 3. | Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33:161-82; quiz 183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 296] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 4. | Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 583] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 5. | Schoenlaub P, Sarraux A, Grosshans E, Heid E, Cribier B. [Survival after cutaneous metastasis: a study of 200 cases]. Ann Dermatol Venereol. 2001;128:1310-1315. [PubMed] |
| 6. | Sanae A, Kaoutar I, Jamal EF, Meryem E. Cutaneous metastasis as a first sign of breast carcinoma. Radiol Case Rep. 2021;16:1899-1901. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | De Giorgi V, Grazzini M, Alfaioli B, Savarese I, Corciova SA, Guerriero G, Lotti T. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Wong CY, Helm MA, Kalb RE, Helm TN, Zeitouni NC. The presentation, pathology, and current management strategies of cutaneous metastasis. N Am J Med Sci. 2013;5:499-504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Cho J, Park Y, Lee JC, Jung WJ, Lee S. Case series of different onset of skin metastasis according to the breast cancer subtypes. Cancer Res Treat. 2014;46:194-199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R, Bianchi G, Steinseifer J, Molloy B, Tokaji E, Gardner H, Phillips P, Stumm M, Lane HA, Dixon JM, Jonat W, Rugo HS. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630-2637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 501] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 11. | Bachelot T, Bourgier C, Cropet C, Ray-Coquard I, Ferrero JM, Freyer G, Abadie-Lacourtoisie S, Eymard JC, Debled M, Spaëth D, Legouffe E, Allouache D, El Kouri C, Pujade-Lauraine E. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718-2724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 543] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 12. | Beaver JA, Park BH. The BOLERO-2 trial: the addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer. Future Oncol. 2012;8:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 13. | Beck JT, Hortobagyi GN, Campone M, Lebrun F, Deleu I, Rugo HS, Pistilli B, Masuda N, Hart L, Melichar B, Dakhil S, Geberth M, Nunzi M, Heng DY, Brechenmacher T, El-Hashimy M, Douma S, Ringeisen F, Piccart M. Everolimus plus exemestane as first-line therapy in HR⁺, HER2⁻ advanced breast cancer in BOLERO-2. Breast Cancer Res Treat. 2014;143:459-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Khan SA, Dougherty TJ, Mang TS. An evaluation of photodynamic therapy in the management of cutaneous metastases of breast cancer. Eur J Cancer. 1993;29A:1686-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Wen X, Li Y, Hamblin MR. Photodynamic therapy in dermatology beyond non-melanoma cancer: An update. Photodiagnosis Photodyn Ther. 2017;19:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Rengeng L, Qianyu Z, Yuehong L, Zhongzhong P, Libo L. Sonodynamic therapy, a treatment developing from photodynamic therapy. Photodiagnosis Photodyn Ther. 2017;19:159-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 17. | Pan X, Wang H, Wang S, Sun X, Wang L, Wang W, Shen H, Liu H. Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics. Sci China Life Sci. 2018;61:415-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 198] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 18. | Cabula C, Campana LG, Grilz G, Galuppo S, Bussone R, De Meo L, Bonadies A, Curatolo P, De Laurentiis M, Renne M, Valpione S, Fabrizio T, Solari N, Guida M, Santoriello A, D'Aiuto M, Agresti R. Electrochemotherapy in the Treatment of Cutaneous Metastases from Breast Cancer: A Multicenter Cohort Analysis. Ann Surg Oncol. 2015;22 Suppl 3:S442-S450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Kong JH, Park YH, Kim JA, Kim JH, Yun J, Sun JM, Won YW, Lee S, Kim ST, Cho EY, Ahn JS, Im YH. Patterns of skin and soft tissue metastases from breast cancer according to subtypes: relationship between EGFR overexpression and skin manifestations. Oncology. 2011;81:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |