Published online Jan 7, 2022. doi: 10.12998/wjcc.v10.i1.227
Peer-review started: June 25, 2021
First decision: July 27, 2021
Revised: August 7, 2021
Accepted: November 28, 2021
Article in press: November 28, 2021
Published online: January 7, 2022
Processing time: 187 Days and 14.9 Hours
Helicobacter pylori (H. pylori) infection is a worldwide problem with increasing burden on the health sector due to its increasing rate of resistance. The conventional triple therapy (TT) is becoming obsolete with a high failure rate of eradication, necessitating the need for better alternatives or regimens.
To investigate H. pylori eradication rate of TT vs modified bismuth quadruple therapy.
Ninety-two patients with dyspepsia symptoms and positive 13C-urea breath test were randomly assigned to two groups. The first group (control group) was treated for 14 d using standard TT protocol: Esomeprazole (40 mg twice daily), amoxicillin (1 g twice daily) and clarithromycin (500 mg twice daily). On the other hand, the second group was prescribed a 10-d course of modified bismuth quadruple therapy fortified with zinc carnosine: TT in addition to bismuth subcitrate (240 mg twice daily) and zinc carnosine (75 mg twice daily). A repeated 13C-urea breath test was done 4 wk after the completion of the eradication therapy.
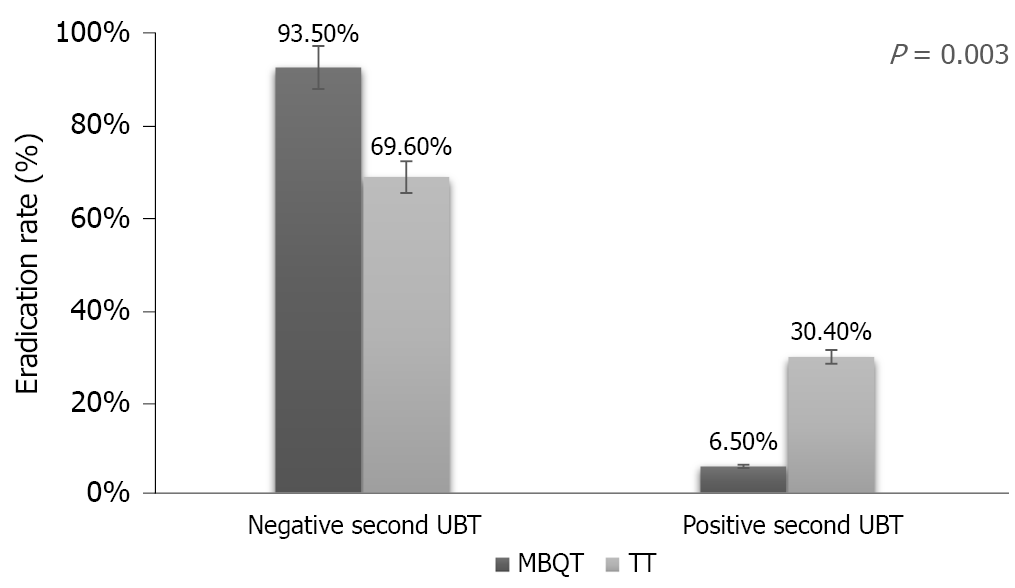
Among the 92 subjects, 67.4% were males and 32.6% were females. There were no differences in demographic characteristics (age, body mass index, smoking history, previous antibiotics use and ethnicity) between the modified bismuth quadruple therapy group and TT group. The eradication rate was higher [93.5% (43/46)] in the modified bismuth quadruple therapy group compared to 69.6% (32/46) in the standard TT group (P = 0.003). Of the tested predictor variables, only nationality, smoking and therapy type were statistically significant. Besides dizziness, which was recorded in modified bismuth quadruple therapy group, there were no significant differences in side effects between the two groups.
Ten days of modified bismuth quadruple therapy fortified with zinc carnosine is superior to 14 d of conventional TT in eradicating H. pylori infection, with no additional significant adverse events.
Core Tip: High eradication failure rate of Helicobacter pylori (H. pylori) infection has been reported due to increasing antibiotic resistance. This necessitates the need for better alternative regimens. The present study revealed higher H. pylori eradication rate with the use of zinc carnosine-based modified bismuth quadruple therapy for 10 d than with 14 d of standard triple therapy.
- Citation: Ibrahim N, El Said H, Choukair A. Zinc carnosine-based modified bismuth quadruple therapy vs standard triple therapy for Helicobacter pylori eradication: A randomized controlled study. World J Clin Cases 2022; 10(1): 227-235
- URL: https://www.wjgnet.com/2307-8960/full/v10/i1/227.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v10.i1.227
Since its first successful culture in the laboratory almost 40 years ago, Helicobacter pylori (H. pylori) infection and gastric diseases have been a source of debate among medical professionals and scientists[1,2]. This bacterium, which is among very few organisms that can survive in the human stomach, has gained much reputation, mostly as a harmful bacterium, based on its association with various gastroduodenal diseases[3]. H. pylori is a highly prevalent helical shaped gram-negative bacterium that colonizes and infects the human gastric mucosa in approximately more than 50% of the world’s population[4]. One conducted cross-sectional study in the United Arab Emirates revealed that the prevalence of H. pylori among healthy children and adults was 40%[5]. Infection can, at a minimum, cause gastritis and is a prominent etiologic agent of gastric and duodenal ulcer diseases, gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma[2,6,7]. The development of peptic ulcers arises in about 10%-20% of patients infected with H. pylori, while advancement to gastric cancer occurs in 1%-3% of cases[8].
Triple therapy (TT), which includes a proton pump inhibitor (PPI) and two antimicrobial agents (clarithromycin and amoxicillin or metronidazole) prescribed for 10 to 14 d, has been the standard first-line eradication therapy since 1996[9]. However, due to the increased rate of clarithromycin or metronidazole resistance, standard TT has been often ineffective in regions with high antibiotic resistance[10,11]. In fact, one systematic review and meta-analysis revealed a high resistance rate (≥ 15%) of H. pylori to clarithromycin and metronidazole in World Health Organization regions[12]. This has led to a detrimental effect on the efficacy of the triple treatment regimen, as its eradication response now falls considerably short between 50%-70%[13]. This is considered far below the minimal acceptable level of intention-to-treat (ITT) eradication rate (> 80%) as recommended by Maastricht guidelines[14]. As a result, four-drug regimens (quadruple, sequential, concomitant and hybrid) and levofloxacin-containing therapies have been studied with variable success[15,16]. Later on, a bismuth based treatment, now known as bismuth quadruple therapy, was suggested as an alternative initial therapy option, especially in regions where high rates of antibiotic resistance exist[13].
Studies have shown that the efficacy of bismuth in H. pylori treatment regimens is mainly associated with its bactericidal effect against H. pylori[17]. Various means that aid bismuth to exert such a role have been proposed[18]. Ultrastructural studies showed that bismuth bind the bacterial wall and periplasmic membrane, thus forming complexes[19]. Moreover, experiments revealed that bismuth is capable of inhibiting various enzymes of H. pylori such as urease, phospholipase and catalase[20]. One other mechanism through which bismuth exerts its anti-H. pylori actions is by inhibiting the pathogen’s protein and adenosine triphosphate synthesis and preventing its adherence to the gastric mucosa[21]. Bismuth compounds also protect the gastric mucosa and aid in ulcer healing[22]. No resistance of strains of H. pylori to bismuth has been reported yet.
Another potential adjuvant therapy that has been evaluated to enhance the eradication of H. pylori is polaprezinc (PZ). PZ, a chelated compound composed of L-carnosine and zinc, is a mucosal protective agent[23] that has been used worldwide as a treatment for ulcers[24]. PZ prevents the formation of gastric mucosal lesions and mucosal cell damage induced by H. pylori–associated gastritis in a dose-dependent manner[25,26]. This role has been attributable to various properties possessed by PZ such as stimulating the production of mucus, exerting its stabilizing-membrane activity and having an antioxidant action[27-30]. Moreover, some studies revealed that PZ led to an improvement in eradication rates of H. pylori[31,32] by inhibiting the growth of H. pylori, in addition to impeding its urease activity and adhesion to gastric mucin.
We, hereby, present a study that compares the eradication responses of H. pylori obtained from a standard TT regimen vs a modified one that constitutes a standard TT regimen enforced by two adjuvants: Bismuth subcitrate and the nutritional supplement zinc carnosine (modified bismuth quadruple therapy or MBQT).
The present study was a prospective, open-label, randomized, controlled trial performed between 2018-2019. Physicians were not blinded to which treatment the subjects received. The patient population comprised 92 consecutive outpatients who presented to outpatient clinic with dyspepsia symptoms and were found to have H. pylori infection. H. pylori infection was diagnosed by 13C urea breath test (UBT) and reassessed 4 wk after the completion of the assigned treatment. The exclusion criteria were: Age < 18 years, existence of severe concomitant diseases, use of medications effective against H. pylori such as bismuth compounds, PPIs, or antibiotics during the last 3 mo, history of gastroduodenal surgery, pregnancy or lactation, chronic corticosteroid or nonsteroidal anti-inflammatory drug use, history of allergy to PPI, macrolides or penicillin, alcohol abuse or drug addiction. Prior to enrolment, a written informed consent was obtained from all patients. This study was approved by the Clinical Research Ethics Committee of NMC specialty hospital.
The enrolled patients were randomized by drawing a sealed envelope that contained pre-assigned treatment instructions. They were allocated to one of the following two groups. Group A (TT group) received esomeprazole 40 mg, clarithromycin 500 mg and amoxicillin 1 g and all the medications were given twice daily for subsequent 14 d. On the other hand, group B (MBQT group) received zinc carnosine (gastrozin) 75 mg and bismuth subcitrate 240 mg in combination with esomeprazole 40 mg, clarithromycin 500 mg and amoxicillin 1 g. All the later medications were given twice daily for subsequent 10 d. Compliance with medication was checked immediately after stopping the treatment by counting the number of returned pills. Four weeks after cessation of the eradication therapy, a repeated UBT was done.
All data entry and statistical analyses were carried out using SPSS version 26.0 for Windows (SPSS, Armonk, NY, United States). The cure rate was then calculated for each arm. Chi-square test, Fisher’s exact test and independent-samples T-test were used to compare the major outcomes between these groups. H. pylori cure rate was evaluated by per protocol (PP) analysis. PP analysis included all patients who took at least 80% of each study medication as prescribed and returned for assessment of H. pylori cure. Multivariable analysis adjusted for sex, age, body mass index, smoking habits, previous antibiotics intake and ethnicity was performed. A P value less than 0.05 was considered statistically significant.
In this study, there were a total of 92 subjects of which 62 (67.4%) were males and 30 (32.6%) were females. Ages ranged from 19 to 56 years (mean of 31.88 ± 8.09 years). Most patients (60.9%) were Asian. This was followed by Arab (28.3%) and African (10.9%). Most subjects (81.5%) were non-smokers. Body mass index ranged from 17.20 to 43.70 kg/m2 (mean of 26.37 ± 4.12 kg/m2). Table 1 shows the demographic characteristics of the two tested groups. Of the two types of therapy, there were 46 (50%) individuals in the MBQT group and 46 (50%) in the TT group (P = 0.003). Among subjects in the MBQT group, 43 tested negative on the repeated UBT test and 3 tested positive. In the TT group, 32 tested negative and 14 tested positive (Figure 1).
| Variable | MBQT (n = 46) | TT (n = 46) |
| (mean ± SD) | ||
| Age | 35.83 ± 7.41 yr | 27.93 ± 6.78 yr |
| Mean body mass index | 26.08 ± 4.57 kg/m2 | 26.67 ± 3.65 kg/m2 |
| % (n) | ||
| Sex | ||
| Female | 32.6% (15) | 32.6% (15) |
| Male | 67.4% (31) | 67.4% (31) |
| Patients who smoke | 17.4% (8) | 19.6% (9) |
| Patients with previous antibiotics intake | 17.4 % (8) | 4.3% (2) |
| Ethnicity | ||
| African | 10.9% (5) | 10.9% (5) |
| Araba | 26.1% (12) | 30.4% (14) |
| Asianb | 63% (29) | 58.7% (27) |
Binary logistic regression models were fitted in order to determine the efficacy of the MBQT in the eradication of H. pylori compared with the standard TT. All assumptions of logistic regression were met. Of the predictor variables, only three were statistically significant: Nationality, smoking and therapy type (Table 2).
| P | aOR | 95%CI | ||
| Lower | Upper | |||
| Age | 0.662 | 1.020 | 0.93 | 1.12 |
| Sex, female | 0.367 | 1.874 | 0.48 | 7.33 |
| Therapy (TT) | 0.004 | 11.440 | 2.18 | 60.07 |
| Smoking | 0.049 | 5.120 | 1.01 | 26.1 |
| Nationality (Asian)a | 0.049 | |||
| Nationality (African) | 0.368 | 0.351 | 0.036 | 3.43 |
| Nationality (Arabs) | 0.019 | 0.114 | 0.019 | 0.67 |
| History of antibiotics | 0.294 | 3.15 | 0.37 | 26.86 |
Patients who received TT were 11 times more likely to have a positive UBT than those who received MBQT [adjusted odds ratio (aOR) = 11.44, P = 0.004, 95% confidence interval (CI): 2.179-60.07]. Moreover, Arabs were more likely to obtain negative UBT than Asians (aOR = 0.19, P = 0.019, 95% CI 0.019-0.696). Furthermore, smoking seemed to increase the odds of persistent H. pylori by 5-fold (aOR = 5.12, P = 0.049, 95% CI: 1.005-26.097).
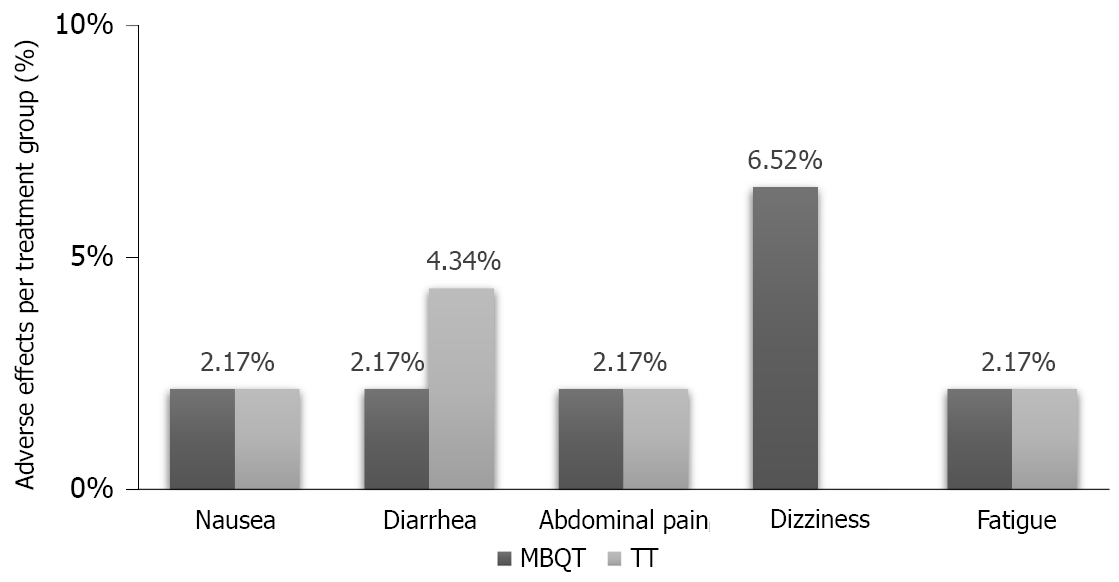
There were some adverse events that occurred in each of the two types of therapies. In the MBQT group, there was one occurrence (2.17% each) of nausea, abdominal pain and fatigue and three occurrences (6.52%) of dizziness. On the other hand, the TT group reported one occurrence (2.17% each) of nausea, diarrhea and fatigue and two occurrences (4.34%) of abdominal pain (Figure 2).
In this prospective study, we aimed to tackle the issue of increased failure rate of standard antimicrobial therapies by combining the benefits and positive effects of both bismuth compound and zinc carnosine in a single regimen protocol. The latter was used to enhance the effect of antimicrobial therapy in eradicating H. pylori infection, while at the same time maintaining a safe profile of the regimen with good patient compliance to the treatment course. In fact, our study was able to show that adding bismuth subcitrate and zinc carnosine to the standard therapy was associated with an increase in negative UBT results, leading to a better eradication rate of H. pylori in that subgroup of patients.
The recent Kyoto global consensus categorized H. pylori-induced gastritis as an infectious disease and recommended performing H. pylori eradication before premalignant changes develop to prevent gastric carcinogenesis[33]. However, the previously assigned first-line choice for H. pylori eradication, which is a clarithromycin-based TT, has become a subject of argument in the medical field due to the worldwide growing resistance to clarithromycin, especially in developing countries[13]. In fact, several regimens have been proposed to overcome this critical concern. One of these regimens that added bismuth as adjuvant to other antimicrobial agents was found to exert synergistic effect that improved eradication rates by almost 30%[34]. In another clinical trial in China, a bismuth-quadruple therapy achieved a 92.7% eradication of H. pylori by ITT analysis[35]. Our study confirms the latter reports on a better eradication rate of the infection with MBQT. Recent studies from other countries suggested that the use of B-quadruple therapy is remarkably effective even in the presence of antibiotic resistance and prior treatment failures[36,37]. On the other hand, a 10-d course of quadruple therapy, consisting of the mucoprotective agent sofalcone added to rabeprazole, amoxicillin and clarithromycin, demonstrated satisfactory treatment outcome with H. pylori eradication rate being not less than 94% on the PP basis[31]. In addition to this, the concomitant use of PZ with TT regimen had previously shown promise in increasing the eradication rate of H. pylori infection. In 1999, Kashimura et al[31] revealed that H. pylori eradication rate can be significantly increased from 77.4% to 94.3% when PZ is added to the TT. In a more recent study, Tan et al[38] (2017) reported that the combined use of PZ with TT improved the eradication rate of H. pylori by 18.4% (ITT analysis) and 19.7% (PP analysis). This has been further validated by the results of our study where eradication rates were higher by 23.9% in MBQT group in comparison with that of TT group. Indeed, this points out the added benefit of using PZ concomitantly with bismuth in increasing H. pylori eradication rates. However, further studies are needed to compare and evaluate the efficacy of PZ solely vs when combined with bismuth for the eradication of H. pylori.
Many factors may affect eradication efficacy such as the physical structure of the patient, smoking habits, adherence to the prescribed regimen, genetic predisposition of cytochrome p450 2C19, which metabolizes PPIs, and frequency of strains resistant to antimicrobials[39,40]. In the present study, there were no significant differences in the baseline characteristics among the trial arms. Although the susceptibility of H. pylori to antibiotics was not assessed in our study, the risk of antibiotic resistance was minimized by excluding patients who had taken previous treatments effective against the organism. In addition to that, our study also showed that being a smoker increased the risk of treatment failure by 5-fold, which comes in concordance with other studies revealing the negative effect of smoking on the eradication rate[41,42]. Another interesting finding was ethnic variability regarding eradication success, where being of Arabic ethnicity increased the odds of eradication success. This could be pertained to ethnic disparities in dietary intake. Hołubiuk et al[43] revealed promising data regarding the anti-H. pylori activity of certain food products present in fruits and vegetables, which is highly consumed in the Middle East[44]. Moreover, another possible factor that may account for such difference is the variability of antibiotics resistant strains among ethnicities or countries due to antibiotics abuse and use. However, the small sample size of our studied population questions the true significance of this finding.
In terms of safety, there has been concerns for bismuth induced neurotoxicity, mostly associated with chronic use[45]. However, in our trial, no bismuth related adverse effects were noted in the MBQT group. In addition, all-cause adverse events in both groups were tolerable and minor and had no influence on patient compliance.
By performing in depth analysis of our study, several limitations were found. Firstly, our study would have benefited from an analysis of H. pylori cultures and antibiograms. This was not feasible for technical and financial causes; hence, the exact role of antibiotic resistance (namely to clarithromycin) in eradication failure could not be evaluated. Another limitation was the study’s lack of double blinding and long-term follow-up period. A third technical limitation was the restricted availability of bismuth, which led to a smaller sample size than what we initially planned. Finally, the information on prior macrolide use was collected from patients using a questionnaire and may have therefore been subject to recall bias.
In conclusion, our study provides more evidence that 10-d modified B-quadruple therapy is a safe and effective therapeutic option for eradicating H. pylori infection. This significant rate of success should promote such therapies to be considered as first line option in place of the old and declining TT protocol.
The rate of resistance of Helicobacter pylori (H. pylori) infection has been increasing worldwide. It is necessary to consider new alternatives to overcome the failure of H. pylori eradication rate.
There is shortage in reports on whether zinc carnosine is effective against H. pylori eradication.
Investigate the effect of triple therapy (TT) vs modified bismuth quadruple therapy against H. pylori eradication rate.
Ninety-two patients with dyspepsia symptoms and positive 13C-urea breath test were randomly assigned in to the following two groups: TT group treated for 14 d using esomeprazole (40 mg twice daily), amoxicillin (1 g twice daily) and clarithromycin (500 mg twice daily). On the other hand, the modified bismuth quadruple therapy fortified with zinc carnosine was prescribed a 10-d of TT in addition to bismuth subcitrate (240 mg twice daily) and zinc carnosine (75 mg twice daily). A 13C-urea breath test was repeated after 4 wk from the completion of the eradication therapy.
The eradication rate was higher in the modified bismuth quadruple therapy group compared to that of the standard TT group (P = 0.003).
Ten-day modified bismuth quadruple therapy is a safe and effective regimen for eradicating H. pylori infection.
The first-line therapy for H. pylori eradication should be re-evaluated. Alternative regimens with higher eradication of H. pylori should be further investigated.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and Hepatology
Country/Territory of origin: Lebanon
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chattopadhyay S S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3302] [Cited by in RCA: 3265] [Article Influence: 79.6] [Reference Citation Analysis (1)] |
| 2. | Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 953] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 3. | Cover TL, Blaser MJ. Helicobacter pylori and gastroduodenal disease. Annu Rev Med. 1992;43:135-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19 Suppl 1:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 344] [Article Influence: 31.3] [Reference Citation Analysis (1)] |
| 5. | Leja M, Grinberga-Derica I, Bilgilier C, Steininger C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter. 2019;24 Suppl 1:e12635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Blaser MJ. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990;161:626-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 478] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 7. | Peek RM Jr, Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol. 2006;208:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 446] [Article Influence: 22.3] [Reference Citation Analysis (2)] |
| 8. | Noto JM, Peek RM Jr. Helicobacter pylori: an overview. Methods Mol Biol. 2012;921:7-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Chey WD, Wong BC; Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 830] [Article Influence: 46.1] [Reference Citation Analysis (3)] |
| 10. | Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 736] [Article Influence: 49.1] [Reference Citation Analysis (1)] |
| 11. | Papastergiou V, Georgopoulos SD, Karatapanis S. Treatment of Helicobacter pylori infection: Past, present and future. World J Gastrointest Pathophysiol. 2014;5:392-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 12. | Savoldi A, Carrara E, Graham DY, Conti M, Tacconelli E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology. 2018;155:1372-1382.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 617] [Cited by in RCA: 823] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 13. | Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, El-Omar EM, Kuipers EJ; European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1719] [Cited by in RCA: 1591] [Article Influence: 122.4] [Reference Citation Analysis (5)] |
| 14. | Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1396] [Cited by in RCA: 1352] [Article Influence: 75.1] [Reference Citation Analysis (1)] |
| 15. | Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, Hassan C, Bernabucci V, Tampieri A, Morini S. Sequential therapy versus standard triple-drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med. 2007;146:556-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 211] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Liou JM, Lin JT, Chang CY, Chen MJ, Cheng TY, Lee YC, Chen CC, Sheng WH, Wang HP, Wu MS. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut. 2010;59:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Alkim H, Koksal AR, Boga S, Sen I, Alkim C. Role of Bismuth in the Eradication of Helicobacter pylori. Am J Ther. 2017;24:e751-e757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Ge R, Chen Z, Zhou Q. The actions of bismuth in the treatment of Helicobacter pylori infections: an update. Metallomics. 2012;4:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Keogan DM, Griffith DM. Current and potential applications of bismuth-based drugs. Molecules. 2014;19:15258-15297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Lambert JR, Midolo P. The actions of bismuth in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1997;11 Suppl 1:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | McColm AA, McLaren A, Klinkert G, Francis MR, Connolly PC, Grinham CJ, Campbell CJ, Selway S, Williamson R. Ranitidine bismuth citrate: a novel anti-ulcer agent with different physico-chemical characteristics and improved biological activity to a bismuth citrate-ranitidine admixture. Aliment Pharmacol Ther. 1996;10:241-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Tanaka S, Guth PH, Paulsen G, Kaunitz JD. Gastroprotective effect of ranitidine bismuth citrate is associated with increased mucus bismuth concentration in rats. Gut. 1996;39:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev. 1993;73:79-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1874] [Cited by in RCA: 1800] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 24. | Hewlings S, Kalman D. A Review of Zinc-L-Carnosine and Its Positive Effects on Oral Mucositis, Taste Disorders, and Gastrointestinal Disorders. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Ishihara R, Iishi H, Sakai N, Yano H, Uedo N, Narahara H, Iseki K, Mikuni T, Ishiguro S, Tatsuta M. Polaprezinc attenuates Helicobacter pylori-associated gastritis in Mongolian gerbils. Helicobacter. 2002;7:384-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Handa O, Yoshida N, Tanaka Y, Ueda M, Ishikawa T, Takagi T, Matsumoto N, Naito Y, Yoshikawa T. Inhibitory effect of polaprezinc on the inflammatory response to Helicobacter pylori. Can J Gastroenterol. 2002;16:785-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Yoshikawa T, Naito Y, Tanigawa T, Yoneta T, Yasuda M, Ueda S, Oyamada H, Kondo M. Effect of zinc-carnosine chelate compound (Z-103), a novel antioxidant, on acute gastric mucosal injury induced by ischemia-reperfusion in rats. Free Radic Res Commun. 1991;14:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Cho CH, Luk CT, Ogle CW. The membrane-stabilizing action of zinc carnosine (Z-103) in stress-induced gastric ulceration in rats. Life Sci. 1991;49:PL189-PL194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Arakawa T, Satoh H, Nakamura A, Nebiki H, Fukuda T, Sakuma H, Nakamura H, Ishikawa M, Seiki M, Kobayashi K. Effects of zinc L-carnosine on gastric mucosal and cell damage caused by ethanol in rats. Correlation with endogenous prostaglandin E2. Dig Dis Sci. 1990;35:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Ohata S, Moriyama C, Yamashita A, Nishida T, Kusumoto C, Mochida S, Minami Y, Nakada J, Shomori K, Inagaki Y, Ohta Y, Matsura T. Polaprezinc Protects Mice against Endotoxin Shock. J Clin Biochem Nutr. 2010;46:234-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Kashimura H, Suzuki K, Hassan M, Ikezawa K, Sawahata T, Watanabe T, Nakahara A, Mutoh H, Tanaka N. Polaprezinc, a mucosal protective agent, in combination with lansoprazole, amoxycillin and clarithromycin increases the cure rate of Helicobacter pylori infection. Aliment Pharmacol Ther. 1999;13:483-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Sakae K, Yanagisawa H. Oral treatment of pressure ulcers with polaprezinc (zinc L-carnosine complex): 8-week open-label trial. Biol Trace Elem Res. 2014;158:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1322] [Cited by in RCA: 1185] [Article Influence: 118.5] [Reference Citation Analysis (0)] |
| 34. | Dore MP, Lu H, Graham DY. Role of bismuth in improving Helicobacter pylori eradication with triple therapy. Gut. 2016;65:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 35. | Liu KS, Hung IF, Seto WK, Tong T, Hsu AS, Lam FY, But DY, Wong SY, Leung WK. Ten day sequential vs 10 day modified bismuth quadruple therapy as empirical firstline and secondline treatment for Helicobacter pylori in Chinese patients: an open label, randomised, crossover trial. Gut. 2014;63:1410-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 36. | Ciccaglione AF, Tavani R, Grossi L, Cellini L, Manzoli L, Marzio L. Rifabutin Containing Triple Therapy and Rifabutin with Bismuth Containing Quadruple Therapy for Third-Line Treatment of Helicobacter pylori Infection: Two Pilot Studies. Helicobacter. 2016;21:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Muller N, Amiot A, Le Thuaut A, Bastuji-Garin S, Deforges L, Delchier JC. Rescue therapy with bismuth-containing quadruple therapy in patients infected with metronidazole-resistant Helicobacter pylori strains. Clin Res Hepatol Gastroenterol. 2016;40:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Tan B, Luo HQ, Xu H, Lv NH, Shi RH, Luo HS, Li JS, Ren JL, Zou YY, Li YQ, Ji F, Fang JY, Qian JM. Polaprezinc combined with clarithromycin-based triple therapy for Helicobacter pylori-associated gastritis: A prospective, multicenter, randomized clinical trial. PLoS One. 2017;12:e0175625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Suzuki T, Matsuo K, Ito H, Sawaki A, Hirose K, Wakai K, Sato S, Nakamura T, Yamao K, Ueda R, Tajima K. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119:217-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Miyoshi M, Mizuno M, Ishiki K, Nagahara Y, Maga T, Torigoe T, Nasu J, Okada H, Yokota K, Oguma K, Tsuji T. A randomized open trial for comparison of proton pump inhibitors, omeprazole versus rabeprazole, in dual therapy for Helicobacter pylori infection in relation to CYP2C19 genetic polymorphism. J Gastroenterol Hepatol. 2001;16:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Camargo MC, Piazuelo MB, Mera RM, Fontham ET, Delgado AG, Yepez MC, Ceron C, Bravo LE, Bravo JC, Correa P. Effect of smoking on failure of H. pylori therapy and gastric histology in a high gastric cancer risk area of Colombia. Acta Gastroenterol Latinoam. 2007;37:238-245. [PubMed] |
| 42. | Itskoviz D, Boltin D, Leibovitzh H, Tsadok Perets T, Comaneshter D, Cohen A, Niv Y, Levi Z. Smoking increases the likelihood of Helicobacter pylori treatment failure. Dig Liver Dis. 2017;49:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 43. | Hołubiuk Ł, Imiela J. Diet and Helicobacter pylori infection. Prz Gastroenterol. 2016;11:150-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 45. | Slikkerveer A, de Wolff FA. Pharmacokinetics and toxicity of bismuth compounds. Med Toxicol Adverse Drug Exp. 1989;4:303-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 137] [Article Influence: 3.8] [Reference Citation Analysis (0)] |