Copyright
©The Author(s) 2021.
World J Clin Cases. Nov 26, 2021; 9(33): 10198-10207
Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10198
Published online Nov 26, 2021. doi: 10.12998/wjcc.v9.i33.10198
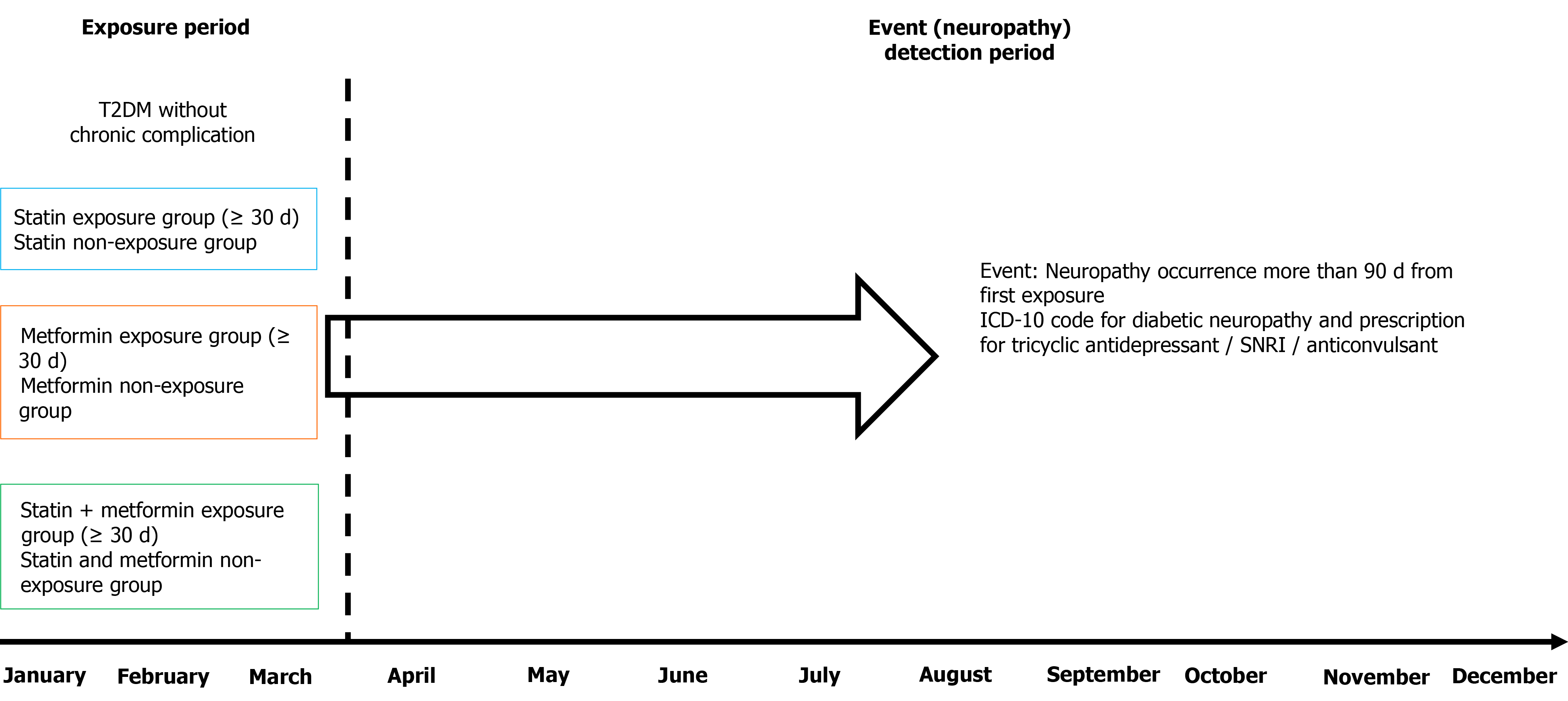
Figure 1 Schematic of the study plan including the medication exposure period and event (newly developed neuropathy) detection period.
Neuropathy was checked as new onset neuropathy that was detected at least 90 d after first exposure to issued medication to reduce detection bias and determine whether new onset neuropathy was affected by these medications. T2DM: Type 2 diabetes mellitus; ICD-10: International Statistical Classification of Diseases and Related Health Problems, 10th revision; SNRI: Serotonin-norepinephrine reuptake inhibitor.
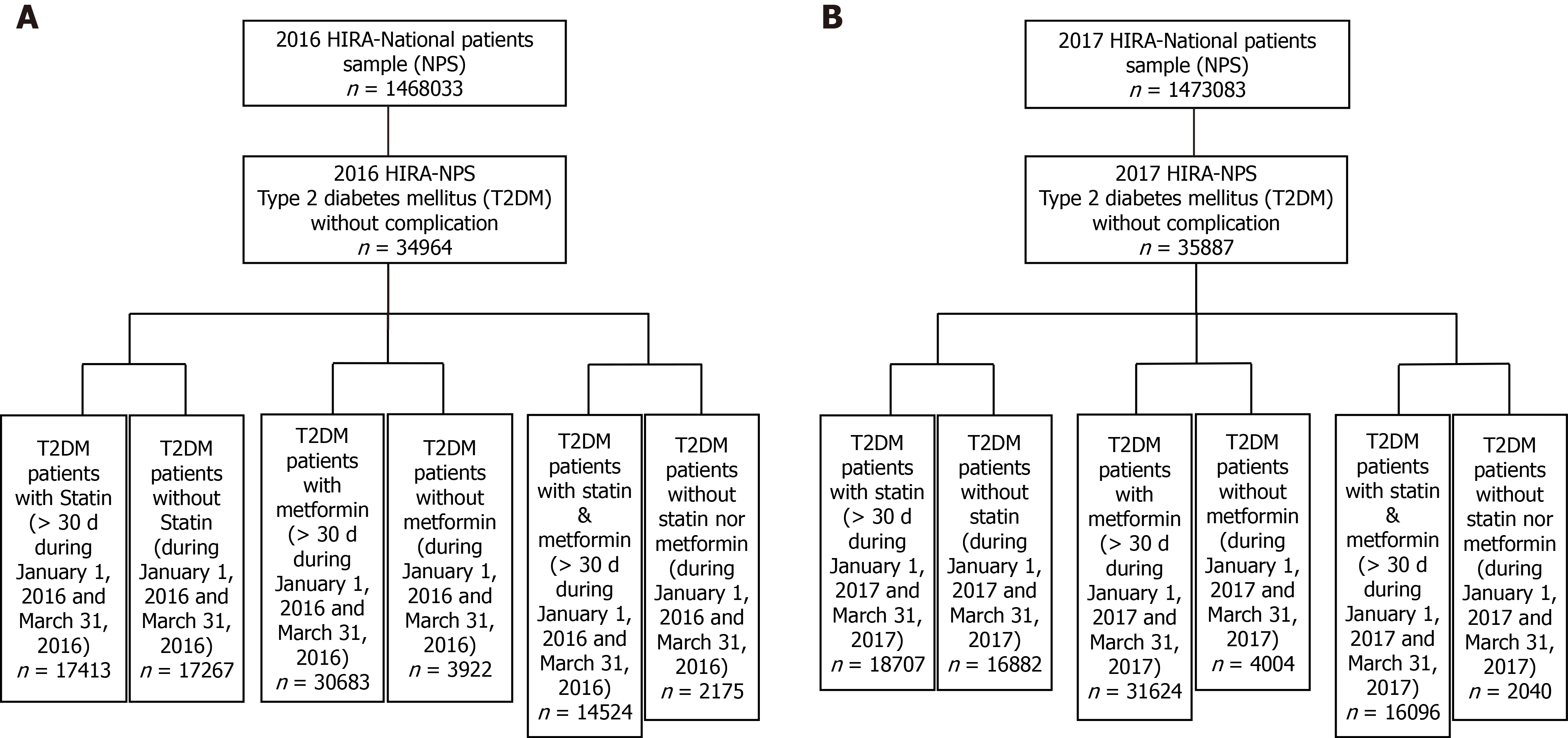
Figure 2 Flow charts of the step-wise inclusion of participants from the 2016 and 2017 Health Insurance Review and Assessment national patient sample.
A: 2016; B: 2017. HIRA: Health Insurance Review and Assessment; NPS: National patient sample; T2DM: Type 2 diabetes mellitus.
- Citation: Min HK, Kim SH, Choi JH, Choi K, Kim HR, Lee SH. Impacts of statin and metformin on neuropathy in patients with type 2 diabetes mellitus: Korean Health Insurance data. World J Clin Cases 2021; 9(33): 10198-10207
- URL: https://www.wjgnet.com/2307-8960/full/v9/i33/10198.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i33.10198