Copyright
©The Author(s) 2021.
World J Clin Cases. Jul 6, 2021; 9(19): 5270-5279
Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5270
Published online Jul 6, 2021. doi: 10.12998/wjcc.v9.i19.5270
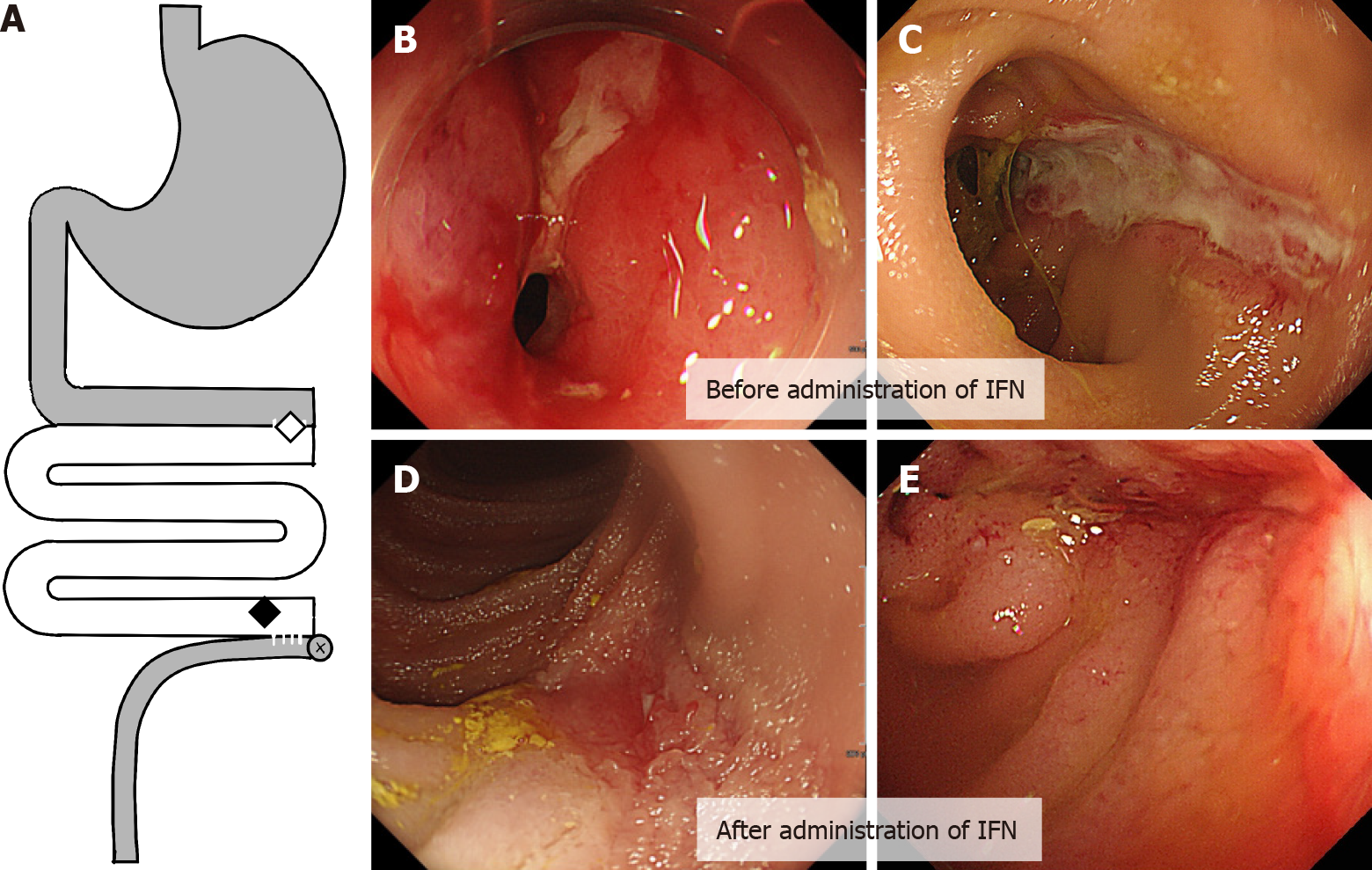
Figure 1 Ulcerative lesion in graft before and after administration infliximab.
A: The location of the diseases in the graft; B and C: Stenosis with inflammation in the proximal anastomosis between the graft and the original intestine (B, indicated by a white diamond in A), and a longitudinal ulcerative lesion in the distal end of the graft (C, indicated by a black diamond in A); D and E: Mucosal healing was observed in the distal ulcerative lesion after the administration of two doses of infliximab. IFN: Infliximab.
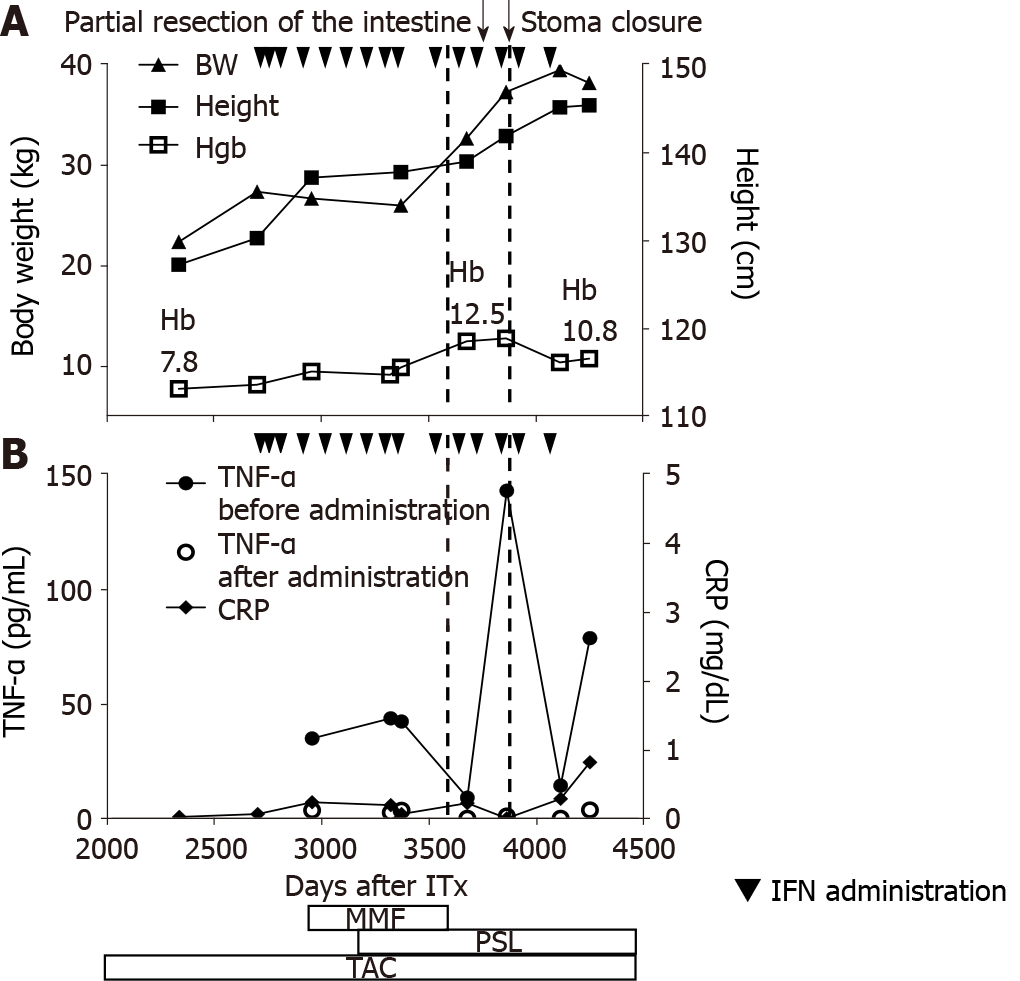
Figure 2 Clinical course during infliximab administration.
A: Height and body weight indicating a steady growth, in line with the initiation of infliximab (5 mg/kg). Hemoglobin (g/dL) level rapidly recovered. Following attenuation in the clinical response, the dose was increased to 7.5 mg/kg and immunomodulatory agents were added (mycophenolate mofetil was later switched with oral prednisolone); B: Dots (●) indicate the trough serum tissue necrosis factor alpha level, which declined immediately after each infliximab infusion (indicated by squares). In contrast, almost normal white blood cell counts and C-reactive protein level were observed throughout the course of treatment. Partial resection and stoma reversal were performed (indicated by dotted lines). IFN: Infliximab; MMF: Mycophenolate mofetil; PSL: Prednisolone; TAC: Tacrolimus; Hb: Hemoglobin.
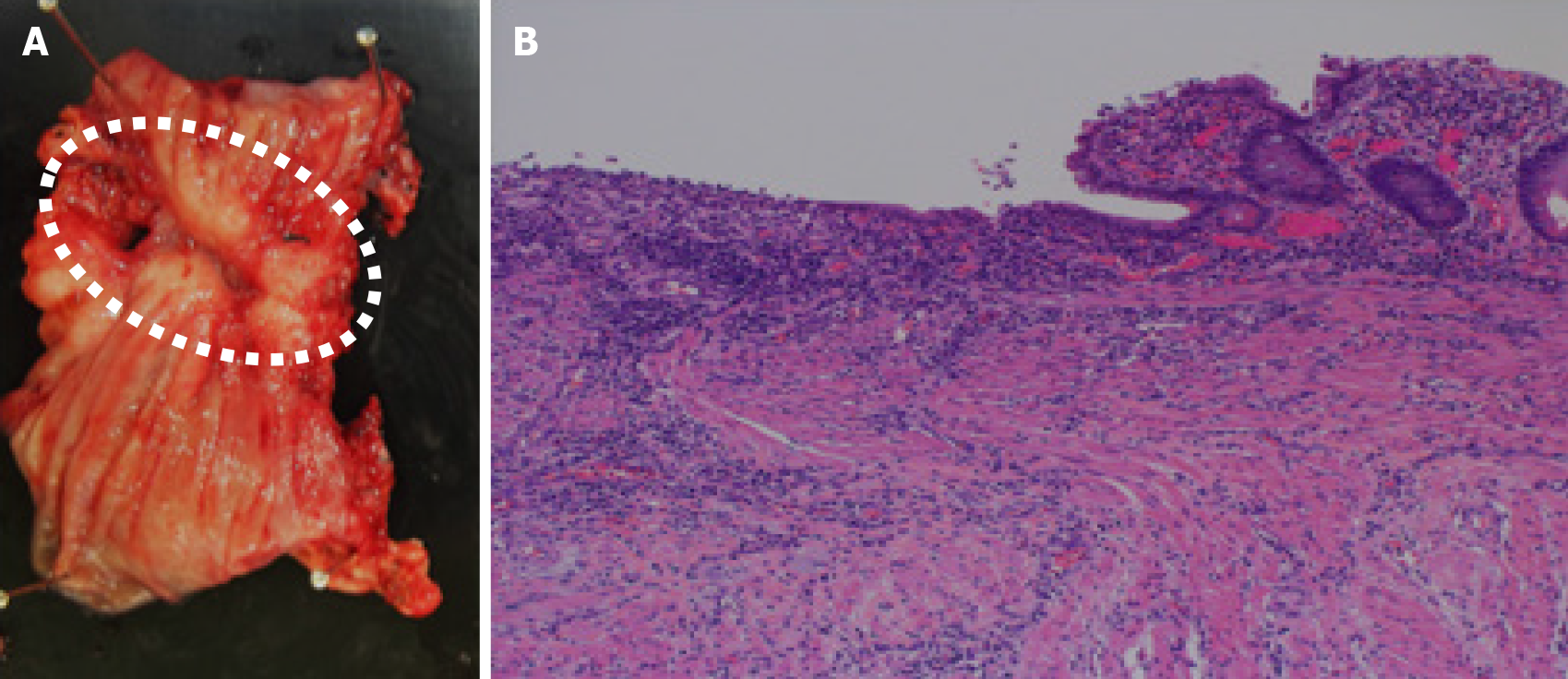
Figure 3 Pathological findings of the ulcerative lesion.
A: The resected specimen of the graft showing ulcerative inflammation with fibrotic tissue; B: No signs of chronic rejection are observed.
- Citation: Fujimura T, Yamada Y, Umeyama T, Kudo Y, Kanamori H, Mori T, Shimizu T, Kato M, Kawaida M, Hosoe N, Hasegawa Y, Matsubara K, Shimojima N, Shinoda M, Obara H, Naganuma M, Kitagawa Y, Hoshino K, Kuroda T. Maintenance treatment with infliximab for ulcerative ileitis after intestinal transplantation: A case report. World J Clin Cases 2021; 9(19): 5270-5279
- URL: https://www.wjgnet.com/2307-8960/full/v9/i19/5270.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v9.i19.5270