Copyright
©The Author(s) 2020.
World J Clin Cases. Apr 26, 2020; 8(8): 1361-1384
Published online Apr 26, 2020. doi: 10.12998/wjcc.v8.i8.1361
Published online Apr 26, 2020. doi: 10.12998/wjcc.v8.i8.1361
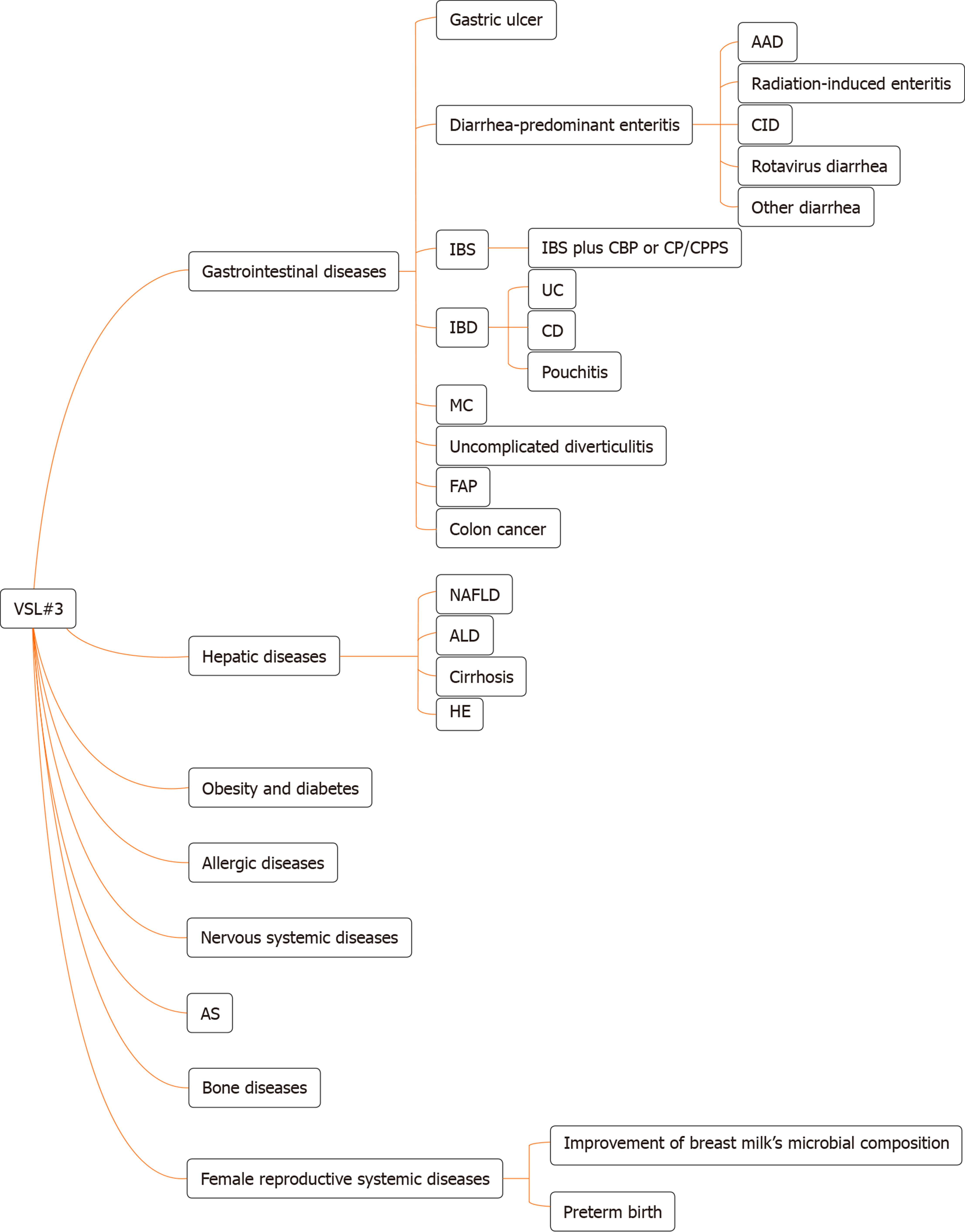
Figure 1 The types of disease for which VSL#3 can work.
AAD: Antibiotic-associated diarrhea; CID: Chemotherapy-induced diarrhea; IBS: Irritable bowel syndrome; CBP: Chronic bacterial prostatitis; CP: Chronic prostatitis; CPPS: Chronic pelvic pain syndrome; IBD: Inflammatory bowel disease; UC: Ulcerative colitis; CD: Crohn’s disease; MC: Microscopic colitis; FAP: Familial adenomatous polyposis; NAFLD: Non-alcoholic fatty liver disease; ALD: Alcoholic liver disease; HE: Hepatic encephalopathy; AS: Atherosclerosis.
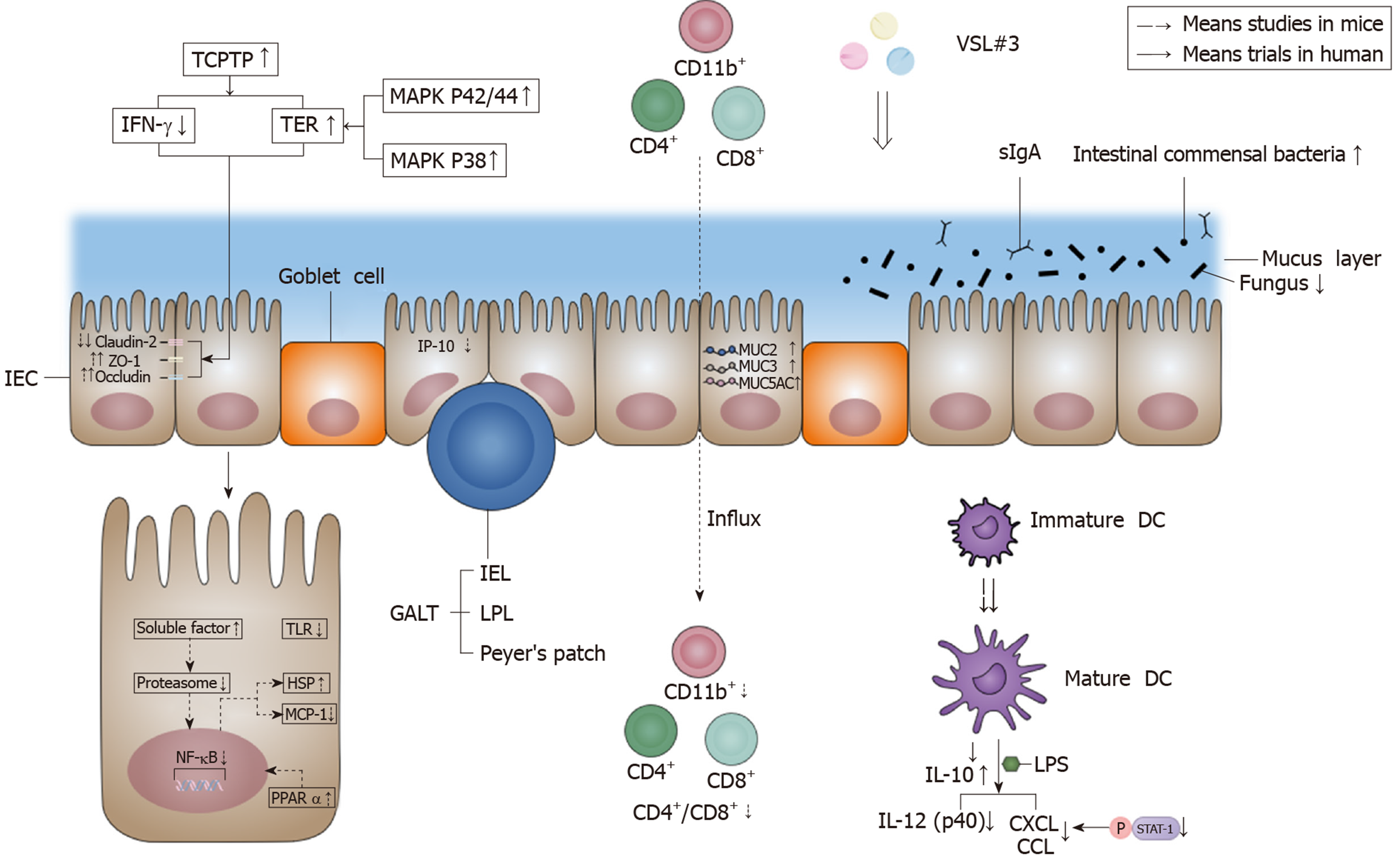
Figure 2 Effects of VSL#3 on intestinal barrier function.
VSL#3 acts on the four components of the intestinal barrier: The mechanical barrier, biological barrier, chemical barrier, and immune barrier. In terms of the mechanical barrier, VSL#3 can increase occludin and zonula occludens-1 and decrease claudin-2 in order to improve tight junction protein function, and the effect is achieved by increasing the activity of T-cell protein tyrosine phosphatase, which is able to decrease T-cell protein tyrosine phosphatase-dependent interferon-γ signaling and increase transepithelial electrical resistance[5-7]. VSL#3 can increase transepithelial electrical resistance by activating the mitogen-activated protein kinase p42/44 and p38 pathway[9]. In terms of the biological barrier, VSL#3 can increase the amount of intestinal commensal bacteria and decrease the amount of fungi[12]. In terms of the chemical barrier, VSL#3 can increase MUC2, MUC3 and MUC5AC gene expression to regulate mucus secretion[9]. In terms of the immune barrier, VSL#3 can inhibit the proinflammatory nuclear factor-κB (NF-κB) pathway, such as inducing heat shock protein (HSP) and reducing monocyte chemoattractant protein-1 (MCP-1). The action mechanism is via the early inhibition of proteasome by producing soluble factors[21]. VSL#3 also up-regulates the peroxisome proliferator-activated receptor α (PPARα) signaling pathway to antagonize the NF-κB pathway[32]. An appropriate dose of VSL#3 can induce the maturation of dendrite cells (DC)[27,28], and VSL#3 can inhibit interferon-inducible protein-10 (IP-10) in intestinal epithelial cells (IEC)[22-24] and the lipopolysaccharide (LPS)-induced expression of chemokines (CXCL9, CXCL10, CCL2, CCL7, and CCL8) by inhibiting STAT-1 phosphorylation[27]. VSL#3 is also able to decrease interleukin (IL)-12 (p40) production induced by LPS[30]. Moreover, VSL#3 can induce IL-10 produced by DC and decrease the influx of innate immune cells (CD11b+) and adaptive immune cells (CD4+/CD8+)[30,31]. The down-regulation of the signaling pathway of Toll-like receptors (TLR) by VSL#3 also has benefits for the intestinal immune barrier[32]. IEC: Intestinal epithelial cells; ZO-1: Zonula occludens-1; TCPTP: T-cell protein tyrosine phosphatase; IFN-γ: Interferon-γ; TER: Transepithelial electrical resistance; MAPK: Mitogen-activated protein kinase; GALT: Gut-associated lymphoid tissue; IEL: Intraepithelial lymphocytes; LPL: Lamina propria lymphocytes; sIgA: secreted immunoglobulin A; NF-κB: Nuclear factor-κB; IL: Interleukin; MCP-1: Monocyte chemoattractant protein-1; HSP: Heat shock protein; PPARα: Peroxisome proliferator-activated receptor α; IP-10: Interferon-inducible protein-10; DC: Dendrite cells; LPS: Lipopolysaccharide; TLR: Toll-like receptors.
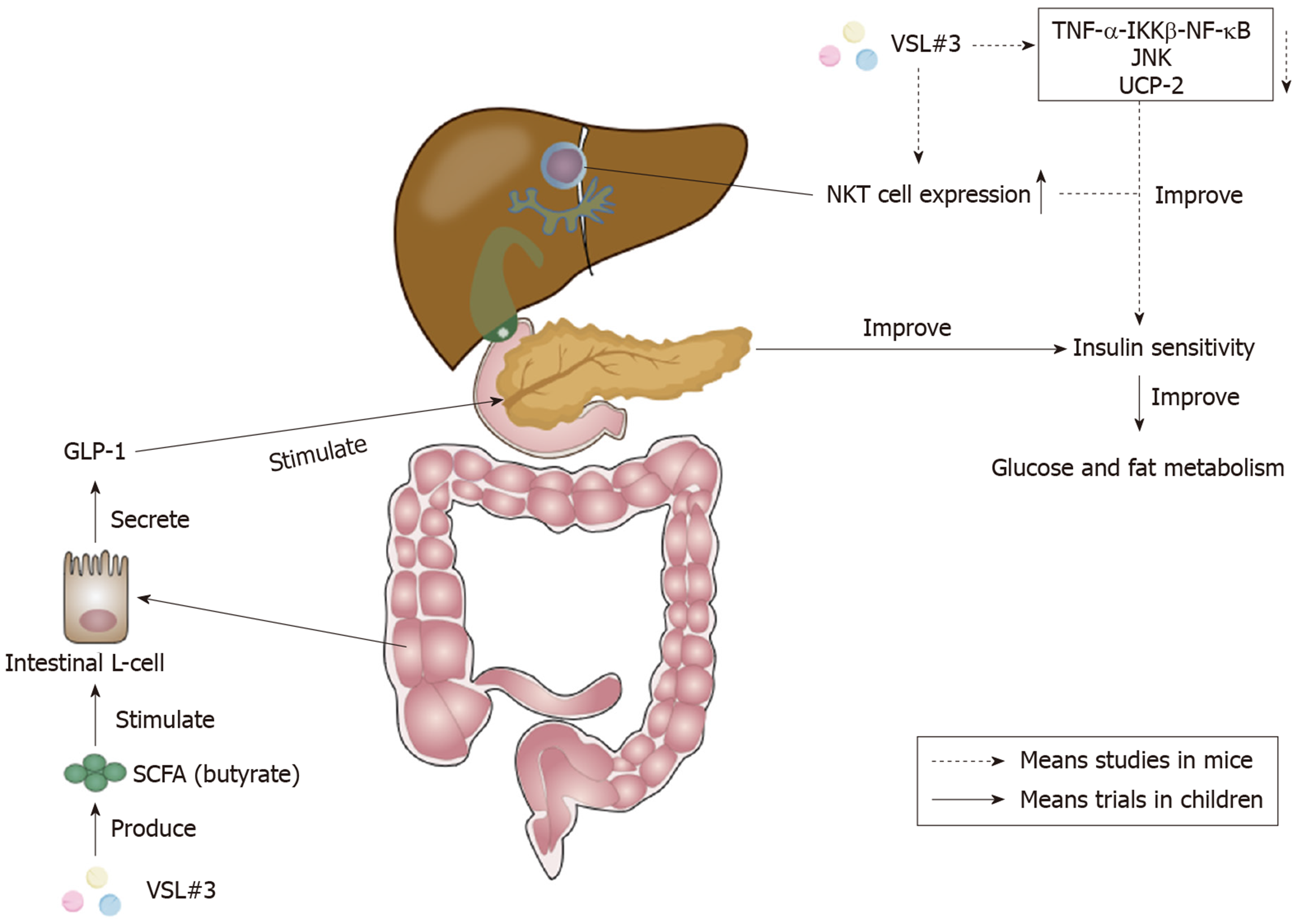
Figure 3 Effects of VSL#3 on insulin sensitivity.
The increase of short-chain fatty acid (SCFA) butyrate caused by VSL#3 is able to stimulate the secretion of glucagon-like peptide 1 (GLP-1) from intestinal L-cells[112]. GLP-1 can stimulate the pancreas and ameliorate insulin sensitivity to improve glucose and fat metabolism[110]. Furthermore, VSL#3 can improve hepatic insulin resistance by reducing tumor necrosis factor-α (TNF-α)-IκB kinase β (IKKβ)-nuclear factor-κB (NF-κB) pathway, the activity of TNF-regulated kinase Jun N-terminal kinase (JNK), and uncoupling protein-2[113-115]. The increase of hepatic natural kill T (NKT) cells caused by VSL#3 also plays a significant role in the improvement of hepatic insulin sensitivity[117,118]. SCFA: Short-chain fatty acid; GLP-1: Glucagon-like peptide 1; TNF-α: Tumor necrosis factor-α; IKKβ: IκB kinase β; NF-κB: Nuclear factor-κB; JNK: Jun N-terminal kinase; UCP-2: Uncoupling protein-2; NKT: Natural kill T.
- Citation: Cheng FS, Pan D, Chang B, Jiang M, Sang LX. Probiotic mixture VSL#3: An overview of basic and clinical studies in chronic diseases. World J Clin Cases 2020; 8(8): 1361-1384
- URL: https://www.wjgnet.com/2307-8960/full/v8/i8/1361.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i8.1361