Copyright
©The Author(s) 2018.
World J Clin Cases. Sep 26, 2018; 6(10): 335-343
Published online Sep 26, 2018. doi: 10.12998/wjcc.v6.i10.335
Published online Sep 26, 2018. doi: 10.12998/wjcc.v6.i10.335
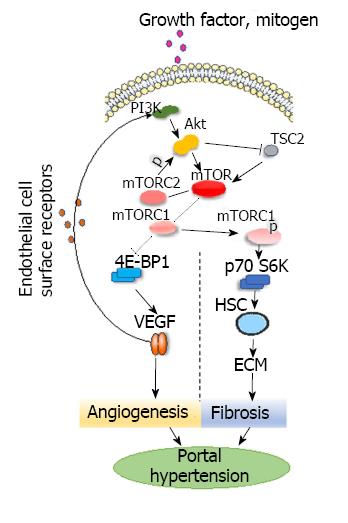
Figure 1 PI3K-AKT-mTOR signaling pathways in the development of hepatic portal hypertension.
PI3K-AKT-mTOR: In vivo, growth factors, mitogens and hormones interact with PI3K, which activates Akt that then activates mTOR. Mammals have two mTOR complexes: mTORC1 and mTORC2. Akt can phosphorylate mTORC1 and then activate p70S6K via phosphorylation to inhibit 4E-BP1. Activated p70S6K promotes the proliferation of HSCs and stimulates both the production of ribosomes and the synthesis of collagen and other extracellular matrix constituents. mTORC1 can inhibit 4E-BP1, promote the splitting of VEGF, and regulate angiogenesis. Akt can inactivate tuberous sclerosis complex 2 and enhance mTOR activity. mTORC2 regulates Akt. Akt: Protein kinase B; ECM: Extracellular matrix; HSC: Hepatic stellate cell; mTOR: Mammalian targets of rapamycin; mTORC: Mammalian targets of rapamycin complex; p70S6K: p70 ribosomal protein S6 Kinase; TSC2: Tuberous sclerosis complex 2; VEGF: Vascular endothelial growth factor; 4E-BP1: 4E-binding protein-1.
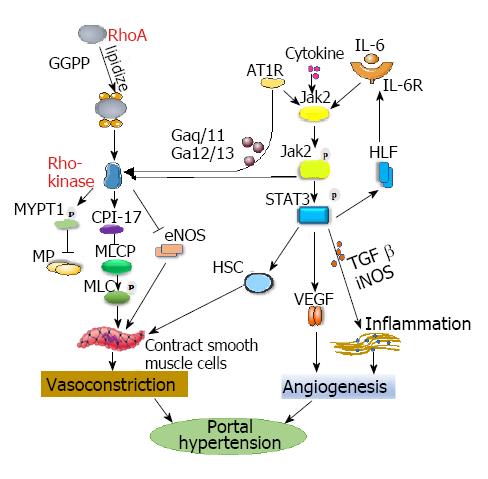
Figure 2 Proposed signaling pathways in the development of hepatic portal hypertension.
RhoA/Rho-kinase: GGPP “lipidizes” RhoA to form membrane-bound RhoA and to activate Rho-kinase. AT1R stimulates JAK2 to phosphorylate and then activate Rho-kinase. Activated Rho-kinase phosphorylates the myosin targeting subunit 1, which inactivates myosin phosphatase. The inactivation of myosin phosphatase increases the phosphorylation of myosin light chain and promotes vasoconstriction. CPI-17 combines with myosin light chain phosphatase to inhibit the activation of the enzymes and promote the contraction of smooth muscle cells. The activation of Rho-kinase could downregulate the expression of eNOS and reduce its activity. The coupling of AT1R to Gaq/11 and Ga12/13 promotes Rho-kinase activation, which is involved in extracellular matrix production. JAK2/STAT3: Cytokines activate the JAK2/STAT3 pathway. Enhanced JAK2/STAT3 signaling upregulates TGF-β and iNOS expression and accelerates intestinal inflammation, which aggravates endothelial dysfunction and angiogenesis. IL-6 binds to its receptor, activates JAK2, phosphorylates it, and then causes the phosphorylation of STAT3 in cells, thereby activating HSC and promoting hypoxia inducible factor expression. These events can upregulate the activation of IL-6 and reactivate the STAT3 pathway, which forms a loop, constantly activating HSC, causing vessel contraction, and ultimately increasing portal pressure. AT1R: Angiotensin-type II receptor 1; CPI-17: C-protein inhibitor protein; eNOS: Endothelial nitric oxide synthase; GGPP: Geranylgeranyl pyrophosphate; HIF: Hypoxia inducible factor; HSC: Hepatic stellate cell; IL-6: Interleukin-6; IL-6R: Interleukin-6 receptor; iNOS: Inducible nitric oxide synthase; JAK2: Janus kinase 2; MLCP: Myosin light chain phosphatase; MP: Myosin phosphatase; MYPT1: Myosin phosphatase target subunit 1; RhoA: Ras homolog gene family member A; STAT3: Signal transducers and activators of transcription; TGF-β: Transforming growth factor β; VEGF: Vascular endothelial growth factor.
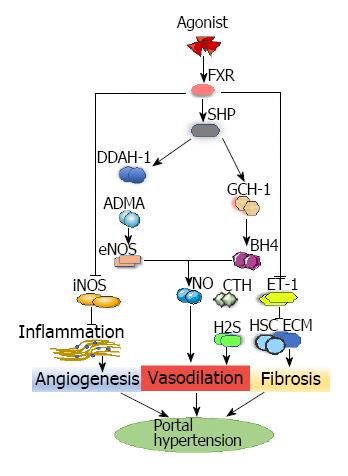
Figure 3 FXR-mediated pathways in angiogenesis, vasodilation and fibrosis during portal hypertension.
FXR pathway: FXR agonist enhances the expression of FXR, which enhances the expression of DDAH-1 and GTP cyclohydrolase-1. DDAH-1 can reduce the levels of ADMA and upregulate the expression of eNOS. GTP cyclohydrolase-1 can increase the expression of BH4. This synergistic enhancement of eNOS and BH4 activity has improved nitric oxide-mediated sinus endothelial function. In addition, FXR agonism reduces the inflammatory response by reducing the expression of iNOS and cyclooxygenase 2 to improve PHT. FXR agonism decreases the expression of endothelin-1 and then inhibits HSC proliferation and extracellular matrix synthesis, which can ameliorate fibrosis. Repressed endothelin-1 can increase the production of cystathionase-mediated hydrogen sulfide, which can cause vasodilation. ADMA: Asymmetric dimethylarginine; BH4: Tetrahydrobiopterin; DDAH-1: Dimethylarginine dimethylamidohydrolase-1; ECM: Extracellular matrix; eNOS: Endothelial nitric oxide synthase; FXR: Farnesoid X receptor; GCH-1: GTP cyclohydrolase-1; H2S: Hydrogen sulfide; HSC: Hepatic stellate cell; iNOS: Inducible nitric oxide synthase; NO: Nitric Oxide; SHR: Small heterodimer partner.
- Citation: Xu W, Liu P, Mu YP. Research progress on signaling pathways in cirrhotic portal hypertension. World J Clin Cases 2018; 6(10): 335-343
- URL: https://www.wjgnet.com/2307-8960/full/v6/i10/335.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i10.335