Copyright
©The Author(s) 2024.
World J Clin Cases. Jun 26, 2024; 12(18): 3515-3528
Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3515
Published online Jun 26, 2024. doi: 10.12998/wjcc.v12.i18.3515
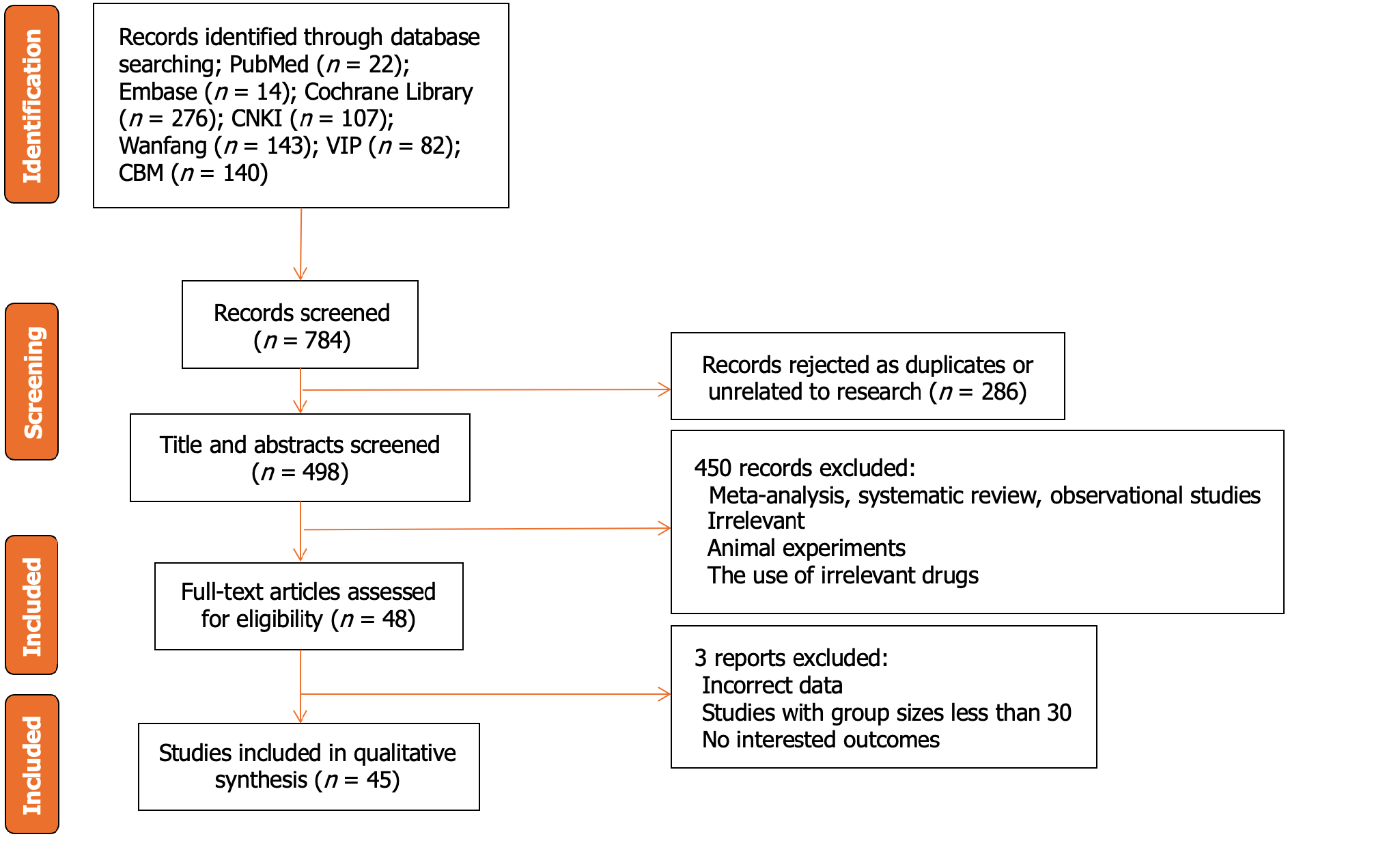
Figure 1 Flow diagram of study inclusion.
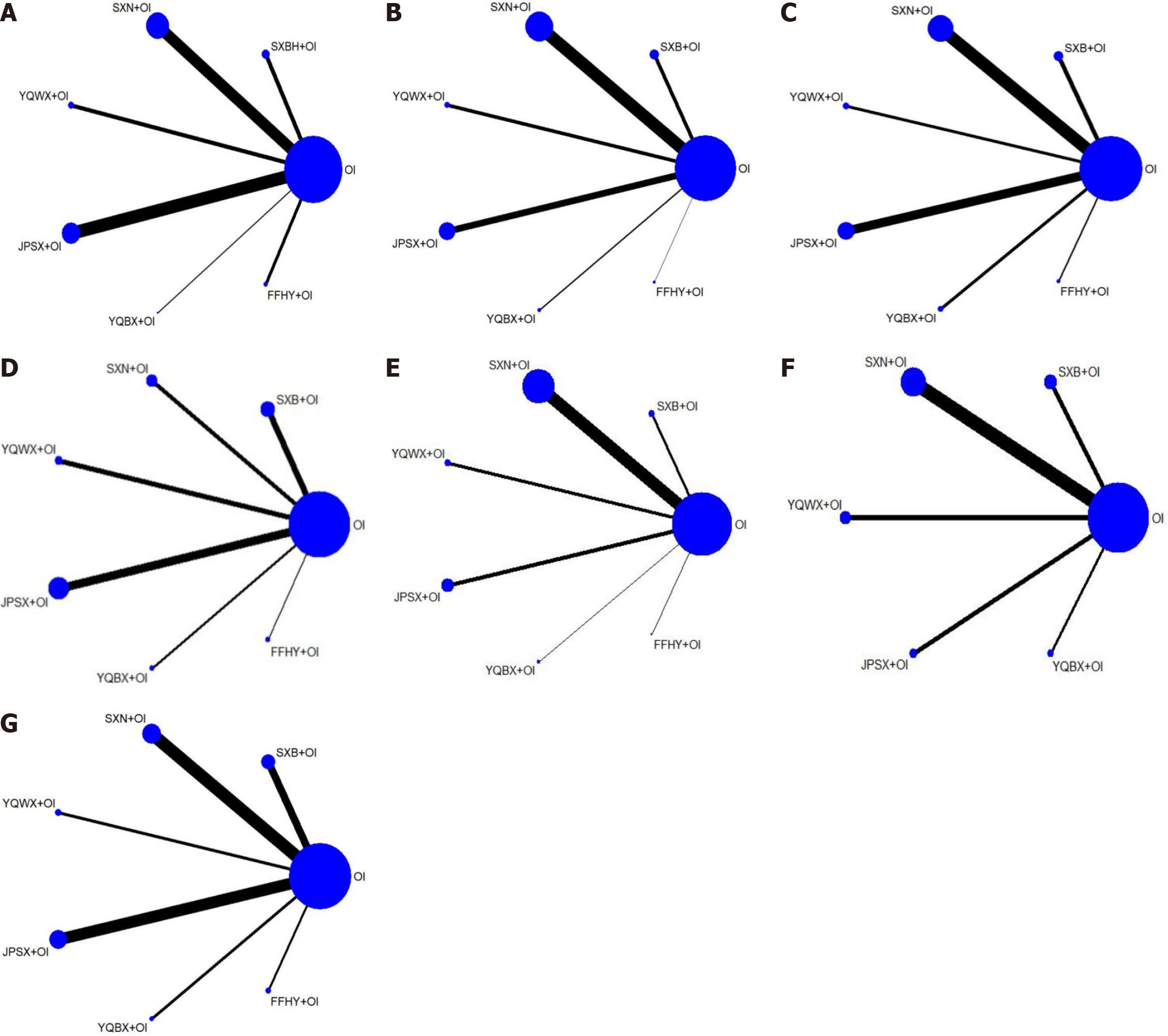
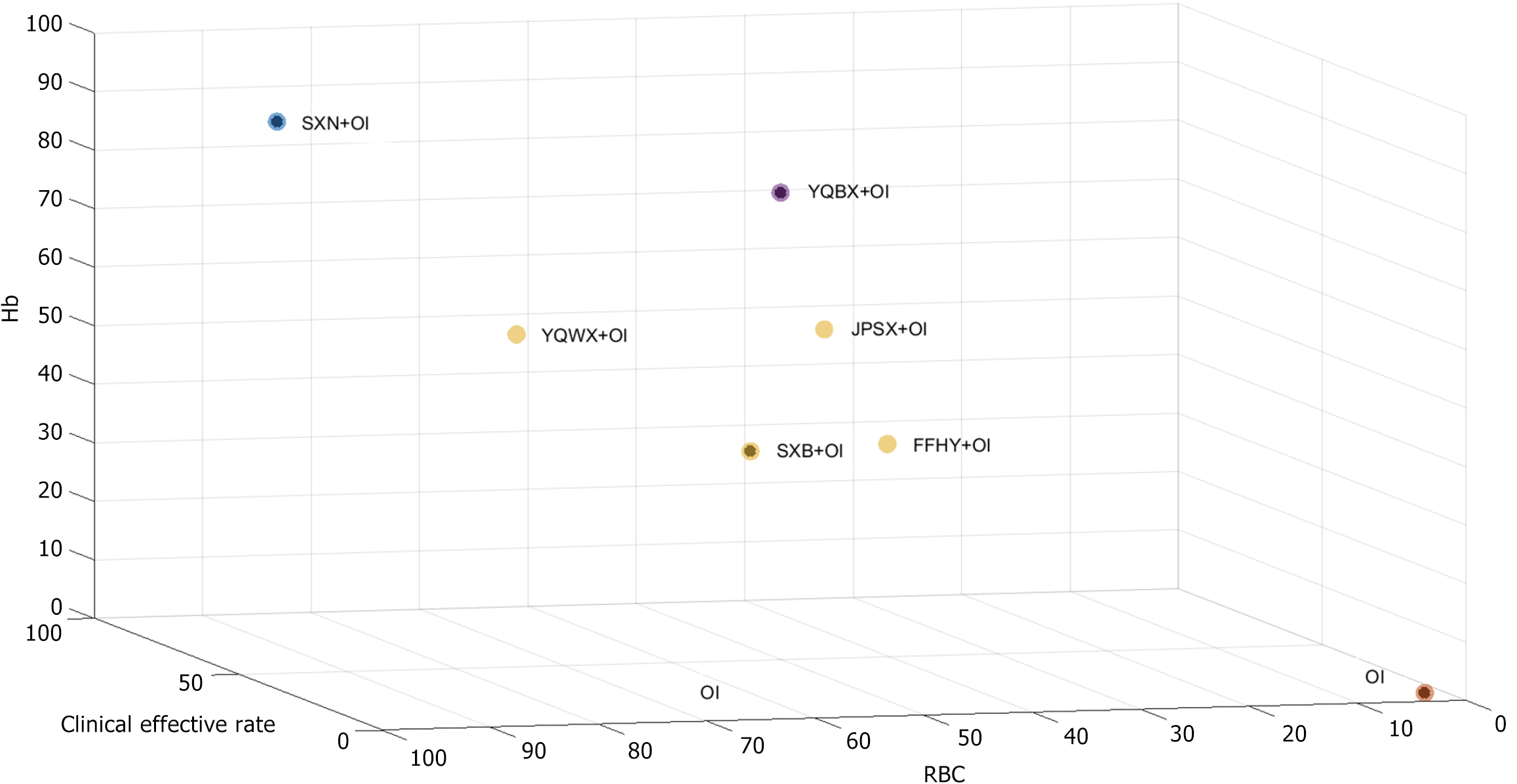
Figure 2 Network graph of the different outcomes.
In each study, the size of the point is determined by the total number of participants and the width of the edge is determined by the standard error. A: Clinical effective rate; B: Red blood cell counts; C: Hemoglobin levels; D: Serum ferritin levels; E: Serum iron levels; F: Adverse pregnancy outcomes; G: Adverse events. OI: Oral iron; SXB: Shengxuebao Mixture; SXN: Shengxianning Tablet; YQWX: Yiqi Weixue Chinese patent medicines; JPSX: Jianpi Shengxue Chinese patent medicines; YQBX: Yiqi Buxue Tablets; FFHY: Compound Hongyi Buxue Oral Liquid.
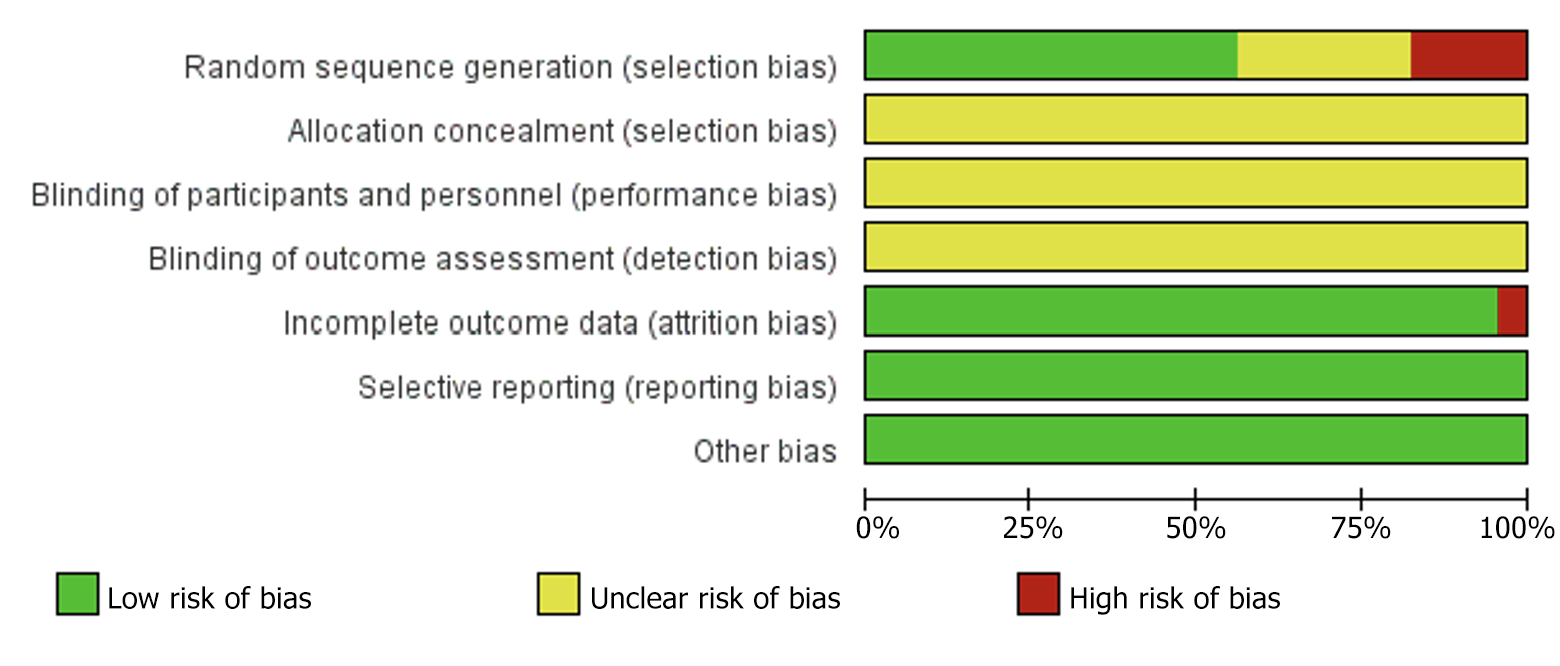
Figure 3 Results of risk of bias graphs.
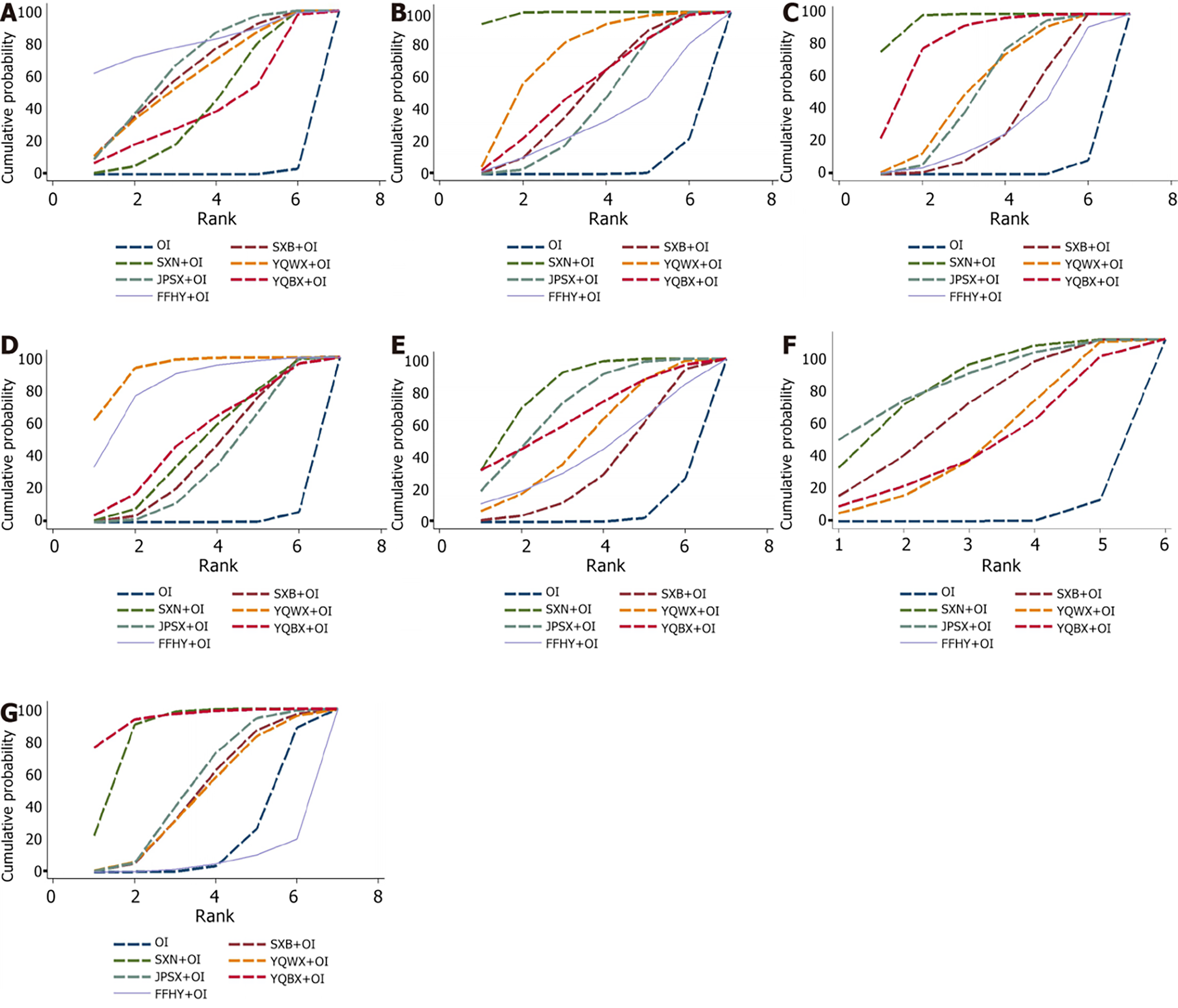
Figure 4 The surface under the cumulative ranking curve plot for all different outcomes.
A: Clinical effective rate; B: Red blood cell counts; C: Hemoglobin levels; D: Serum ferritin levels; E: Serum iron levels; F: adverse pregnancy outcomes; G: Adverse events. OI: Oral iron; SXB: Shengxuebao Mixture; SXN: Shengxianning Tablet; YQWX: Yiqi Weixue Chinese patent medicines; JPSX: Jianpi Shengxue Chinese patent medicines; YQBX: Yiqi Buxue Tablets; FFHY: Compound Hongyi Buxue Oral Liquid.
Figure 5 Cluster analysis plot.
OI: Oral iron; SXB: Shengxuebao Mixture; SXN: Shengxianning Tablet; YQWX: Yiqi Weixue Chinese patent medicines; JPSX: Jianpi Shengxue Chinese patent medicines; YQBX: Yiqi Buxue Tablets; FFHY: Compound Hongyi Buxue Oral Liquid.
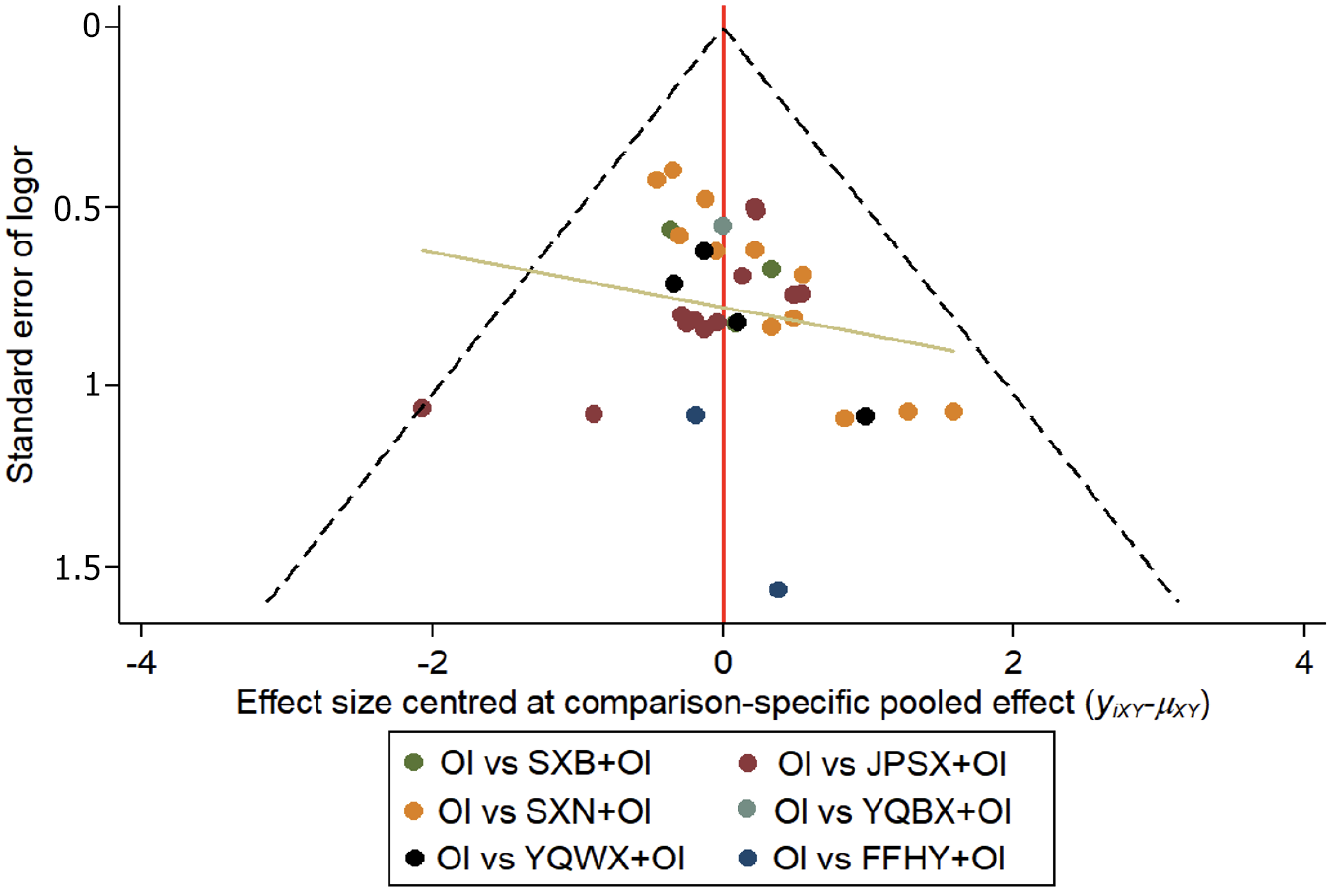
Figure 6 Funnel plot of clinical effective rate.
OI: Oral iron; SXB: Shengxuebao Mixture; SXN: Shengxianning Tablet; YQWX: Yiqi Weixue Chinese patent medicines; JPSX: Jianpi Shengxue Chinese patent medicines; YQBX: Yiqi Buxue Tablets; FFHY: Compound Hongyi Buxue Oral Liquid.
- Citation: Zou JC, Jia XL, Wang HX, Su YJ, Zhu JY. Comparative efficacy and safety of Chinese patent medicines of iron deficiency anemia during pregnancy: A network meta-analysis. World J Clin Cases 2024; 12(18): 3515-3528
- URL: https://www.wjgnet.com/2307-8960/full/v12/i18/3515.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i18.3515