Copyright
©The Author(s) 2023.
World J Clin Cases. Jan 6, 2023; 11(1): 1-6
Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.1
Published online Jan 6, 2023. doi: 10.12998/wjcc.v11.i1.1
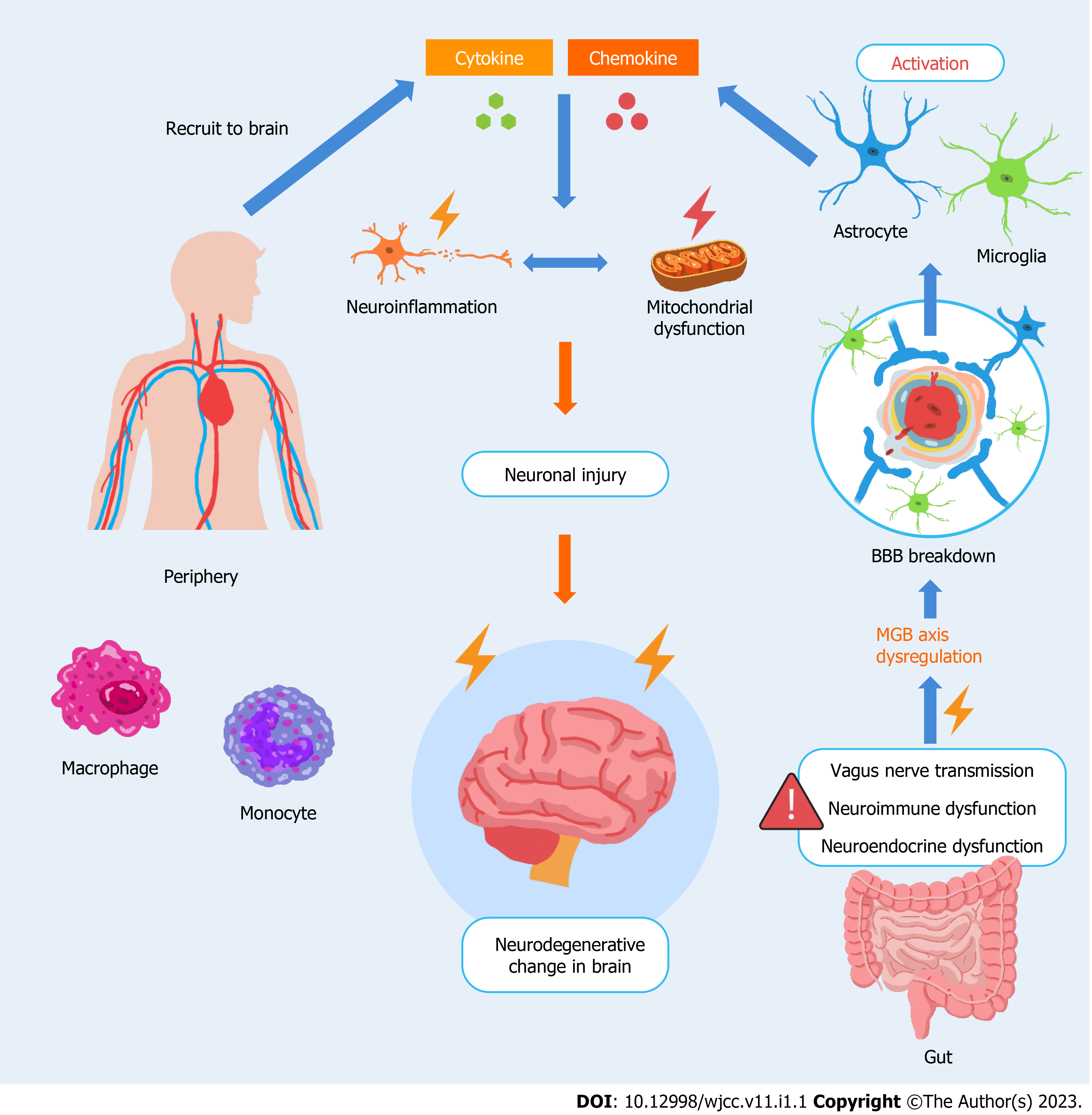
Figure 1 Dysregulation of the microbiome–gut–brain axis contributes to the vicious amplification of neuroinflammatory circuits in the brain.
Astrocytes, microglia (glial cells), and the blood-brain barrier (BBB) have supportive and defensive functions to ensure neuronal stability and maintain constancy in the brain. Through a neuroinflammatory response that is dominated by glial cells in the central nervous system, the neurological system is committed to preserve cell metabolism, repair, and renewal to maintain microenvironment homeostasis and integrity/long-term viability of the nervous system. In the event of microbiome–gut–brain axis dysfunction, the self-fulfilling regions of the brain are implicated and disturbed. The leakage of microbiota or microbial-derived intermediates from the gut likely causes a systemic immune response that affects the neuroendocrine system. These pathological factors may destabilize the brain directly from the gut via the vagus nerve. These factors may modify BBB permeability, causing a shift in peripheral immune cells toward the brain (originally blocked by the BBB), such as macrophages and monocytes. Meanwhile, glial cells are activated, and more proinflammatory cytokines and chemokines are secreted, inevitably leading to neuroinflammation and progressing to mitochondrial dysfunction. These events aggravate the neurodegenerative changes in the brain.
- Citation: Lin MS, Wang YC, Chen WJ, Kung WM. Impact of gut–brain interaction in emerging neurological disorders. World J Clin Cases 2023; 11(1): 1-6
- URL: https://www.wjgnet.com/2307-8960/full/v11/i1/1.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i1.1