Published online Mar 25, 2025. doi: 10.5527/wjn.v14.i1.101930
Revised: December 24, 2024
Accepted: January 7, 2025
Published online: March 25, 2025
Processing time: 111 Days and 2.1 Hours
The underlying molecular changes that result in minimal change disease (ne
Core Tip: Minimal change disease (MCD) represents a unique type of nephrotic syn
- Citation: Govindarajan KK. Current understanding of adult nephrotic syndrome: Minimal change disease. World J Nephrol 2025; 14(1): 101930
- URL: https://www.wjgnet.com/2220-6124/full/v14/i1/101930.htm
- DOI: https://dx.doi.org/10.5527/wjn.v14.i1.101930
Minimal change disease (MCD) represents a small percentage (10%-20%) of the adult population suffering from nephrotic syndrome. The etiology of MCD is suspected to be immunologically driven. Triggers such as insect bites, pollen, and certain foods that initiate allergic reactions can induce MCD. These factors also account for multiple relapses. MCD patients are known to have a relatively higher incidence of atopic dermatitis and elevated immunoglobulin E levels. Together, these data have led to the hypothesis that atopy and MCD are closely linked. More than the causation, the underlying dysregulation of immune mechanisms in both must be appreciated[1].
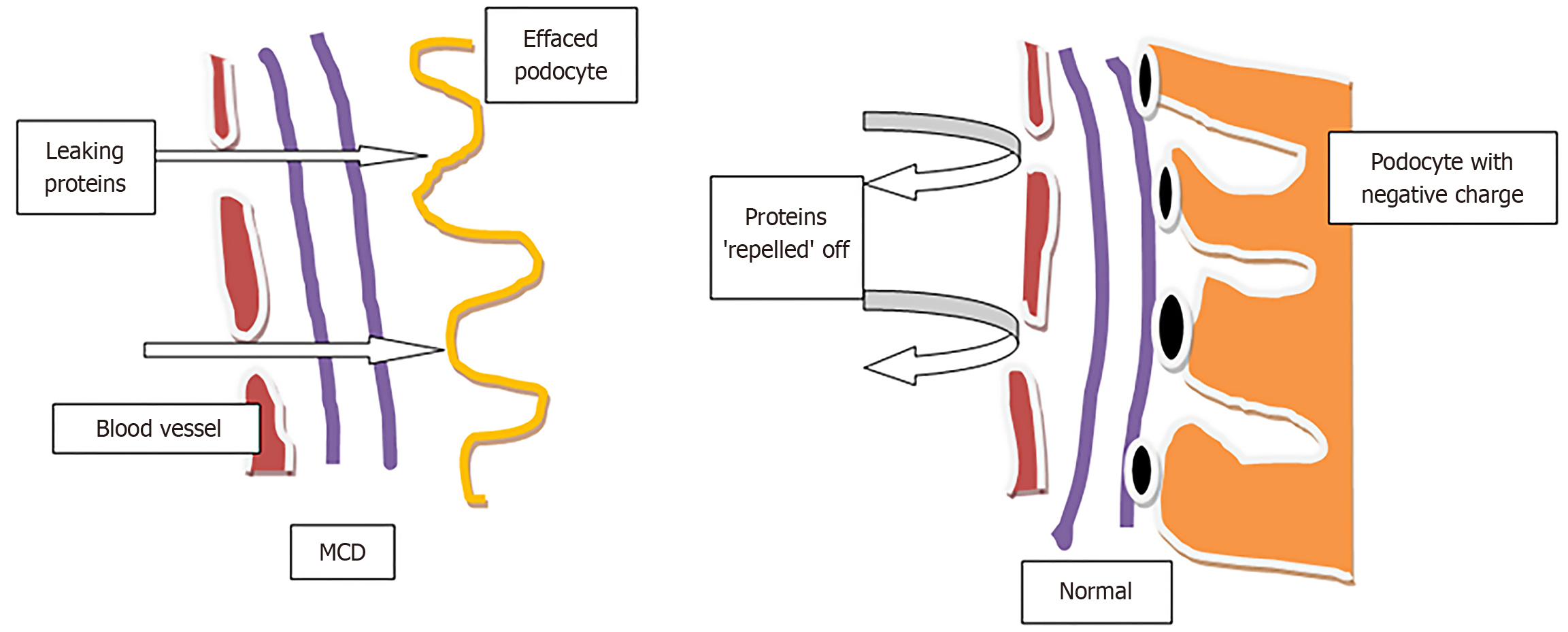
In the kidney, podocytes form an essential part of the glomerular filtration barrier. Podocyte foot processes are integral to maintaining the barrier, and damaging them results in the typical clinical manifestations of MCD, namely albuminuria. Upon upregulation of the Zinc fingers and Homeoboxes (ZHX) and Angiopoietin like 4 (ANGPTL4) pathway, hyposialylated ANGPTL4 is released, inducing microcellular level injury to podocytes. Certain genetic defects involving ZHX2 place podocytes at risk of injury caused by cytokine storms from the common cold. The cytokine storm displaces ZHX1 from the podocyte membrane to the nucleus, leading to the ANGPTL4 upregulation. This cascade results in the secretion of hyposialylated ANGPTL4 protein, ultimately damaging the glomerulus through podocyte foot process effacement and loss of glomerular basement membrane charge (Figure 1). Hence, the current focus in understanding MCD is on ZHX and ANGPTL4 [2].
Earlier animal model studies proposed that MCD pathogenesis was associated with the administration of specific proteins, podocyte structural alterations, alternate pathways and T-cell-mediated damage. T cell dysfunction is known to be a component in MCD, but whether it plays a causative role is unclear. Through their ability to activate B cells and cytotoxic T8 cells and to release cytokines, T4 helper contribute to the autoimmune mechanism involved in targeting podocytes. T4 regulator cells, which help restore normalcy, are reduced in numbers in MCD, resulting in immune dysregulation and an exaggerated inflammatory response[3]. The complex interactions between the T cell and B cell are significant in pathogenesis. However, the available studies have failed to elucidate the exact sequence of events orchestrating the disease process of MCD. In addition, the multiple MCD relapses were attributable to the complex interactions of many proteins rather than a single protein[2,4,5]. The B lymphocyte dysregulator Telitacicept prevents its differentiation and maturation in recalcitrant MCD and has been demonstrated to elicit a therapeutic response, reserving its use in select cases[6].
The key component of the ZHX and ANGPTL4 pathway in MCD is the depletion of sialic acid due to podocyte injury. The management strategy of steroid use can rescue the ANGPTL4 upregulation, decreasing the chances of its hyposialylation and thus eliciting a favorable clinical response[5].
Although MCD and focal segmental glomerulosclerosis (FSGS) represent the extremes along a spectrum, they are both steroid-responsive. The differences in molecular mechanisms underlying MCD and FSGS deserve attention. ZHX2 expression is reduced in both, but there is an increase in nuclear ZHX1 in MCD, and an increase in nuclear ZHX2 and ZHX3 in FSGS. The former (rise in nuclear ZHX1) leads to disturbance in the podocyte body, whereas the latter (rise in nuclear ZHX2, ZHX3) results in altered podocyte foot processes[2].
Newer studies have been investigating the role of anti-nephrin autoantibodies in MCD pathogenesis, which is considered immune-mediated podocytopathy. Nephrin is an integral component of podocyte architecture, and it plays a central role in cellular signaling. Autoantibodies against Nephrin display a good correlation with disease activity and are also etiological in the evolution of the disease process. Rituximab, a B cell-targeted agent, can induce clinical remission by exhausting the anti-nephrin antibodies. Pharmacological agents aimed at the anti-nephrin antibodies are potential future targets in the management of MCD[7,8].
Interestingly, the degree of proteinuria severity does not appear to influence MCD outcomes. However, elevated 24-hour albumin clearance levels are predictive of MCD recurrence. The impaired charge barrier at the glomerular level directly affects albumin clearance and is the basis for the utility of albumin clearance value in prognosis[9,10].
Targeted molecular interventions, or personalized precision nephrology, are recent developments that help identify MCD patients with unconventional outcomes, such as poor responders. Customizing the therapeutic approach to suit the individual patient with inputs from the disease progression, genetics, and other personal factors defines how personalized precision nephrology works. A typical example is tumor necrosis factor, identified by markers such as monocyte chemoattractant protein-1 and tissue inhibitor of metalloproteinases-1 and known to be associated with a subtype of poor responders amongst MCD. Such an individualized approach represents a significant move towards recognizing those with poor prognosis upfront and planning suitable therapeutic strategies for better outcomes[11].
The documentation of MCD by Shakeel et al[12], including the clinicopathologic characteristics, treatment response and outcome evaluation, offers an institutional viewpoint. In addition, the various aspects of MCD, such as the presentation and management approach, can be understood from the background of a single center perspective.
| 1. | Hadhri A, Mrabet S, Ben Aicha N, Fradi A, Azzabi A, Sahtout W, Boukadida R, Guedri Y, Zellama D, Abdessaied N, Ben Saad H, Achour A. Nephrotic syndrome with Minimal Change Disease and Atopy in NorthAfrican adults. Tunis Med. 2023;101:253-258. [PubMed] |
| 2. | Chugh SS, Clement LC. "Idiopathic" minimal change nephrotic syndrome: a podocyte mystery nears the end. Am J Physiol Renal Physiol. 2023;325:F685-F694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Bertelli R, Bonanni A, Caridi G, Canepa A, Ghiggeri GM. Molecular and Cellular Mechanisms for Proteinuria in Minimal Change Disease. Front Med (Lausanne). 2018;5:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Liu S, Bush WS, Miskimen K, Gonzalez-Vicente A, Bailey JNC, Konidari I, McCauley JL, Sedor JR, O'Toole JF, Crawford DC. T-cell receptor diversity in minimal change disease in the NEPTUNE study. Pediatr Nephrol. 2023;38:1115-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Macé C, Del Nogal Avila M, Marshall CB, Kharlyngdoh J, Das R, Molina-Jijon E, Donoro Blazquez H, Shastry S, Soria E, Wetzels J, Dijkman H, Avila-Casado C, Clement LC, Chugh SS. The zinc fingers and homeoboxes 2 protein ZHX2 and its interacting proteins regulate upstream pathways in podocyte diseases. Kidney Int. 2020;97:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Li S, Ding L, Yang YJ, Yang XD. Telitacicept for minimal change disease. Kaohsiung J Med Sci. 2023;39:748-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Hengel FE, Dehde S, Lassé M, Zahner G, Seifert L, Schnarre A, Kretz O, Demir F, Pinnschmidt HO, Grahammer F, Lucas R, Mehner LM, Zimmermann T, Billing AM, Oh J, Mitrotti A, Pontrelli P, Debiec H, Dossier C, Colucci M, Emma F, Smoyer WE, Weins A, Schaefer F, Alachkar N, Diemert A, Hogan J, Hoxha E, Wiech T, Rinschen MM, Ronco P, Vivarelli M, Gesualdo L, Tomas NM, Huber TB; International Society of Glomerular Disease. Autoantibodies Targeting Nephrin in Podocytopathies. N Engl J Med. 2024;391:422-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 95] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 8. | Jiang H, Shen Z, Zhuang J, Lu C, Qu Y, Xu C, Yang S, Tian X. Understanding the podocyte immune responses in proteinuric kidney diseases: from pathogenesis to therapy. Front Immunol. 2023;14:1335936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 9. | Son HE, Yun G, Kwon EJ, Park S, Jeong JC, Kim S, Na KY, Paik JH, Chin HJ. Outcomes of minimal change disease without nephrotic range proteinuria. PLoS One. 2023;18:e0289870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kuno H, Kanzaki G, Sasaki T, Haruhara K, Okabe M, Yokote S, Koike K, Tsuboi N, Yokoo T. High Albumin Clearance Predicts the Minimal Change Nephrotic Syndrome Relapse. Kidney360. 2023;4:e787-e795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 11. | Mariani LH, Eddy S, AlAkwaa FM, McCown PJ, Harder JL, Nair V, Eichinger F, Martini S, Ademola AD, Boima V, Reich HN, El Saghir J, Godfrey B, Ju W, Tanner EC, Vega-Warner V, Wys NL, Adler SG, Appel GB, Athavale A, Atkinson MA, Bagnasco SM, Barisoni L, Brown E, Cattran DC, Coppock GM, Dell KM, Derebail VK, Fervenza FC, Fornoni A, Gadegbeku CA, Gibson KL, Greenbaum LA, Hingorani SR, Hladunewich MA, Hodgin JB, Hogan MC, Holzman LB, Jefferson JA, Kaskel FJ, Kopp JB, Lafayette RA, Lemley KV, Lieske JC, Lin JJ, Menon R, Meyers KE, Nachman PH, Nast CC, O'Shaughnessy MM, Otto EA, Reidy KJ, Sambandam KK, Sedor JR, Sethna CB, Singer P, Srivastava T, Tran CL, Tuttle KR, Vento SM, Wang CS, Ojo AO, Adu D, Gipson DS, Trachtman H, Kretzler M. Precision nephrology identified tumor necrosis factor activation variability in minimal change disease and focal segmental glomerulosclerosis. Kidney Int. 2023;103:565-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 12. | Shakeel S, Rashid R, Jafry NH, Mubarak M. Adult minimal change disease: Clinicopathologic characteristics, treatment response and outcome at a single center in Pakistan. World J Nephrol. 2024;13:99643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |