Published online Jun 25, 2024. doi: 10.5527/wjn.v13.i2.93322
Revised: May 24, 2024
Accepted: June 18, 2024
Published online: June 25, 2024
Processing time: 119 Days and 11.2 Hours
Obstructive uropathy is defined as the structural or functional interruption of urinary outflow at any level in the urinary tract. It is regarded as one of the most prevalent causes of acute kidney injury (AKI), accounting for 5%–10% of cases. Acute severe obstruction of the urinary tract is a potentially threatening situation for the kidneys and therefore requires prompt identification and management to relieve obstruction. The aim of the present article is to review and synthesize available evidence on obstructive uropathy, providing a clinical guideline for clinicians. A literature review on obstructive uropathy in the context of AKI was performed, focusing on the least clarified aspects regarding diagnosis and management. Recent literature searching was conducted in English and top-level evidence articles including systematic reviews, metanalyses and large series were prioritized. Acute obstruction of the urinary tract is a diagnostic and therapeutical challenge that may lead to important clinical complications together with direct structural and hemodynamic damage to the kidney. Early recognition of the leading cause and its exact location is essential to ensure prompt urinary drainage together with the most suitable drainage technique selection. A multidisciplinary approach, including urologists, nephrologists, and other medical specialties, is best suited to correctly manage concomitant hemodynamic changes, fluid and electrolyte imbalances, and other related issues. Obstructive uropathy is one of the leading causes of AKI. Recognition of patients suitable for early diversion and feasibility or adequate selection of the indicated technique is sometimes challeng
Core Tip: Obstructive uropathy is a prevalent cause of acute kidney injury that can potentially lead to death or irreversible and permanent tissue damage leading to chronic kidney disease. It is of vital importance to perform a correct initial assessment in order to identify patients that may benefit from early urinary diversion. Acute obstruction of the urinary tract leads to volume overload, electrolyte imbalances and infectious complications that need to be correctly addressed, therefore, a multidisciplinary team is key. Management of urinary tract obstruction does not end after urinary diversion. Timing and adequate management of such condition will determine renal recovery following obstruction.
- Citation: Pérez-Aizpurua X, Cabello Benavente R, Bueno Serrano G, Alcázar Peral JM, Gómez-Jordana Mañas B, Tufet i Jaumot J, Ruiz de Castroviejo Blanco J, Osorio Ospina F, Gonzalez-Enguita C. Obstructive uropathy: Overview of the pathogenesis, etiology and management of a prevalent cause of acute kidney injury. World J Nephrol 2024; 13(2): 93322
- URL: https://www.wjgnet.com/2220-6124/full/v13/i2/93322.htm
- DOI: https://dx.doi.org/10.5527/wjn.v13.i2.93322
Obstructive renal failure is defined by impaired urinary flow due to an obstruction along the urinary tract. It is important to clarify associated terminology as it is not uncommon to see different terms used indistinctively referring to urinary obstruction and the secondary kidney injury produced. Classically, obstructive uropathy has been considered the presence of structural or functional changes in the urinary tract altering the normal flow of urine. On the other hand, obstructive nephropathy has been regarded as the secondary renal disease to the alteration of the normal flow of urine that leads to renal failure[1]. Impaired renal function may then lead to potential long-term sequelae. Understanding the different mechanisms underlying obstructive renal failure is of vital importance, as it guides diagnostic strategies and therapeutic interventions required.
Despite acute kidney injury (AKI) being a very common condition observed both in the hospital and the outpatient setting, the incidence of urinary tract obstruction (UTO) as a main cause for AKI is not known with certainty. A global incidence of 1.7/1000 with an estimated proportion of 5%-10% of AKI secondary to UTO is often considered, however, a clear age-related incidence variation has been described with incidence peaks during infancy and in late life[2,3]. The largest series of elderly patients and AKI often show significantly higher rates of UTO as a leading cause for their condition, with incidence rates as high as 22% in some series[4]. AKI secondary to UTO is more common in men than women due to male-exclusive conditions, benign prostatic hyperplasia and prostate cancer predominantly[5]. This article aims to provide a comprehensive review of the current state of knowledge surrounding obstructive renal failure, focusing on the mechanisms contributing to its development and progression.
Although obstructive uropathy is a well-known condition, there is a notable deficiency in the literature concerning comprehensive exploration of the issue from a urological standpoint. Most published research emphasizes its path
Furthermore, the consequences of urinary obstruction extend beyond the immediate threat to renal function. Complications may include electrolyte imbalances or infectious complications, together with potentially irreversible structural damage to the kidneys[8]. Therefore, such a condition urges the need for prompt recognition and intervention to mitigate adverse outcomes. In terms of therapeutic approaches, an understanding of obstructive mechanisms is crucial for tailoring effective interventions. Therefore, our aim was to compile and summarize the existing evidence on obstructive uropathy into a single article, spanning from its etiology to potential sequelae. This comprehensive approach is sought to guide clinicians in making informed decisions based on the unique characteristics and clinical circumstances of each patient.
Acute UTO results in a disruption of urine flow, causing an elevation in pressure within the urinary tract. Pressure increase is transmitted retrogradely, ultimately impacting renal intratubular flow and inflicting injury on the kidney leading to changes in the kidneys and urinary tract[9]. An initial compensatory hemodynamic response produces functional changes in the kidney, which, in the absence of obstruction relief, may structurally affect the kidneys resulting in permanent damage[10]. These undesirable effects derived from urinary obstruction have been widely studied since the mid 1900s. However, not many advances have been made in the clinical understanding of obstruction since the first experimental studies were published[11,12].
Hemodynamics in UTO may vary depending on the degree and site of obstruction.
In unilateral UTO (UUO) three different stages may be observed[13,14].
The resulting elevation of intratubular pressure is compensated with increased renal blood flow secondary to the secretion of intrinsic prostaglandin-E2 by the kidneys to maintain an adequate glomerular filtration rate (GFR).
This compensatory mechanism lasts no longer than 1-2 h; renal blood flow starts to decrease. On the contrary, intratubular pressure keeps increasing.
After 3-4 h, a pronounced decrease in renal blood flow is observed as a consequence of increasing intratubular pressure. With this decrease in renal vascular supply, intratubular pressure also declines. Renal blood flow impairment produces a decrease of GFR and a redistribution of intrarenal blood circulation from the cortex to the medulla.
In bilateral UTO (BUO) only two phases are observed[15].
An initial increase in renal blood flow that lasts for 90 min approximately.
Accused renal blood flow impairment following the same mechanisms described above. Intrarenal blood circulation is redistributed in the opposite way from the medulla to the cortex.
These changes lead to vascular impairment of renal nephrons, resulting in acute tubular necrosis and, if maintained over time, may produce permanent damage to renal tissue[16].
The establishment of renal injury starts with the retrograde transmission of the elevation of urinary tract pressure, which produces dilation of the urinary tract and consequently hydronephrosis. High intratubular pressure produces an interstitial expansion of the kidney extracellular matrix which triggers an inflammatory cascade with cellular infiltrates and interstitial fibrosis, finally producing tubular cell apoptosis[17]. The persistence of unrelieved obstruction results in tubulointerstitial necrosis with possible associated glomerulosclerosis[18].
The effects of UTO on the kidney mainly affect three aspects of tubular function; sodium transport, urinary concentrating ability and urinary acidification[19]. These changes are more pronounced in cases of BUO than in UUO. With the onset of obstruction, aquaporin tubular channels in charge of the transport of water are downregulated. In vivo studies show a 50% decrease of these channels, even 7 d after the relief of obstruction[20]. This mechanism has been proposed as one of the ultimate causes of post-obstructive polyuria and the inability to reabsorb water and concentrate urine of the obstructed kidney. Sodium channels are also affected by obstructive uropathy; diminished levels are observed 24 h after the onset of obstruction[21]. These alterations are translated into an increased natriuresis/salt-wasting syndrome due to the inability to reabsorb sodium by the obstructed kidney(s). Hyperkalemia is the most life-threatening ionic alteration derived from obstruction. First, the amount of potassium filtered by the glomerulus is diminished due to the decrease of GFR derived from impaired renal blood flow. This situation is aggravated by the lack of sodium at the distal tubule secondary to obstructive natriuresis, reducing its intraluminal concentration. Sodium-potassium exchange is therefore impaired and potassium cannot be excreted[22]. Finally, urinary acidification is also affected by the inability of the affected kidney to secrete hydrons in the distal tubule (type I distal tubular acidosis)[5].
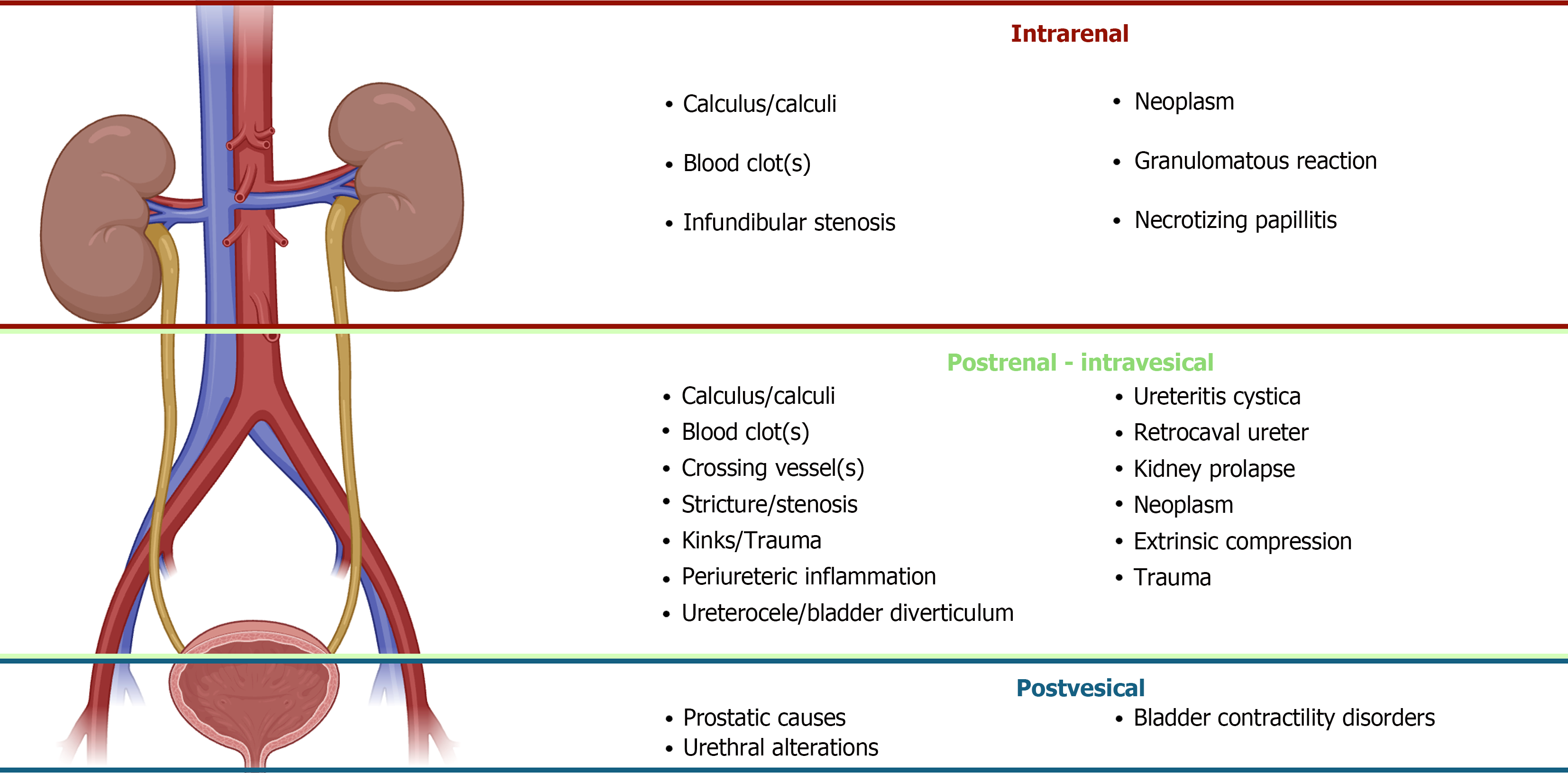
Establishing the site at which UTO occurs is of vital importance for diagnosis and posterior management. From a func
Intrarenal: Translated as retrograde dilation of individual calyces or caliectasis. Kidney stones, infundibular stenosis secondary to infection or stones, urothelial tumors, blood clots or idiopathic causes can be possible causes. Treatment often involves, if necessary, drainage of the affected area proximally (stenting if feasible or nephrostomy).
Postrenal - intravesical: Produces ureterohydronephrosis proximally to the site of obstruction. May affect both kidneys in some cases, as in retroperitoneal fibrosis. The most common causes ca be intrinsic; ureteral stones, ureteral tumors, pelviureteric joint obstruction (UPJ syndrome), thrombi or extrinsic; retroperitoneal masses or fibrosis and in the female patient compression from gynecologic cancer. Intravesical causes, such as bladder tumors or prostate cancer with infiltration of the trigone may produce UUO or BUO depending on the specific affectation of ureteral orifices[1].
Post vesical: Causes BUO with bilateral ureterohydronephrosis. Bladder outlet obstruction often as a result of an alteration at the level of the prostate or urethra; prostatic enlargement/cancer, urethral stenosis or neurogenic bladder with associated contractility disorder[23].
The urinary tract is a peristaltic organ. Starting from the renal papillae, where the concentration of myoblastic interstitial cells is highest, it extends 25-30 cm cranially until its insertion in the urinary bladder at the level of the ureteral orifices. These cells are known for their pacemaker activity, allowing the ureter to work as a manometric multiplier, producing a constant pressure peristaltic wave along the ureter until it reaches the bladder[24]. This wave is transmitted at a speed of 2-5 cm/s, starting at a low resting pressure of 0-5 cm H2O at its site of origin and progressively increasing to a maximum of 20-60 cm H2O at the level of the ureteral orifices[25]. In vivo studies of ureteral peristalsis have revealed the presence of an anatomical structure, sometimes referred to as “ureteral displacement sheath”, inside which the ureter performs the action of peristalsis[26].
Partial or complete disturbance of such structure in extrinsic causes of UTO, such as retroperitoneal fibrosis, impairs the correct flow of urine from the kidneys to the bladder. On the other hand, intrinsic causes of UTO impair the correct flow of the peristaltic bolus due to a blockage of the normal flow of urine inside the urinary tract. The timing and degree of obstruction leads to differences in physiopathological pathways and consequently the clinical implications derived from the initial insult. Previously described hemodynamic changes are more notable when complete or bilateral obstruction occurs, leading to lower adaptability derived from compensatory mechanisms[13,14]. Similarly, complete and abrupt obstruction will more frequently lead to acute renal failure, while partial and progressive obstruction will permit the onset of compensatory mechanisms with a more gradual course as observed in cases of chronic obstructive uropathy.
Physical examination is the initial approach to diagnosing obstructive uropathy. Depending on the location of obstr
Initial assessment after physical examination and a correct anamnesis includes laboratory testing and imaging. Regarding laboratory tests, it is important to check serum creatinine, potassium and acid-base balance to determine the functional effect of hydronephrosis[27]. Determining accurately the GFR, the hallmark of kidney function, is cumbersome and time-consuming with the available technology. Thus, it is usually assessed in a clinical setting by monitoring different solutes that are normally cleared by the kidney (mainly creatinine and others, such as cystatin C)[28]. Nevertheless, variations in creatinine levels lack sensitivity for AKI detection in an otherwise healthy individual. Approximately 50% of GFR must be lost before detectable creatinine changes[29], thus new biomarkers such as cyclophilin are being investigated for improved diagnostic precision[30,31]. Creatinine is also a product of muscle catabolism and levels may vary. Interpretation in the context of AKI might be difficult in individuals with very high or very low levels of muscle mass[32]. Potassium should also be assessed; hyperkalemia is the main electrolyte imbalance derived from obstructive uropathy and represents one of the main indications for prompt urinary drainage. In cases of unclear etiology of AKI, other measurements as urinary sodium and fractional excretion of sodium (FeNa: Urine sodium/Serum sodium) may play a role. Typically, a urine sodium < 20 mEq/mol and FeNa < 1% are markers of a prerenal cause of AKI[33].
Initial radiologic assessment must include abdominal X-ray and, if no clear images of urinary stones are seen, a kidney-bladder ultrasound must be performed. Renal ultrasonography represents the main and less invasive imaging technique in the initial assessment of obstructive uropathy. It allows for visualizing the presence of ureterohydronephrosis or retrograde dilation of the ureterocallyceal system, and it also permits the diagnosis of reno ureteral stones, bladder tumors or the presence of acute urinary retention with a filled bladder[1]. It is important to remark that hydronephrosis indicates that the collecting system is dilated, therefore it is an anatomical finding, and it does not imply the cause of dilation or its nature. The cause of hydronephrosis can be obstructive or non-obstructive, as observed in cases of excessive hydration or a prominent extra-renal pelvis. Thus, hydronephrosis can be present in the absence of obstruction. In patients with a high clinical suspicion of obstructive uropathy, the finding of hydronephrosis has a positive predictive value of almost 70% and declines up to 6% in cases with a low clinical suspicion[34]. On the other hand, obstructive uropathy may also be present in the absence of hydronephrosis. A false negative rate of up to 35% has been recorded, usually in the event of acute ureteric colic. Diagnostic performance of ultrasound in the obstructive uropathy setting is therefore not perfect, and findings must be considered together with the previous degree of clinical suspicion. The main advantage of ultrasonography is the exclusion of obstruction in the absence of hydronephrosis with low clinical suspicion providing a negative predictive value of 98%[27]. Consequently, considering reno ureteral stones as the most prominent case of obstructive uropathy, initial ultrasonography has been proven to have a similar efficacy to initial abdominopelvic CT with less associated radiation in high-risk diagnosis[35]. Additionally, with the use of Doppler Ultrasound, it is possible to evaluate the presence of ureteral jets in the bladder; the absence or decreased frequency of these suggests the presence of urinary obstruction[36].
If initial assessment does not reveal a determined etiology, usually with the finding of urinary tract dilation in the absence of a specific obstructive cause, further imaging must be considered. Besides, ultrasonography has limited view of the middle part of the ureters and usually fails to correctly evaluate them, even if they are dilated. The following step in the diagnostic algorithm of obstructive uropathy after an undetermined ultrasound should be performing a non-contrast-enhanced CT scan[34]. It has long been proven that non-contrast CT is more effective than the long-time-gold-standard intravenous urography in the determination of the presence of ureteric obstruction[37]. Non-contrast CT is the initial most important diagnostic method following ultrasonography in obstructive uropathy. However, as we previously mentioned, despite urinary stones being the main cause for ureteric obstruction, there are many other causes which may not be detected by conventional non-contrast CT (blood clots, upper tract malignancy or other intraluminal causes…). Thus, the addition of an excretory phase in doubtful cases may reveal further information. Modern multidetector CT scans with additional excretory phase images offer a multiplanar imaging modality with good spatial resolution, allowing to locate the site and source of obstruction. Additionally, they offer further information on renal functioning due to the evaluation of the excretion of infused contrast by the kidneys[6]. Functional tests, such as diuretic isotopic renogram or other invasive tests such as Whitaker, usually do not have their place in the acute setting. They are time-consuming, difficult to interpret, and are often relegated to uncertain cases after initial stabilization or in the event of subacute/chronic onset of obstruction. It is important to highlight that these functional tests represent the sole diagnostic modality capable of diagnosing obstruction on their own[38].
The treatment of obstructive uropathy comprises three main factors. Firstly, a series of general measures should be considered. These include a general assessment of hemodynamic instability, mainly in the event of associated urosepsis, and the rapid approach of emergent life-threatening complications derived from obstruction. Once clinical stability is assured, attention should be directed towards symptomatic control which usually involves pain control. Finally, a decision on whether the patient should undergo urgent urinary diversion should be promptly made. Urinary diversion is the most important aspect of the treatment of obstructive uropathy and it should be considered as soon as diagnosis is confirmed and generally not be deferred.
Early recognition of some obstruction-related aspects is vital. Firstly, the possibility of associated infectious complications should be assessed. There are a series of signs and symptoms (fever, malaise, chills) that suggest the presence of urosepsis and evidence-proven identification clinical scales can be used (qSOFA, SOFA, NEWS…) to aid with early recognition[39]. In case urosepsis is suspected, immediate antibiotic treatment should be started either empirically or pathogen-directed in case of previous positive cultures. If empirical treatment is started, it should consider the most common pathogens producing infection in the urinary tract in accordance with the different geographical patterns of antimicrobial resistance following the local antimicrobial stewardship recommendations[40]. Laboratory tests should be performed, acute phase reactants help both in the diagnosis and give prognostic information in cases when associated urosepsis is present. Other alterations, such as hyperkalemia, should also be addressed. If moderate-severe hyperkalemia is present (K > 6 mEq/L), antihyperkalemic measures should be started. It is important to detect and suspend medications that elevate potassium plasma levels [beta-blockers, non-steroidal anti-inflammatory drugs (NSAIDs), antimineralocorticoids] and consider the use of loop diuretics if eGFR < 30 mL/min and fluid overload is present[41]. If the patient fails to respond or develops anuria, additional measures should be considered. A solution to enhance transcellular potassium shift should be started (50% glucose solution + a maximum of 10UI of regular acting Insulin), and sodium bicarbonate intravenous infusion (1 mEq/kg for 10-15 min). Beta-adrenergic agonists (albuterol, salbutamol) are also quite effective but are perhaps somewhat more controversial and more likely to produce side effects. If EKG changes are present, a cardiac membrane stabilizing agent such as calcium gluconate 10% IV should be used[42]. In patients failing to these measures, further agents such as ion exchange resins may be employed; however, it is vital to remark the need for urgent urinary diversion consideration, as the relief of obstruction will normally lead to the resolution of the derived electrolyte imbalances. All of the previously mentioned measures should not defer the need for prompt urinary diversion[43].
The obstruction of the urinary tract results in the retrograde transmission of pressure dilating intrarenal cavities against a non-distensible capsule and producing pain. In the case of bladder outlet obstruction, both the bladder and upper urinary tract are dilated and progressively painful[26]. NSAIDs decrease pressure in the collecting system during obstructive uropathy secondary to the inhibition prostaglandin secretion and their vasodilating effect on the afferent renal arteriole, which increases renal blood flow[14]. Proinflammatory signals during the acute phase of urinary obstruction have shown to increase the medullar expression of COX-2 enzyme. Parecoxib, a COX-2 specific inhibitor NSAID, has shown to decrease the downregulation of AQP2 and AQP3 and other renal transmembrane transport channels if used during bilateral obstruction or in the post obstructive phase[19]. However, this in vitro observed effect has not been associated with less polyuria or urinary concentration inability reversal during the post obstructive phase[44]. Despite NSAIDs should be used with caution in patients with a certain degree of renal failure, they represent the first-line treatment for pain control in urinary obstruction[45].
Diversion of the urinary tract is the most important aspect in the treatment of obstructive uropathy. If a decision of urinary diversion is made, it should be performed as soon as possible. The chosen technique will vary depending on the site of obstruction, patient characteristics, and the treating team preferences. Upper tract obstruction is usually managed by retrograde diversion using a ureteral double-J stent. If not feasible, diversion with percutaneous nephrostomy should be considered. Extrinsic compression of the urinary tract causing urinary obstruction (retroperitoneal mass, fibrosis…) retrograde diversion with a ureteral stent is associated with up to 42% failure rates with 29% of these patients requiring a subsequent diversion with a nephrostomy tube. Other independent bad prognostic markers of urinary diversion with stents are cancer diagnosis, basal creatinine values > 1.3 mg/dL or the need for systemic treatment after diversion[46]. The use of metallic stents (Resonance®) may help in these particular cases, being able to successfully divert the urinary tract in cases of extrinsic compression more efficiently than conventional stents[47]. In the case of bladder outlet obstruction, urinary diversion with a urinary catheter should be performed. Following unsuccessful urinary catheterization, a decision to insert a suprapubic catheter should be considered.
The timing of urinary diversion is also substantially important to assess. A distinction between emergent need for urinary diversion and other deferrable situations should be made. Immediate need for emergent diversion include the presence of high-risk infectious complications (urosepsis, pyonephrosis), solitary kidney, upper tract bilateral obstruction, previously marked renal impairment or hyperkalemia. Bacteriemia without associated sepsis does not require emergent diversion but rather urgent; it could be safely deferred 6-8 h[23]. Assuming urolithiasis as the most prominent cause of upper UTO, early urinary diversion even in the absence of the previously mentioned indications has also been considered. A recent study by Innes et al[48] in a multicentric cohort of patients admitted to emergency departments throughout Canada explored early urinary diversion even in the absence of emergent diversion criteria vs a more conservative approach. Early diversion showed benefits for larger stones (> 7 mm) and medium-sized stones (5-7 mm) located in the proximal or mid-ureter, reducing emergency department admissions and/or the need for intervention within the first 60 d after diagnosis. In smaller (< 5 mm) or medium-size stones (5-7 mm) in the distal ureter, a conservative approach was found to be superior over early diversion.
In patients amenable to conservative management, medical expulsive therapy is often considered as the preferred treatment of choice. Spontaneous passage of urinary stones depends on stone size and their relative location within the urinary tract. Regarding their location, spontaneous passage rates of up to 68% at the distal ureter, 58% mid-ureter and 49% proximal ureter have been recorded. Smaller stones (< 5 mm) are prone to spontaneous passage in up to 75% cases. However, bigger stones (> 5 mm), are less likely to pass, in up to 62% cases[49]. Medical expulsive therapy (MET) involves the use of an alpha-adrenergic blocker (tamsulosin, silodosin) based on the smooth-muscle relaxation effect it enhances at the level of the ureteric wall[50]. This class effect of alpha-blockers has been demonstrated, although it is an off-label indication[51]. Other drug classes, such as phosphodiestarase-5 inhibitors, calcium channel inhibitors or corticosteroids have also been proposed in combination with alpha-blockers. However, based on small studies with contradictory evidence, no recommendations have been made in most clinical guidelines regarding their use. Regarding alpha-blockers, the available evidence is also contradictory. Several well-designed, double-blinded randomized controlled trials show limited or no efficacy, except for some advantage in treating distal ureteral stones larger than 5 mm[52,53]. A recent meta-analysis comparing the use of tamsulosin or tadalafil as MET for distal ureteral stones found a higher stone expulsion rate in the patients treated with tadalafil, with similar stone expulsion times[54]. Based on current evidence, European guidelines recommend the use of alpha-blockers, not other drug classes, as a treatment option for patients amenable to conservative treatment with distal > 5 mm ureteral stones[55].
Another matter of debate regarding urinary tract diversion is the risk of tumor seeding or metachronous tumor development in the presence of malignancy. Upper urinary tract carcinoma or bladder tumors with ureteral orifice invasion are another prominent cause of obstructive uropathy and sometimes require urinary diversion. The insertion of a ureteral stent may facilitate tumor spreading along the urinary tract or cause a reflux mechanism at the level of the ureteral orifice which may ease the translocation of bladder tumoral cells to the ureter or kidney[56]. A recent meta-analysis explored the association between prophylactic stenting of patients with bladder tumors involving the ureteral orifice during transurethral resection of bladder tumor (TURBT) or radical cystectomy, and the development of metach
Hematuria following urinary tract drainage is based on the assumption that rapid or sudden decompression of a high-pressure dilated cavity might lead to vessel breakdown and hemorrhage. To date, debate persists on whether decompression should be rapid or gradual. Hematuria ex-vacuo, as it is often referred to, occurs mainly after catheterization following bladder outlet obstruction, although it may also be observed following upper urinary tract diversion. It is more common to be observed in obstructive uropathy affecting the bladder due to its higher capacity and distensibility favoring the rapid and significant variation in volume and pressure that stresses bladder wall vascularization[58]. The first randomized clinical trial to study the need for gradual or rapid urinary decompression, found no differences between gradual and rapid emptying of the bladder for urinary retention[59]. A later meta-analysis confirmed these findings and concluded that currently available data suggest that rapid urinary decompression is an effective and safe method with a complication rate similar to that of gradual decompression[60].
Post-obstructive polyuria is a common finding after urinary tract drainage with an estimated prevalence of up to 50% in some studies[61]. Its physiopathological basis resides on fluid overload and AQP2-3 and sodium channel downregulation in the obstructed kidney leading to the inability of urinary concentration, resulting in the loss of water and solutes due to lack of reabsorption[20]. Similarly to hematuria ex-vacuo, it is more frequently observed in cases of bilateral or bladder outlet obstruction. Post-obstructive polyuria is often defined as a urinary output > 200 cc/h during the first 2 h following urinary tract drainage[62]. It can also be defined by other authors as a urinary output > 3000 cc during the first 24 h following drainage[63].
The polyuric phase following diversion often lasts about 48 h, during which strict monitoring is mandatory. Management often involves close monitoring of vital signs, urinary output, and electrolyte imbalances together with volume reposition. In stable patients, oral reposition is preferred over intravenous administration of fluids. The aim is to approximately replace 50%-75% of hourly urinary output with balanced solutions such as lactated Ringer’s solution. In patients with sodium overload, compensation should be minimally applied (30%-50% on the first day) because polyuria already allows for sodium levels correction. In patients with dehydration, fluid compensation should be aggressively managed (125%-150% during the first day) because the initial output is negative[64].
The functional recovery of the obstructed kidney following urinary decompression will depend on the degree of obstruction, total obstruction time and the presence of associated urinary infection. Renal recovery after obstruction has been studied in a variety of animal models, experimentally reproducing a range of scenarios comparing different types of obstruction based on their origin, time or onset[65]. Following a complete unilateral occlusion for a period of 7 d, classic studies in dogs found a 100% renal function recovery. If the obstructive period was prolonged for 14 d, renal recovery following decompression declined to 70% and to a further 30% if obstruction was maintained for 4 wk. After 6 wk of complete unilateral obstruction, absence of renal recovery was observed following drainage of the urinary tract[66]. Other studies in rat models, have observed a decline in GFR and renal blood flow following 7 d of complete unilateral obstruction which remains diminished to 40% up to 30 d following urinary drainage[67]. Other experimental human studies have demonstrated an even more rapid deleterious effect during the first 24-72 h and in the first 2 wk, which may be irreversible[11]. Therefore, even though renal recovery might be complete following treatment, there are several changes that may be unrecoverable following even a 3-d long unilateral obstruction. Damage to the kidney, which initially might not be reflected in renal function tests, might condition a worse response and recovery in subsequent obstructive episodes. A total period of 6 wk of obstruction is often considered the threshold for non-recoverable permanent kidney injury.
In addition to the previously mentioned causes of renal function loss, which respond to hemodynamic changes during obstructive uropathy resulting in impaired renal blood flow and acute tubular necrosis, there are other pathways that may hinder renal recovery following obstruction. A proinflammatory immune cellular response is triggered which results in interstitial expansion and fibrosis which may lead to tubulointerstitial necrosis. This fibrotic response leads to the deposition of elastin, collagen and other pro-fibrotic molecules which may lead to irreparable tissue damage in early phases of obstruction[17]. Collagen and elastin deposition leads to scarring and thinning of the renal parenchyma which can be radiologically assessed in order to determine the extent of damage and future prognosis[68]. Human studies on patients undergoing pyeloplasty, have also determined that higher concentrations of elastin[69] and collagen[70] in resected ureter specimens are associated with a slower functional and anatomical recovery following surgery.
Literature provides insights into therapies aimed at mitigating kidney damage in cases of chronic renal failure[23]. These treatment options serve as potential adjuncts following the management of obstructive uropathy. Among these, Angiotensin Receptor 1 Blockers (ARBs) emerge as nephroprotective agents in chronic kidney disease. Early initiation of ARB therapy holds promise in preserving renal function[71]. Furthermore, research has illustrated the advantageous impact of ARBs on glomerular injury. These benefits are attributed to the blockade of the AT1 receptor, and the amplified effects of angiotensin mediated through the AT2 receptor[72].
Obstructive uropathy is a prevalent cause of AKI. If incorrectly managed, it can potentially lead to death or irreversible permanent tissue damage leading to chronic kidney disease. It is of vital importance to perform a correct initial assessment in order to identify patients that may benefit from early urinary diversion to avoid potential complications. Acute obstruction of the urinary tract leads to volume overload, electrolyte imbalances and infectious complications that need to be correctly addressed, therefore, a multidisciplinary team is key. Management of UTO does not end after urinary diversion, there are several side effects and complications derived from the intervention that need to be early identified and corrected. Timing and adequate management of such condition will determine renal recovery following obstruction.
| 1. | Chávez-Iñiguez JS, Navarro-Gallardo GJ, Medina-González R, Alcantar-Vallin L, García-García G. Acute Kidney Injury Caused by Obstructive Nephropathy. Int J Nephrol. 2020;2020:8846622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Yaxley J, Yaxley W. Obstructive uropathy - acute and chronic medical management. World J Nephrol. 2023;12:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (7)] |
| 3. | Wang Y, Wang J, Su T, Qu Z, Zhao M, Yang L; ISN AKF 0by25 China Consortium. Community-Acquired Acute Kidney Injury: A Nationwide Survey in China. Am J Kidney Dis. 2017;69:647-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 4. | Yang L, Xing G, Wang L, Wu Y, Li S, Xu G, He Q, Chen J, Chen M, Liu X, Zhu Z, Yang L, Lian X, Ding F, Li Y, Wang H, Wang J, Wang R, Mei C, Xu J, Li R, Cao J, Zhang L, Wang Y, Xu J, Bao B, Liu B, Chen H, Li S, Zha Y, Luo Q, Chen D, Shen Y, Liao Y, Zhang Z, Wang X, Zhang K, Liu L, Mao P, Guo C, Li J, Wang Z, Bai S, Shi S, Wang Y, Wang J, Liu Z, Wang F, Huang D, Wang S, Ge S, Shen Q, Zhang P, Wu L, Pan M, Zou X, Zhu P, Zhao J, Zhou M, Yang L, Hu W, Wang J, Liu B, Zhang T, Han J, Wen T, Zhao M, Wang H; ISN AKF 0by25 China Consortiums. Acute kidney injury in China: a cross-sectional survey. Lancet. 2015;386:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 330] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 5. | Caddeo G, Williams ST, McIntyre CW, Selby NM. Acute kidney injury in urology patients: incidence, causes and outcomes. Nephrourol Mon. 2013;5:955-961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 6. | Khandelwal S, Dhande R, Sood A, Parihar P, Mishra GV. Role of Multidetector Computed Tomography Urography in the Evaluation of Obstructive Uropathy: A Review. Cureus. 2023;15:e48038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 7. | Nørregaard R, Mutsaers HAM, Frøkiær J, Kwon TH. Obstructive nephropathy and molecular pathophysiology of renal interstitial fibrosis. Physiol Rev. 2023;103:2827-2872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 8. | Klahr S. Obstructive nephropathy. Intern Med. 2000;39:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 9. | Cai PY, Lee RS. Ureteropelvic Junction Obstruction/Hydronephrosis. Urol Clin North Am. 2023;50:361-369. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 10. | Klahr S, Morrison A, Buerkert J. Effects of urinary tract obstruction on renal function. Contrib Nephrol. 1980;23:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Vela-Navarrete R. Percutaneous intrapelvic pressure determinations in the study of hydronephrosis. Invest Urol. 1971;8:526-533. [PubMed] |
| 12. | Hammad FT. The long-term renal effects of short periods of unilateral ureteral obstruction. Int J Physiol Pathophysiol Pharmacol. 2022;14:60-72. [PubMed] |
| 13. | Allen JT, Vaughan ED Jr, Gillenwater JY. The effect of indomethacin on renal blood flow and uretral pressure in unilateral ureteral obstruction in a awake dogs. Invest Urol. 1978;15:324-327. [PubMed] |
| 14. | Vaughan ED Jr, Sorenson EJ, Gillenwater JY. The renal hemodynamic response to chronic unilateral complete ureteral occlusion. Invest Urol. 1970;8:78-90. [PubMed] |
| 15. | Gulmi FA, Matthews GJ, Marion D, von Lutterotti N, Vaughan ED. Volume expansion enhances the recovery of renal function and prolongs the diuresis and natriuresis after release of bilateral ureteral obstruction: a possible role for atrial natriuretic peptide. J Urol. 1995;153:1276-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 384] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 17. | Diamond JR, Kees-Folts D, Ding G, Frye JE, Restrepo NC. Macrophages, monocyte chemoattractant peptide-1, and TGF-beta 1 in experimental hydronephrosis. Am J Physiol. 1994;266:F926-F933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Pascual L, Oliva J, Vega-P J, Príncipi I, Vallés P. Renal histology in ureteropelvic junction obstruction: are histological changes a consequence of hyperfiltration? J Urol. 1998;160:976-9; discussion 994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Nørregaard R, Jensen BL, Li C, Wang W, Knepper MA, Nielsen S, Frøkiaer J. COX-2 inhibition prevents downregulation of key renal water and sodium transport proteins in response to bilateral ureteral obstruction. Am J Physiol Renal Physiol. 2005;289:F322-F333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 86] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 20. | Li C, Wang W, Kwon TH, Isikay L, Wen JG, Marples D, Djurhuus JC, Stockwell A, Knepper MA, Nielsen S, Frøkiaer J. Downregulation of AQP1, -2, and -3 after ureteral obstruction is associated with a long-term urine-concentrating defect. Am J Physiol Renal Physiol. 2001;281:F163-F171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Klein J, Gonzalez J, Miravete M, Caubet C, Chaaya R, Decramer S, Bandin F, Bascands JL, Buffin-Meyer B, Schanstra JP. Congenital ureteropelvic junction obstruction: human disease and animal models. Int J Exp Pathol. 2011;92:168-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Sutherland RW. Obstructive Uropathy. National Kidney Foundation Primer on Kidney Diseases. 2014;. [DOI] [Full Text] |
| 23. | Mourmouris. Obstructive Uropathy: From Etiopathology to Therapy. World J Nephrol Urol. 2014;. [DOI] [Full Text] |
| 24. | Lapides J, Woodburne RT. Configuration of ureteral lumen during peristalsis. J Urol. 1972;108:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Constantinou CE, Granato JJ Jr, Govan DE. Dynamics of the upper urinary tract: accommodations in the rate and stroke volume of ureteral peristalsis as a response to transient alteration in urine flow rate. Urol Int. 1974;29:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Martínez-ballesteros C, Martínez-salamanca J, Sola Galarza I, Carballido Rodríguez J. Uropatía obstructiva. Medicine - Programa de Formación Médica Continuada Acreditado. 2011;10:5595-5600. [DOI] [Full Text] |
| 28. | Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 633] [Cited by in RCA: 1148] [Article Influence: 191.3] [Reference Citation Analysis (0)] |
| 29. | Delanaye P, Cavalier E, Pottel H. Serum Creatinine: Not So Simple! Nephron. 2017;136:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 212] [Article Influence: 26.5] [Reference Citation Analysis (1)] |
| 30. | Hou W, Leong KG, Ozols E, Tesch GH, Nikolic-Paterson DJ, Ma FY. Cyclophilin D promotes tubular cell damage and the development of interstitial fibrosis in the obstructed kidney. Clin Exp Pharmacol Physiol. 2018;45:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Cabello R, Fontecha-Barriuso M, Martin-Sanchez D, Lopez-Diaz AM, Carrasco S, Mahillo I, Gonzalez-Enguita C, Sanchez-Niño MD, Ortiz A, Sanz AB. Urinary Cyclophilin A as Marker of Tubular Cell Death and Kidney Injury. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Bouquegneau A, Vidal-Petiot E, Vrtovsnik F, Cavalier E, Rorive M, Krzesinski JM, Delanaye P, Flamant M. Modification of Diet in Renal Disease versus Chronic Kidney Disease Epidemiology Collaboration equation to estimate glomerular filtration rate in obese patients. Nephrol Dial Transplant. 2013;28 Suppl 4:iv122-iv130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Pépin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Leclerc E, Sakai Y, Fujii T. Microfluidic PDMS (polydimethylsiloxane) bioreactor for large-scale culture of hepatocytes. Biotechnol Prog. 2004;20:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 160] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 35. | Smith-Bindman R, Aubin C, Bailitz J, Bengiamin RN, Camargo CA Jr, Corbo J, Dean AJ, Goldstein RB, Griffey RT, Jay GD, Kang TL, Kriesel DR, Ma OJ, Mallin M, Manson W, Melnikow J, Miglioretti DL, Miller SK, Mills LD, Miner JR, Moghadassi M, Noble VE, Press GM, Stoller ML, Valencia VE, Wang J, Wang RC, Cummings SR. Ultrasonography versus computed tomography for suspected nephrolithiasis. N Engl J Med. 2014;371:1100-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 417] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 36. | Hassan W, Sharif I, El Khalid S, Ellahibux K, Sultan S, Waqar A, Zohaib A, Yousuf F. Doppler-Assessed Ureteric Jet Frequency: A Valuable Predictor of Ureteric Obstruction. Cureus. 2021;13:e18290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Smith RC, Rosenfield AT, Choe KA, Essenmacher KR, Verga M, Glickman MG, Lange RC. Acute flank pain: comparison of non-contrast-enhanced CT and intravenous urography. Radiology. 1995;194:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 422] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 38. | Lien WC, Chang YC, Chou HH, Lin LC, Liu YP, Liu L, Chan YT, Kuan FS. Detecting Hydronephrosis Through Ultrasound Images Using State-of-the-Art Deep Learning Models. Ultrasound Med Biol. 2023;49:723-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 39. | Jones AE, Trzeciak S, Kline JA. The Sequential Organ Failure Assessment score for predicting outcome in patients with severe sepsis and evidence of hypoperfusion at the time of emergency department presentation. Crit Care Med. 2009;37:1649-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 518] [Cited by in RCA: 454] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 40. | Kranz J, Bartoletti R, Bruyère F, Cai T, Geerlings S, Köves B, Schubert S, Pilatz A, Veeratterapillay R, Wagenlehner FME, Bausch K, Devlies W, Horváth J, Leitner L, Mantica G, Mezei T, Smith EJ, Bonkat G. European Association of Urology Guidelines on Urological Infections: Summary of the 2024 Guidelines. Eur Urol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 62] [Reference Citation Analysis (0)] |
| 41. | Moore PK, Hsu RK, Liu KD. Management of Acute Kidney Injury: Core Curriculum 2018. Am J Kidney Dis. 2018;72:136-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 42. | Palmer BF, Clegg DJ. Physiology and Pathophysiology of Potassium Homeostasis: Core Curriculum 2019. Am J Kidney Dis. 2019;74:682-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 43. | Wang CJ, Hsu CS, Chen HW, Chang CH, Tsai PC. Percutaneous nephrostomy versus ureteroscopic management of sepsis associated with ureteral stone impaction: a randomized controlled trial. Urolithiasis. 2016;44:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Nørregaard R, Jensen BL, Topcu SO, Diget M, Schweer H, Knepper MA, Nielsen S, Frøkiaer J. COX-2 activity transiently contributes to increased water and NaCl excretion in the polyuric phase after release of ureteral obstruction. Am J Physiol Renal Physiol. 2007;292:F1322-F1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Gu HY, Luo J, Wu JY, Yao QS, Niu YM, Zhang C. Increasing Nonsteroidal Anti-inflammatory Drugs and Reducing Opioids or Paracetamol in the Management of Acute Renal Colic: Based on Three-Stage Study Design of Network Meta-Analysis of Randomized Controlled Trials. Front Pharmacol. 2019;10:96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 46. | Chung SY, Stein RJ, Landsittel D, Davies BJ, Cuellar DC, Hrebinko RL, Tarin T, Averch TD. 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. J Urol. 2004;172:592-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 47. | Chow PM, Chiang IN, Chen CY, Huang KH, Hsu JS, Wang SM, Lee YJ, Yu HJ, Pu YS, Huang CY. Malignant Ureteral Obstruction: Functional Duration of Metallic versus Polymeric Ureteral Stents. PLoS One. 2015;10:e0135566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Innes GD, Scheuermeyer FX, McRae AD, Law MR, Teichman JMH, Grafstein E, Andruchow JE. Which Patients Should Have Early Surgical Intervention for Acute Ureteral Colic? J Urol. 2021;205:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 49. | Yallappa S, Amer T, Jones P, Greco F, Tailly T, Somani BK, Umez-Eronini N, Aboumarzouk OM. Natural History of Conservatively Managed Ureteral Stones: Analysis of 6600 Patients. J Endourol. 2018;32:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 50. | Yilmaz E, Batislam E, Basar MM, Tuglu D, Ferhat M, Basar H. The comparison and efficacy of 3 different alpha1-adrenergic blockers for distal ureteral stones. J Urol. 2005;173:2010-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 170] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 51. | Liu XJ, Wen JG, Wan YD, Hu BW, Wang QW, Wang Y. Role of silodosin as medical expulsive therapy in ureteral calculi: a meta-analysis of randomized controlled trials. Urolithiasis. 2018;46:211-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Bai Y, Yang Y, Wang X, Tang Y, Han P, Wang J. Tadalafil Facilitates the Distal Ureteral Stone Expulsion: A Meta-Analysis. J Endourol. 2017;31:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 53. | Porpiglia F, Vaccino D, Billia M, Renard J, Cracco C, Ghignone G, Scoffone C, Terrone C, Scarpa RM. Corticosteroids and tamsulosin in the medical expulsive therapy for symptomatic distal ureter stones: single drug or association? Eur Urol. 2006;50:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 54. | Belkovsky M, Zogaib GV, Passerotti CC, Artifon ELA, Otoch JP, da Cruz JAS. Tamsulosin vs. Tadalafil as medical expulsive therapy for distal ureteral stones: a systematic review and meta-analysis. Int Braz J Urol. 2023;49:668-676 [. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 55. | Türk C, Knoll T, Seitz C, Skolarikos A, Chapple C, McClinton S; European Association of Urology. Medical Expulsive Therapy for Ureterolithiasis: The EAU Recommendations in 2016. Eur Urol. 2017;71:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 56. | Hupe MC, Dormayer L, Ozimek T, Struck JP, Hennig MJP, Klee M, von Klot CAJ, Kuczyk MA, Merseburger AS, Kramer MW. Impact of double J stenting or nephrostomy placement during transurethral resection of bladder tumour on the incidence of metachronous upper urinary tract urothelial cancer. BMC Cancer. 2020;20:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Sountoulides P, Pyrgidis N, Brookman-May S, Mykoniatis I, Karasavvidis T, Hatzichristou D. Does Ureteral Stenting Increase the Risk of Metachronous Upper Tract Urothelial Carcinoma in Patients with Bladder Tumors? A Systematic Review and Meta-analysis. J Urol. 2021;205:956-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 58. | Nyman MA, Schwenk NM, Silverstein MD. Management of urinary retention: rapid vs gradual decompression and risk of complications. Mayo Clin Proc. 1997;72:951-956. [PubMed] [DOI] [Full Text] |
| 59. | Boettcher S, Brandt AS, Roth S, Mathers MJ, Lazica DA. Urinary retention: benefit of gradual bladder decompression - myth or truth? A randomized controlled trial. Urol Int. 2013;91:140-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 60. | Wu MY, Chang JR, Lee YK, Lin PC, Tsai TY. The Effect and Safety of Rapid and Gradual Urinary Decompression in Urine Retention: A Systematic Review and Meta-Analysis. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 61. | Roth JD, Lesier JD, Casey JT, Szymanski KM, Whittam BM, Misseri R, Rink RC, Cain MP. Incidence of pathologic postobstructive diuresis after resolution of ureteropelvic junction obstruction with a normal contralateral kidney. J Pediatr Urol. 2018;14:557.e1-557.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 62. | Leslie SW, Sajjad H, Sharma S. Postobstructive Diuresis. 2024 Feb 16. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. [PubMed] |
| 63. | Halbgewachs C, Domes T. Postobstructive diuresis: pay close attention to urinary retention. Can Fam Physician. 2015;61:137-142. [PubMed] |
| 64. | Harrison S, Lasri A, Jabbour Y, Slaoui A, Djamal J, Karmouni T, Khader KE, Koutani A, Andaloussi AIA. Post-Obstructive Diuresis: Physiopathology, Diagnosis and Management after Urological Treatment of Obstructive Renal Failure. OJU. 2018;08:267-274. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 65. | Hernando Arteche A, Chávez Roa C, González-Enguita C. Fisiopatología de la obstrucción urinaria [Pathophysiology of urinary obstruction]. In: Tratado de Urología de la AEU [Spanish Urological Association Urologic Treaty]. Madrid: AEU; 2020. p. 1-29. . |
| 66. | Vaughan ED Jr, Gillenwater JY. Recovery following complete chronic unilateral ureteral occlusion: functional, radiographic and pathologic alterations. J Urol. 1971;106:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Chaabane W, Praddaude F, Buleon M, Jaafar A, Vallet M, Rischmann P, Galarreta CI, Chevalier RL, Tack I. Renal functional decline and glomerulotubular injury are arrested but not restored by release of unilateral ureteral obstruction (UUO). Am J Physiol Renal Physiol. 2013;304:F432-F439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 68. | Feder MT, Blitstein J, Mason B, Hoenig DM. Predicting differential renal function using computerized tomography measurements of renal parenchymal area. J Urol. 2008;180:2110-2115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Kim DS, Noh JY, Jeong HJ, Kim MJ, Jeon HJ, Han SW. Elastin content of the renal pelvis and ureter determines post-pyeloplasty recovery. J Urol. 2005;173:962-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 70. | Kiratli PO, Orhan D, Gedik GK, Tekgul S. Relation between radionuclide imaging and pathologic findings of ureteropelvic junction obstruction in neonatal hydronephrosis. Scand J Urol Nephrol. 2008;42:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | Álvarez-Prats A, Hernández-Perera O, Díaz-Herrera P, Ucero ÁC, Anabitarte-Prieto A, Losada-Cabrera A, Ortiz A, Rodríguez-Pérez JC. Combination therapy with an angiotensin II receptor blocker and an HMG-CoA reductase inhibitor in experimental subtotal nephrectomy. Nephrol Dial Transplant. 2012;27:2720-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Naito T, Ma LJ, Yang H, Zuo Y, Tang Y, Han JY, Kon V, Fogo AB. Angiotensin type 2 receptor actions contribute to angiotensin type 1 receptor blocker effects on kidney fibrosis. Am J Physiol Renal Physiol. 2010;298:F683-F691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |